Abstract
Background
Temperature and light are two critical environmental stimuli that greatly impact our agricultural industry. We explored the effects of varying temperature and light conditions on the metabolomics of basil, a plant widely used in cuisine and for medicinal purposes worldwide. Basil plants were subjected to elevated temperatures of 30 °C, 35 °C, and 40 °C, along with two light intensities (D11 and D23).
Results
Our results showed that the maximum shoot length (42.98 cm) was achieved at T30 and D23, the maximum leaf area (6.92 cm) at T30/D11, and the highest number of leaves (472) at T40/D11. However, at the higher temperature of T40, the number of leaves increased, but shoot length and leaf size significantly decreased (p < 0.05). Maxim shoot biomass (81 gm) and root biomass (91.2 gm) was recorded at T35/D23 treatments. Metabolomic studies of basil revealed that rosmarinic acid (RA), and linalool and eugenol were the major phenolic and volatile organic compounds (VOCs) under different growth conditions. RA levels were higher at 30 °C, correlated with elevated shikimate levels, indicating a carbon supply through the TCA cycle. In contrast, the growth conditions of 35 °C/D11 (μmol/m2/d) up-regulated the glyoxylate cycle (fumaric acid and malic acid) as part of stomatal closure, preventing ROS accumulation and resulting in increased VOC emission. Exposure to the higher temperature of 40 °C induced higher amino acid accumulation, improving temperature stresstolerance.
Conclusions
This study suggests that elevated temperature and light intensity affected the overall health of basil plants by modulating underlying growth mechanisms. Furthermore, elevated temperature plays a vital role in plant defense mechanisms by mediating the production of secondary metabolites associated with plant defense responses.
Graphical Abstract

Similar content being viewed by others
Introduction
Plants are sensitive to environmental stimuli and adaptable to changes in their surroundings [1]. Temperature and light are the most critical environmental cues that influence the growth and development of plants by providing seasonal information [2]. The interrelated and complex effects of temperature and light trigger various plant responses. Light significantly contributes to acclimation responses to both low and high temperatures and can influence the extent of stress damage [3]. Plant photoreceptors, like phytochrome and cryptochrome, crucially recognize the quality, quantity, and direction of light, as well as temperature sensing. They differentially affect phytohormone signaling based on temperature levels [1, 4, 5]. Photoreceptors are responsible for both light quality and high temperatures, showing that photo- and thermo-morphogenesis share a core signaling pathway, indicating their interdependence [6,7,8,9,10,11]. While normal conditions tend to counteract light signals in stem growth adjustment, a temporary temperature increase supports the light-induced inhibition of hypocotyl growth [12]. Karayekov, Sellaro [12] demonstrated that the high-temperature response in Arabidopsis depends on the presence or absence of light, with a significant effect in response to light but not in the dark. Additionally, various developmental processes, such as seed germination, seedling de-etiolation, vegetative growth, and flowering, are induced by unfavorable conditions [6, 7]. These responses are considered part of the defense mechanism activated by plants to perceive environmental stimuli and minimize damage from challenging conditions [2, 13].
Under unfavourable environmental conditions, such as heat stress, plants experience reduced productivity and shortened life cycles due to diminished photosynthetic efficiency and compromised photochemical reactions [14]. Additionally, secondary metabolite accumulation contributes to environmental adaptation and acts as a defense mechanism against external stimuli [15]. Temperature stress can also lead to the accumulation of reactive oxygen species (ROS) in plant cells, resulting in lipid peroxidation, enzyme inactivation, nucleic acid and protein degradation [Full size image
Status of VOCs under elevated temperature and light
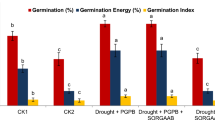
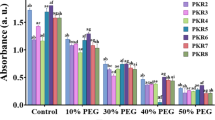
We detected 44 VOCs that included 5 phenylpropenes, 16 monoterpenes, 15 sesquiterpenes, 5 aldehydes, and 3 other compound groups (Table 2). Linalool (monoterpene) and eugenol (phenylpropene) were identified as the major constituents of VOCs in basil, as displayed in Additional file 1: Fig. S2. Among the other components, relatively major compounds accounting for more than 1% on average were (E)-α-bergamotene (4.4%), eucalyptol (6.5%), α-terpineol (3.1%), and epi-α-cadinol (2.5%) and their quantification results were presented in Fig. 4. However, the distribution of total VOCs content, ranging from 163.8 µg/20 mg to 394.3 µg/20 mg, was determined by two main constituents (linalool and eugenol), which accounted for 26.2% and 48.1% on average. Under the lowest temperature condition (T30), a high DLI had a positive effect on phenolic compounds (TPC and RAC) as shown in Fig. 1, however had a negative effect on VOCs, as indicated in Table 3. Meanwhile, temperature stress-induced conditions (T35 and T40) and the lowest DLI (D11) stimulated VOCs production (Fig. 4). The highest content was observed in T35/D11 (361.9 ± 33.7 µg/20 mg), followed by T35/D23 (305.3 ± 25.7 µg/20 mg) and T40/D11 (293.4 ± 27.9 µg/20 mg). These opposite trends resulted in a negative correlation between the main VOCs, including linalool, eugenol, eucalyptol, (E)-α-bergamotene, α-terpineol, and epi-α-cadinol, with TPC (r = 0.1860, − 0.4271, − 0.6910, − 0.5997, − 0.7479, and − 0.3415, respectively) and RAC (r = 0.2366, − 0.4271, − 0.7481, − 0.6196, − 0.8166, and − 0.4138, respectively), as presented in Fig. 2.
Content (µg/g) of main compositions of VOCs. A Eugenol, B linalool, C eucalyptol (C), (E)-α-bergamotene (D), epi-α-cadinol (E), α-terpineol (F), etc. (G), and total VOCs (H) in basil were analyzed using HS-SPME-GC-ToF–MS. In box-plots, each group consisted of five biological replicates represented by individual scores and different light conditions were indicated by color (D11, orange; D23, blue). Statistical differences between temperature groups were determined using Student's T-test. ***p < 0.005; NS, non-significant
Analysis of antioxidant activities
DPPH and ABTS radical assays have been developed to spectrophotometrically monitor antioxidant activities as such assays are considered effective for screening and identifying the natural bioactivity of complex mixtures. We recorded higher activity in the ABTS assay (RC50 = 65.7 ± 15.8 μg/mL) compared to the DPPH assay (RC50 = 133.2 ± 3.2 μg/mL) as shown in Fig. 3. Notably, temperature was found a significant factor in antioxidant activity. Based on the temperature grou** (Fig. 3C, D), the T30 treatment exhibited stronger DPPH (RC50 = 107.1 ± 19.4 μg/mL) and ABTS radical scavenging activities (RC50 = 55.2 ± 5.7 μg/mL) compared to T35 (DPPH RC50 = 141.6 ± 29.7 μg/mL; ABTS RC50 = 66.3 ± 16.3 μg/mL) and T40 (DPPH RC50 = 150.9 ± 29.3 μg/mL; ABTS RC50 = 75.5 ± 16.3 μg/mL).
Under the same DLI conditions, higher activity was observed at lower temperatures, and this temperature-dependent trend became more pronounced at lower DLI levels, regardless of the experimental method. Therefore, strong correlations (p < 0.0001) were observed between phenolic contents and antioxidant activities (Fig. 2), as follows: TPC-DPPH: r = 0.6666; TPC-ABTS: r = 0.6632; RAC-DPPH: r = 0.5960; and RAC-ABTS: r = 0.5803. Considering the results categorized by temperature conditions, the highest functional value, as judged by TPC, RAC, and antioxidant activities of basil grown under T30 conditions, was clearly demonstrated.
ANOVA analysis
A two-way ANOVA analysis was performed to evaluate the effects of variables (temperature and DLI) on growth patterns, secondary metabolites (TPC, RAC, and VOCs), and antioxidant activities. While the production based on shoot weight was significantly influenced by the combination of temperature and DLI, not by single factors, a significant effect of temperature was observed in most cases (p < 0.05). In particular, a highly significant effect of temperature on TPC, RAC, and total VOCs was determined (p < 0.005). TPC and RAC showed a p value of less than 0.005 only with temperature as a factor, but total VOCs significantly changed with temperature alone and when combined with DLI.
Profiling of primary metabolites
Based on the secondary metabolite analysis and two-way ANOVA, it was elucidated that temperature primarily regulates the secondary metabolism of basil. We also conducted an analysis of primary metabolites in this study. The variables within the same category (such as organic acids, amino acids, sugars, and fatty acids) displayed positive correlations with one another, as indicated in Fig. 2. Furthermore, the results revealed that primary metabolites exhibited variations based on temperature, as illustrated in Fig. 5. Consequently, we averaged the results by temperature to facilitate interpretation. The total organic content and total sugar content decreased with rising temperatures, while the trend for total amino acids was the opposite. Notably, the highest total organic acids content was observed in the T30 condition (128.5 ± 21.3 μg/10 mg), which was approximately 1.17- and 1.29- times higher than the T35 (110.2 ± 16.2 μg/10 mg) and T40 (55.2 ± 16.4 μg/10 mg) conditions. The content of all observed organic acids (fumaric acid, malic acid, shikimic acid, and citric acid) related to the TCA cycle contributed significantly to the total organic acids content, with positive correlations (r = 0.7180, 0.8260, 0.8860, and 0.5643, respectively). In the T30 condition, it was observed that as DLI increased, the content of these organic acids and sugars also increased. Similarly, the total sugar content followed the same trend. T30 (90.5 ± 32.8 μg/10 mg) exhibited 1.64- and 3.64- times higher content compared to T35 (55.2 ± 16.4 μg/10 mg) and T40 (24.9 ± 3.5 μg/10 mg), respectively. Most sugar components, except for sucrose, displayed significantly positive correlations with total sugar content. In particular, glucose, which represented over 50% of the total sugar content, exhibited a very strong correlation with total sugar content (r value of 0.999). This is why the highest total sugar content was determined in T30/D23 (120.6 ± 8.0 μg/10 mg), where the glucose content was the highest (99.0 ± 6.9 μg/10 mg). Conversely, the total amino acids content exhibited a significant correlation within the total amino acids content. All detected amino acids, except for 5-oxoproline, showed positive correlations. The content of total amino acids was found to be 1.33 times higher in T40 (27.5 ± 2.2 μg/10 mg) compared to T30 (20.6 ± 2.7 μg/10 mg) and 1.32 times higher compared to T35 (20.8 ± 7.0 μg/10 mg).
Heatmap analysis between cultivation conditions and metabolites. Visulization was conducted un including (A) growth parameters, (B) organic acids, (C) fatty acids, (D) amino acids, (E) sugars, (F) phenolics and antioxidants, and (G) VOCs for each sample. The data were normalized, and a color-bar positioned above the heatmap indicated the Pearson correlation coefficient, with blue representing lower content and red representing higher content. Rows represented the variables, while columns represented the samples, which were color-coded according to the respective growth conditions: red for T30/D11, orange for T30/D23, blue for T35/D11, pink for T35/D23, green for T40/D11, and yellow for T40/D23