Abstract
Background
Fruit and vegetables are highly perishable. In an era where reducing food waste is absolutely essential, packaging is important for maintaining the postharvest quality of these fresh products. Research is working to reduce the use of synthetic materials, not safe for the environment and human health. In this perspective, chitosan emerges as a viable solution for this purpose, as it is biodegradable, biocompatible and also safe for food application. The growing interest in using insects as a source of chitin has allowed for increased exploitation of insect-based waste products to recover valuable materials, such as biopolymers. The black soldier fly (Hermetia illucens L.) is the most widely reared species in Europe for feed production and waste management.
Results
In this work, fresh mature apricots (Prunus armeniaca L.), nectarines (Prunus persica vulgaris Mill.) and yellow peaches (Prunus persica var. laevis Gray) were coated with 0.5% and 1% chitosan from the pupal exuviae of Hermetia illucens, applied by spraying and stored at room temperature or 4 °C until they decay. Then, to validate the effectiveness of chitosan as a polymer for fruit preservation, several parameters including pH, TSS and weight loss were evaluated.
Conclusions
The results showed that chitosan derived from the black soldier fly is as effective as or better than the commercially available crustacean chitosan in maintaining more stable some storage parameters in fresh apricots, nectarines and peaches. Thus, insects, especially Hermetia illucens, are confirmed as a viable alternative source of the polymer.
Graphical Abstract

Similar content being viewed by others
Background
Fruit and vegetables are highly perishable commodities that undergo postharvest deterioration due to weight loss, physiological disorders, and diseases [1,2,3]. Different packaging methodologies, including edible coating, are relevant for maintaining the postharvest quality of these fresh products. Synthetic polymers, traditionally used for food packaging, have serious problems related to their environmental impact as non-biodegradable materials, despite their good effectiveness as a protective barrier [4]. In 2020, the global value of the packaged food market was estimated at $1.9 trillion, with an expected annual growth rate of 5% [5]. Therefore, research is making increasing efforts to reduce the environmental impact of conventional packaging develo** edible preservative coatings based on natural, biodegradable polymers (e.g., proteins, lipids, polysaccharides). Chitosan is one of the most promising biopolymers for this application [6]. It is a linear cationic polysaccharide derived from the deacetylation of chitin, the main structural component of arthropods exoskeleton [7]. Chitosan-based coatings have already demonstrated good efficacy in enhancing the shelf-life of a variety of fresh fruits and vegetables, including strawberries [8], plums [9], pears [10], mangoes [11], berries [12, 13], tomatoes [14], carrots [15], cucumbers [16], bell peppers [17], mushrooms [18], aubergines [19], and also commodities of animal origin, such as meat [20, 21], fish fillets [22, 23] and eggs [24]. Chitosan coatings act as a barrier able to reduce dehydration and weight loss of fresh fruit and vegetables, also delaying maturation and senescence of these products during storage. Chitosan also shows antioxidant and antimicrobial activities [25], thus retarding microbial spoilage and growth of fungal populations [26]. Chitosan has a broad variety of uses due to its distinctive features, versatility, and helpful qualities. It is able to form films and chelate metal ions [27, 28]. Chitosan is also suitable for cosmetic applications [29, 30]. Furthermore, it can be used in the treatment of burns and wounds and as a drug delivery system [31]. Due to its low toxicity, chitosan is even used in the food industry as a natural preservative. It can inhibit the growth of some bacteria, fungi, and yeasts, thus extending the shelf life of food products [32]. In agriculture, chitosan is employed as a biopesticide and a stimulant of plant development [33, 34].
Chitosan is traditionally produced by alkaline hydrolysis of chitin extracted from fishery waste, mainly crustacean shells [35]. Recently, a growing interest in using insects as a source of chitin and chitosan has developed, driven by the numerous large-scale insect breeding facilities that have arisen in many countries. Waste materials from these farms, mainly pupal exuviae generated by the molting of insects from one developmental stage to another, represent a chitin-rich biomass that can be exploited [36, 37]. The black soldier fly (Hermetia illucens L.) (BSF) is the most widely reared species in Europe for feed production and waste management [38]. Indeed, BSF larvae, attracted by specific organic volatile compounds [39], can fed on a variety of organic substrates, resulting in a body mass with a high protein and lipid content that can be used for the production of animal feed, biofuels, in cosmetic and biomedical industry [40,41,42,43,44,45,46,47,48,49,50,51]. The pupal exuviae released by BSF during the transition from the pupal to the adult stage is one of the main waste products of its breeding and contains up to 25% chitin that can be purified to produce chitosan [52].
The aim of this preliminary investigation was to evaluate, for the first time, the ability of chitosan produced from an insect biomass (BSF pupal exuviae) to perform as a protective coating for the preservation of three fresh fruits (apricots, nectarines and peaches). The purpose of the work was to test its suitability to be used as an alternative to the commercially available chitosan to validate the valorisation of a H. illucens breeding waste for an application in the agri-food sector. Obtaining a biopolymer so sought-after on the market, and so useful in the agri-food sector, from an alternative and widely available source, is particularly relevant. Producing chitosan from H. illucens not only satisfies the commercial demand for the product but also makes it possible to counteract problems related to the processing of the commercial source, i.e., crustaceans. Indeed, the exploitation of the ocean depths is causing a major environmental problem, prospectively very damaging for the planet. However, the use of this alternative source makes it possible to obtain a biopolymer that provides the same characteristics of the crustacean one, but with a zero ecological footprint. The effect of commercial chitosan from crustaceans used in the preservation of fresh fruit has already been widely studied. In contrast, there are few studies evaluating the effect of insect chitosan. This is the first study evaluating the effect of chitosan derived from H. illucens in improving the shelf life of peaches, apricots, and nectarines. Comparing its effect with commercial chitosan, insect chitosan proved to be a viable alternative to the use of crustacean chitosan. The novelty of the work also lies in the utilization of one of the few waste products generated by the breeding of H. illucens. We would not only report the production of chitosan and its characterisation [53], but also its application in a valuable economic sector.
Methods
Chitin and chitosan preparation
BSF pupal exuviae were provided by Xflies s.r.l (Potenza, Italy). Larvae were reared on a standard Gainesville diet (30% alfalfa, 50% wheat bran, 20% corn meal), supplied by the animal feed factory Mangimi Losasso s.r.l.—Balvano (Potenza, Italy) [54]. Chitin was extracted from BSF pupal exuviae, both bleached (Bl) and unbleached (Unbl), and heterogeneously deacetylated, as reported in Triunfo et al. [53]. Two heterogeneous chitosan samples were obtained from pupal exuviae chitin: unbleached and bleached chitosan. Commercial crustacean-derived chitin and chitosan, used as benchmark, as well as all reagents were purchased from Merck Millipore (Burlington, MA, USA).
Chitin and chitosan characterization
Characterization of BSF chitin and chitosan was determined using FTIR Jasco 460Plus and scanned in the range of wavelength from 4000 to 500 cm-1. Chitin and chitosan functional groups were defined from the fingerprint of each compound.
For each BSF chitosan samples, the deacetylation degree (DD) by potentiometric titration, according to Jiang et al. method [55], and the viscosity-average molecular weight (Mv) by intrinsic viscosity (η), following the Singh et al. method [56], were determined.
BSF chitosan film-forming ability
The film-forming ability of BSF chitosan was also assessed. The 1% (w/v) chitosan was dissolved in a 1% (v/v) aqueous solution of acetic acid, stirred until complete solubilization of chitosan and poured into a Petri dish. The film-forming solution was allowed to dry at room temperature for 72 h, and then films were removed from the Petri dishes [54].
Fruits
Fresh mature apricots (Prunus armeniaca L.) and two varieties of the persica species, such as yellow nectarines (Prunus persica vulgaris Mill.) with smooth skin and crunchy pulp, and yellow peaches (Prunus persica var. laevis Gray), i.e., glabrous fruits with spongy pulp, were supplied by a local agricultural cooperative (APOFRUIT Italia soc. coop. agricola, Scanzano Jonico, Matera, Italy). Fruits without signs of mechanical damage or spoilage, and of similar size, were selected for the experiments.
Preparation and application of coating solutions
Coating solutions were prepared according to the method by Hassan et al. and Tafi et al. [8, 57]. Chitosan (0.5% w/v or 1% w/v) was dissolved in a solvent solution consisting of 1% v/v acetic acid, 2% v/v glycerol and 0.2% v/v Tween-80. Two control conditions were set up: untreated fruits (negative control) and treatment with the chitosan-free coating solution (solvent alone). A commercial chitosan (Comm CS) sample was also tested. Fruits were coated by spraying using an aerograph (Martellato s.r.l., Rovigo, Italy) and then dried at room temperature for 30 min. The spraying with the coating solution was repeated twice to ensure uniform surface coverage, as reported by Tafi et al. [57]. One half of the fruit of each treatment was stored at room temperature (RT), while the other half was stored at refrigeration temperature (4 °C). For the evaluation, the fruits were carried to decay.
Weight loss determination
All fruits were weighed every 3 days, using an electronic weighing balance (Sartorius- BCE ENTRIS II, Göttingen, Germany). The weight loss was calculated as the percentage ratio between the weight difference at the beginning (T0) and at the end (Tf) of the storage period, and the initial weight of the fruit:
Measurement of total soluble solids (TSS) and pH
Fruit pulp was homogenized using a laboratory blender and then diluted, suspending 5 g of pulp in 25 ml of distilled water. TSS was determined using a hand refractometer, according to the standard method EN ISO 2173:2003 [58] and expressed as Brix°. The pH of the fruit pulp was measured at RT with a pH meter (Orion Research Inc., Boston, USA). TSS and pH were measured at the beginning and the end of the experiment and the variation of these parameters during the storage period was evaluated.
Statistical analysis
All measurements were performed in triplicate and data were expressed as average ± standard deviation. Data were analyzed by one-way Anova and Bonferroni post-hoc test. Statistical analyses were performed using a GraphPad Prism version 6.0 for Windows (GraphPad Software, San Diego, California USA).
Results
Chitin and chitosan characterization
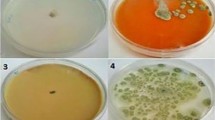
Spectra of chitin and chitosan obtained by FTIR are provided in Fig. 1a, b. All samples were structurally similar to the commercial polymers. The α form was identified for chitin by observing the split of the amide I band into two peaks at 1650 and 1620 cm−1 [59]. Chitosan spectra presented the characteristic bands at 1650 cm−1 (amide I) and 1590 cm−1 (amide II) [60], confirming its formation after chitin deacetylation. In accordance with Kumirska et al. [59], the higher intensity of the band at 1650 cm−1 (amide I) than the one at 1590 cm−1 (NH2 bending) is a qualitative indicator of a lower deacetylation of the chitosan obtained from BSF in comparison with the commercial sample. Chemical characterization of chitosan is reported in Table 1. Chitosan from BSF pupal exuviae features a similar deacetylation degree compared to crustacean-derived chitosan, but with a much lower molecular weight, as reported in Table 1. Both chitosan samples produced from BSF form a uniform and homogeneous film, in terms of surface integrity, thickness, and transparency (Fig. 2). Films were strong enough to be handled without breaking.
Evaluation of the influence of BSF chitosan coating on fruit decay
To evaluate the effectiveness of the chitosan coating from BSF on the Prunus species tested, several parameters were analyzed, such as weight loss, TSS content, and pH. The test was considered completed when the fruits reached decay at both storage conditions tested: approximately 8–15 days at RT and 15–26 days at 4 °C. In the present work, a crucial effect of the chitosan coating in inhibiting spontaneous mold growth on apricots, nectarines, and peaches was demonstrated (Fig. 3a, b). Performing a visual evaluation, chitosan treatments were effective in maintaining the shelf life of all fruits, in terms of physical deterioration. Particularly, at RT and for the same period, the control and solvent showed a visible deterioration compared to the BSF chitosan-treated fruits (Fig. 3a). Therefore, the introduction of an appropriate solvent-only control allowed one to recognize whether the effect was attributable to the active polymer alone or to other components of the solution used. In the current instance, since the solvent-only control always gave a higher presence of mold than the other treatments, the effect observed in reducing the incidence of moldy fruits can be attributed to the chitosan itself. In storage at 4 °C, the low temperature has a crucial influence in preventing decay. However, even in this case, all fruits treated with chitosan, particularly BSF chitosan, were better than the untreated control and the solvent alone (Fig. 3b).
Pictures of apricots, yellow nectarines and peaches coated by spraying at the beginning (T0) and at the end (Tf) of their storage period at (a) RT and (b) 4 °C. Treatments: untreated fruits (Ctrl-), solvent, coating with 0.5% and 1% of commercial chitosan (Comm CS), unbleached (Unbl CS) and bleached (Bl CS), chitosan from H. illucens pupal exuviae
Weight loss
In apricots stored at RT, a similar weight loss was observed for all treatments (Table 2). Although not statistically significant compared to the others, the lowest loss was detected in the fruits coated with bleached chitosan. At 4 °C, a significant reduction in the weight loss was observed in apricots treated with both chitosan samples from the BSF pupal exuviae, particularly with 0.5% bleached chitosan and with the commercial one, compared to the negative control. Nevertheless, the solvent-alone coating had a similar but slightly worse effect to the chitosan treatments.
In nectarines, no treatment had a different effect than the negative control, neither at RT nor at 4 °C (Table 3). Although not statistically significant compared to the others, the lowest loss was found in fruits coated with bleached chitosan at both storage conditions.
In peaches, treatment with both samples of chitosan from BSF pupal exuviae was effective in significantly reducing weight loss compared to both the negative control and solvent-coated fruit, regardless of storage temperature (Table 4). In addition, chitosan from BSF also showed a significant effect compared to commercial chitosan, particularly with 0.5% bleached sample, at both storage conditions.
Variation of TSS content
TSS content of all fruits increased during storage, at both temperatures.
In apricots stored at RT, the greatest TSS increase occurred in the negative control (Table 2). Fruits treated with solvent alone, commercial and unbleached chitosan from pupal exuviae, had a slightly smaller increase in TSS than the negative control, although statistically similar. Only coating with 0.5% bleached chitosan gave a significant reduction in the TSS increase compared to the negative control. Although no significant differences were detected among treatments on apricots stored at 4 °C (Table 2), the bleached chitosan coating, particularly the 0.5% chitosan sample, proved to be the most powerful in limiting the TSS increase during storage.
In nectarines stored at RT, BSF chitosan (particularly 0.5% Unbl CS and 0.5% Bl CS), and commercial chitosan (particularly coating with 1% Comm CS) were effective in significantly reducing the TSS variation compared to both the negative control and the solvent control (Table 3). At 4 °C storage, although no significant differences were observed among treatments, the bleached chitosan and only 1% unbleached chitosan from BSF showed the lowest TSS variation.
In peaches stored at RT, the highest increase in TSS occurred in negative control which was statistically different from both commercial and BSF chitosan treatments (Table 4). Compared to the commercial chitosan coating, the unbleached chitosan from BSF was more effective in reducing the TSS increase than the bleached one at both concentrations tested. No significant differences were detected among treatments on peaches stored at 4 °C. However, the lowest variation was observed in fruits coated with 0.5% unbleached chitosan from BSF.
Variation of pH
pH of apricots increased during storage, at both temperatures. At RT, pH of apricots coated with all chitosan samples from pupal exuviae and with 1% commercial chitosan increased significantly less than that of fruits belonging to both negative and solvent control (Table 2). Bleached chitosan from BSF had a better effect than commercial one. At 4 °C, all treatments, including the solvent alone, gave a significantly smaller increase in pH than the negative control, to a similar extent.
In nectarines kept at RT, the greatest pH increase was observed in the solvent control (Table 3). All chitosan treatments gave a smaller pH increase than the solvent alone, but only the pupal exuviae chitosan treatments (except 0.5% unbleached chitosan) significantly reduced the pH variation compared to the negative control. All pupal exuviae chitosan coatings had a better effect compared to the commercial one. All treatments with chitosan from pupal exuviae had a better effect than the commercial one. At 4 °C, all treatments reduced the pH variation compared to the negative control and the solvent-alone control. Chitosan from BSF proved to be more effective than the commercial sample. In particular, among pupal exuviae chitosan treatments, the 1% unbleached chitosan and the 0.5% bleached chitosan were the most effective in maintaining the pH of nectarines stable during cold storage.
In peaches, none of the applied treatment significantly reduced the pH increase compared to the negative control (Table 4). Although not statistically significant, bleached chitosan (1% Bl CS at RT and 0.5% Bl CS at 4 °C) was more effective in maintaining a stable pH of the peaches during both storage conditions. Particularly, at 4 °C, treatment with BSF chitosan, both unbleached and bleached, was better than with commercial chitosan.
Discussion
Film-forming ability of chitosan
After validating their film-forming capability, the chitosan samples from BSF were used for the planned application. Indeed, filmogenic ability is a crucial property that chitosan is required to possess to be applied as coating. The chemical features of the biopolymer itself, however, can have an impact on the properties of this coating. Viscosity and relative molecular weight are among the parameters influencing the characteristics of the coating solution, and they were determined to be the main differences between the chitosan from BSF and the commercial one. Therefore, the expected variations in response of coated fruits might be mainly due to the molecular weight of the chitosan applied. Furthermore, the ability of the chitosan coating to inhibit fruit pathogens in the postharvest storage has been largely investigated. An effective inhibition action of chitosan-based coatings against fungal growth was already assessed in different fruits, such as strawberry [8], tomato [61], papaya [62], pear [63], mango [64], blueberry [65].
Weight loss
Loss of weight occurs in fresh fruits, mainly due to transpiration and respiration processes that cause moisture evaporation between the fruit tissue and surrounding air. Chitosan coating acts as a semipermeable barrier against oxygen, carbon dioxide and moisture, thereby reducing respiration and water loss and counteracting the dehydration and shrinkage of the fruit [66, 67]. In the present work, a significant effect of the BSF-based chitosan coating was observed in all treated fruits, with the exception of peaches, compared to untreated fruits; particularly bleached chitosan was shown to be a better treatment than the unbleached one, already at a 0.5% concentration, mainly at 4 °C. Crustacean-derived chitosan, at a concentration from 0.5% to 2%, have already been effective in reducing weight loss of fresh apricots [68,69,70], as well as whole and fresh-cut nectarines [71, 72], all stored at cold temperature. The effect of weight loss reduction mediated by chitosan coatings was demonstrated for many other fruits, including tomatoes [61], berries [12, 13], citrus fruits [73, 74], grapes [75] and mangoes [76]. On the contrary, no significant effect of the chitosan treatment was detected in cold-stored nectarines by Ramirez et al. [77], in accordance with the present results. Ghasemnezhad et al. [78] found a significative difference in the efficacy of the coating depending on its chitosan concentration: only 0.25% chitosan significantly reduced the apricot weight loss compared to the control and to the higher chitosan concentrations (0.5% and 0.75%). It was assumed that the anaerobic respiration of the fruit could be increased by high concentrations of chitosan, resulting in greater weight loss [78]. This was in agreement with the reported results, in which the BSF-bleached sample performed better at 0.5% chitosan than at 1%. This suggests testing further concentrations of BSF chitosan, below 0.5%, in future experiments.
Variation of TSS content
TSS is an estimation of the sugar content of the fruit, serving as an indicator of fruit ripening. TSS increases during ripening, due to the hydrolytic conversion of starch stored by the fruit into sugars, mainly glucose, fructose, and sucrose [11, 79]. As the fruit reaches full ripeness, the TSS content decreases because of the reduction of ethylene production and respiration rate. As storage further proceeds, with the advancement of the postharvest ripening process, TSS increases again because of the restarting of ethylene synthesis and fruit respiration, with consequent hydrolysis of starch into sugars. This process occurs in “climacteric” fruits (e.g., apricots, peaches, apples, pears), in which the biochemical ripening mechanisms continue even after the detachment of fruit from the plant [80, 81]. In the current experiments, the TSS of apricots, nectarines, and peaches increased during storage, in accordance with other works [69, 71, 72]. On the contrary, other authors reported a decrease in TSS in the same fruits [68, 70, 77, 78, 82]. These differences could be due to the different physiological stages of fruits at the time of the experiment. According to the literature, the increase in TSS could indicate that fruits are either ripening or at an advanced storage phase. The fruits supplied by the grower were at the right stage of ripeness to be marketed [11, 80]. Therefore, it can be assumed that the TSS increased in the first phase of the experiment until full ripeness was reached, followed by a decrease, and then a further increase again in the final storage phase [11, 80]. In addition, TSS rises during storage because of sugar concentration due to moisture loss [83]. In this work, only in fruits stored at RT, BSF chitosan was effective in reducing the TSS increase compared to the negative control. Especially, 0.5% bleached chitosan was the best treatment for all three tested fruits, whereas in nectarines and peaches, even 0.5% unbleached chitosan contributed. Accordingly, in most experiments carried out on apricots and nectarines, chitosan coating did not give different effects to the controls [68, 70, 71, 77, 78]. Only in a few cases, chitosan effectively kept TSS more stable, but combined with modified atmosphere packaging [69, 72]. In some other fruits, chitosan treatment was more effective in reducing TSS variation, including blueberry [12], mango [11], blackberry [13], lemon [74] and pomegranate [84]. The chitosan coating can modify the internal atmosphere of the fruit, with a reduction in the oxygen level and/or an increase in the carbon dioxide level, thus reducing the respiration rate and metabolic activity, and delaying both the accumulation of sugars and the starch degradation [67, 76].
Variation of pH
Organic acids accumulate in the fruit cells during ripening, as they are the main substrate for the enzymes involved in the respiration process of fruits [11, 85]. An increase in the respiration rate accelerates glycolytic metabolism and tricarboxylic acid cycle, thus increasing the acid content of fruit [81]. Acidity often reduces during postharvest storage because of acid metabolism, which turns acid and starch into sugars [12]. This is in agreement with the increase in pH observed in our experiments. Through this work, it was possible to find that pH was the parameter on which BSF chitosan had the greatest effect. In both peaches at 4 °C and nectarines and apricots at both storage temperatures, all BSF chitosan coatings significantly reduced or avoided the increase in pH compared to both the negative and solvent controls. BSF chitosan also had a significantly better effect than the commercial polymer. Thus, a slowing down of the acidity decrease, due to a deceleration in the acid metabolism of the fruit, is revealed by the buffering in the chitosan-mediated pH variation [12]. Similar results on the same fruits were obtained by Marvdashti et al., Morsy & Rayan and Chiabrando & Giacalone [68, 69, 71]. In other works, in contrast, no differences in pH evolution were found between coated and uncoated apricots or nectarines [70, 71, 79]. An alkalization-reducing effect mediated by commercial chitosan was observed also in different fruits, such as plums, blueberries, and mangoes [11, 12, 86]. The pH can also be affected by the presence of fungal populations, as fungi and moulds can use organic acids as a growth substrate, thus increasing the pH of the fruit [11].
Conclusions
The results of this investigation revealed that BSF chitosan was more effective than the commercially available crustacean chitosan in food preservation, particularly by maintaining more stable some important post-harvest physico-chemical parameters in fresh apricots, nectarines and peaches, especially at room temperature. The coating properties of BSF chitosan could be further improved by acting on the characteristics of the chitosan: chitosan used in the present experiments, with a similar DD, had a lower molecular weight than commercial chitosan, which, according to the literature, might affect the barrier properties of the coating [87, 88].
Significant differences were found between the two tested concentrations of chitosan, and between the bleached and the unbleached polymer. Specifically, bleached chitosan was found to be a better treatment than unbleached one, already at 0.5% concentration; in contrast, unbleached chitosan is more active at the higher concentration. This is probably due to the presence of pigments that hide the activity of the polymer, requiring a higher concentration for the same effectiveness. Based on the experiments carried out, it is clear that chitosan from BSF performed significantly better than solvent treatment alone, underlining the inherently good effect of the biopolymer in slowing down the spoilage of fresh fruit and thus retaining its storage.
Further work could also investigate coatings with different concentrations of chitosan or blending with other natural active components (e.g., starch, proteins, plant extracts) that can enhance its preservative effect. These preliminary results represent an encouraging starting point for the validation of insect biomass for the production of chitosan for use in the agri-food chain. Indeed, with a view to the future, the potential of producing a natural polymer for packaging with this preservation performance turns out to be crucial for both environmental safeguard and human health. Actually, prolonging the shelf life of fresh food reduces food waste, a huge issue of our millennium. Besides that, the bioconverter insect H. illucens, being a highly sustainable source and having its breeding always readily available, represents an alternative and valuable source for the production of chitosan, thus obtained in a completely zero-waste circular economy system. The innovation of our work will thus allow, in the future, preserving food, taking care of the environment and also recovering what is normally wasted, thereby becoming a product with high biological value.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BSF:
-
Black soldier fly
- Bl:
-
Bleached
- Bl CS:
-
Bleached chitosan
- CT:
-
Chitin
- CS:
-
Chitosan
- Comm CT:
-
Commercial chitin
- Comm CS:
-
Commercial chitosan
- DD:
-
Deacetylation degree
- Ctrl-:
-
Negative control
- RT:
-
Room temperature
- TSS:
-
Total soluble solids
- Unbl:
-
Unbleached
- Unbl CS:
-
Unbleached chitosan
References
Mahajan BC, Tandon R, Kapoor S, Sidhu MK. Natural coatings for shelf-life enhancement and quality maintenance of fresh fruits and vegetables—a review. J Postharvest Technol. 2018;6(1):12–26.
Zhou Y, Zhong Y, Li L, Jiang K, Gao J, Zhong K, Pan M, Yan B. A multifunctional chitosan-derived conformal coating for the preservation of passion fruit. LWT. 2022. https://doi.org/10.1016/j.lwt.2022.113584.
Zhou C, Bai J, Zhang F, Zhang R, Zhang X, Zhong K, Yan B. Development of mussel-inspired chitosan-derived edible coating for fruit preservation. Carbohydr Polym. 2023. https://doi.org/10.1016/j.carbpol.2023.121293.
Azeredo HM, de Britto D, Assis OB. Chitosan edible films and coatings—a review. Mater Sci Eng: C. 2010. https://doi.org/10.1016/j.msec.2013.01.010.
Kumar S, Deshmukh R. Packaged Food Market by Type (Dairy Products, Confectionery, Packaged Produce, Bakery & Snacks, Meat, Poultry & Seafood, Ready Meals, and Others), Sales Channel (Supermarket/Hypermarket, Specialty Stores, Grocery Stores, Online Stores, and Others): Global Opportunity Analysis and Industry Forecast 2021–2030. Allied Market Research. 2021, https://www.alliedmarketresearch.com/packaged-food-market.
Zhou Y, Liu R, Zhou C, Gao Z, Gu Y, Chen S, Yang Q, Yan B. Dynamically crosslinked chitosan/cellulose nanofiber-based films integrated with γ-cyclodextrin/curcumin inclusion complex as multifunctional packaging materials for perishable fruit. Food Hydrocoll. 2023. https://doi.org/10.1016/j.foodhyd.2023.108996.
Dutta PK, Dutta J, Tripathi VS. Chitin and chitosan: chemistry, properties and applications. J Sci Ind Res. 2004. https://doi.org/10.1016/j.progpolymsci.2006.06.001.
Hassan J, Anwar R, Khan AS, Ahmad S, Malik AU, Nafees M, Hussain Z, Inam-ur-Raheem M. Chitosan-based edible coating delays fungal decay and maintains quality of strawberries during storage. Intl J Agric Biol. 2020;24:486–92. https://doi.org/10.17957/IJAB/15.1463.
Kumar P, Sethi S, Sharma RR, Srivastav M, Varghese E. Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci Horti. 2017;226:104–9. https://doi.org/10.1016/j.scienta.2017.08.037.
Wang J, Yang B, Zhang S, Cao J, Jiang W. Effect of thymol on antifungal ability of chitosan coating against Penicillium expansum in Yali pear. Emir J Food Agric. 2016;28(10):725–31. https://doi.org/10.9755/ejfa.2015-09-788.
Shah S, Hashmi MS. Chitosan–aloe vera gel coating delays postharvest decay of mango fruit. Hortic Environ Biotechnol. 2020;61:279–89. https://doi.org/10.1007/s13580-019-00224-7.
Vieira JM, Flores-López ML, de Rodríguez DJ, Sousa MC, Vicente AA, Martins JT. Effect of chitosan–Aloe vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol Technol. 2016;116:88–97. https://doi.org/10.1016/j.postharvbio.2016.01.011.
Vilaplana R, Guerrero K, Guevara J, Valencia-Chamorro S. Chitosan coatings to control soft mold on fresh blackberries (Rubus glaucus Benth.) during postharvest period. Sci Hortic. 2020. https://doi.org/10.1016/j.scienta.2019.109049.
Sultana N, Zakir HM, Parvin MA, Sharmin S, Seal HP. Effect of chitosan coating on physiological responses and nutritional qualities of tomato fruits during postharvest storage. Asian J Adv Agric Res. 2019. https://doi.org/10.9734/ajaar/2019/v10i230027.
Leceta I, Molinaro S, Guerrero P, Kerry JP, De la Caba K. Quality attributes of map packaged ready-to-eat baby carrots by using chitosan-based coatings. Postharvest Biol Technol. 2015;100:142–50. https://doi.org/10.1016/j.postharvbio.2014.09.022.
Olawuyi IF, Lee W. Influence of chitosan coating and packaging materials on the quality characteristics of fresh-cut cucumber. Korean J Food Preserv. 2019;26(4):371–80. https://doi.org/10.1100/kjfp.2019.26.4.371.
Ali A, Noh NM, Mustafa MA. Antimicrobial activity of chitosan enriched with lemongrass oil against anthracnose of bell pepper. Food Packag Shelf Life. 2015;3:56–61. https://doi.org/10.1016/j.fpsl.2014.10.003.
Eissa HAA. Effect of chitosan coating on shelf-life and quality of fresh-cut mushroom. Pol J Food Nutr Sci. 2008;58(1):95–105. https://doi.org/10.1111/j.1745-4557.2007.00147.x.
Sharma S, Prasad RN, Tiwari S, Chaurasia SNS, Shekhar S, Singh J. Effect of chitosan coating on postharvest quality and enzymatic activity of eggplant (Solanum melongena L.) cultivars. J Food Process Preserv. 2021;45(1):e15098. https://doi.org/10.1111/jfpp.15098.
Fang Z, Lin D, Warner RD, Ha M. Effect of gallic acid/chitosan coating on fresh pork quality in modified atmosphere packaging. Food Chem. 2018;260:90–6. https://doi.org/10.1016/j.foodchem.2018.04.005.
Duran A, Kahve HI. The effect of chitosan coating and vacuum packaging on the microbiological and chemical properties of beef. Meat Sci. 2020. https://doi.org/10.1016/j.meatsci.2019.107961.
Lee JS, Jahurul MHA, Pua VC, Shapawi R, Chan PT. Effects of chitosan and ascorbic acid coating on the chilled tilapia fish (Oreochromis niloticus) fillet. In J Phys Conf Ser. 2019;1358(1):012009. https://doi.org/10.1080/10408399609527720.
Li T, Sun X, Chen H, He B, Mei Y, Wang D, Li J. Effect of the combination of vanillin and chitosan coating on the microbial diversity and shelf-life of refrigerated turbot (Scophthalmus maximus) filets. Front Microbial. 2020;11:462. https://doi.org/10.3389/fmicb.2020.00462.
Bhale S, No HK, Prinyawiwatkul W, Farr AJ, Nadarajah K, Meyers SP. Chitosan coating improves shelf life of eggs. J Food Sci. 2003;68(7):2378–83. https://doi.org/10.1111/j.1365-2621.2003.tb05776.x.
Guarnieri A, Triunfo M, Scieuzo C, Ianniciello D, Tafi E, Salvia R, De Bonis A, Falabella P. Antimicrobial properties of the chitosan from different developmental stages of the bioconverter Hermetia illucens. Sci Rep. 2022;12:8084. https://doi.org/10.1038/s41598-022-12150-3.
Kabanov VL, Novinyuk LV. Chitosan application in food technology: a review of recent advances. Food Syst. 2020;3(1):10–5. https://doi.org/10.2132/2618-9771-2020-3-1-10-15.
Roy JC, Salaün F, Giraud S, Ferri A, Chen G, Guan J. Solubility of chitin: solvents, solution behaviors and their related mechanisms. Solubility Polysacch. 2017;3:20–60.
Kumar MNR. A review of chitin and chitosan applications. React Funct Polym. 2000;46(1):1–27. https://doi.org/10.1016/S1381-5148(00)00038-9.
Guzmán E, Ortega F, Rubio RG. Chitosan: a promising multifunctional cosmetic ingredient for skin and hair care. Cosmetics. 2022;9(5):99. https://doi.org/10.3390/cosmetics9050099.
Triunfo M, Tafi E, Guarnieri A, Scieuzo C, Hahn T, Zibek S, Salvia R, Falabella P. Insect chitin-based nanomaterials for innovative cosmetics and cosmeceuticals. Cosmetics. 2021;8(2):40. https://doi.org/10.3390/cosmetics8020040.
Jafernik K, Ładniak A, Blicharska E, Czarnek K, Ekiert H, Wiącek AE, Szopa A. Chitosan-based nanoparticles as effective drug delivery systems—a review. Molecules. 2023;28(4):1963. https://doi.org/10.3390/molecules28041963.
Shurmasti DK, Kermani PR, Sarvarian M, Awuchi CG. Egg shelf life can be extended using varied proportions of polyvinyl alcohol/chitosan composite coatings. Food Sci Nutr. 2023. https://doi.org/10.1002/fsn3.3394.
Alfy H, Ghareeb RY, Soltan ELSAYED, Farag DA. Impact of chitosan nanoparticles as insecticide and nematicide against Spodoptera littoralis, Locusta migratoria, and Meloidogyne incognita. Plant Cell Biotechnol Mol Biol. 2020;21:126–40.
Kemboi VJ, Kipkoech C, Njire M, Were S, Lagat MK, Ndwiga F, Tanga CM. Biocontrol potential of chitin and chitosan extracted from black soldier fly pupal exuviae against bacterial wilt of tomato. Microorganisms. 2022;10(1):165.
Arbia W, Arbia L, Adour L, Amrane A. Chitin extraction from crustacean shells using biological methods–a review. Food Technol Biotechnol. 2013;51(1):12–25.
Van Huis A. Potential of insects as food and feed in assuring food security. Annu Rev entomol. 2013;58:563–83. https://doi.org/10.1146/annurev-ento-120811-153704.
Hahn T, Tafi E, Paul A, Salvia R, Falabella P, Zibek S. Current state of chitin purification and chitosan production from insects. J Chem Technol Biotechnol. 2020;95(11):2775–95. https://doi.org/10.1002/jctb.6533.
Derrien C, Boccuni A. Current status of the insect producing industry in Europe. Edible Insects Sustain Food Syst. 2018. https://doi.org/10.1007/978-3-319-74011-9_30.
Scieuzo C, Nardiello M, Farina D, Scala A, Cammack JA, Tomberlin JK, Vogel H, Salvia R, Persaud K, Falabella P. Hermetia illucens (L.) (Diptera: Stratiomyidae) odorant binding proteins and their interactions with selected volatile organic compounds: an in silico approach. Insects. 2021;12:814. https://doi.org/10.3390/insects12090814.
Scala A, Cammack JA, Salvia R, Scieuzo C, Franco A, Bufo SA, Tomberlin JK, Falabella P. Rearing substrate impacts growth and macronutrient composition of Hermetia illucens (L.) (Diptera: Stratiomyidae) larvae produced at an industrial scale. Sci Rep. 2020;10:1–8. https://doi.org/10.1038/s41598-020-76571-8.
Franco A, Scieuzo C, Salvia R, Petrone AM, Tafi E, Moretta A, Schmitt E, Falabella P. Lipids from Hermetia illucens, an innovative and sustainable source. Sustain. 2021;13:10198. https://doi.org/10.3390/su131810198.
Franco A, Salvia R, Scieuzo C, Schmitt E, Russo A, Falabella P. Lipids from insects in cosmetics and for personal care products. Insects. 2022;13:41. https://doi.org/10.3390/insects1301004.
Triunfo M, Tafi E, Guarnieri A, Scieuzo C, Hahn T, Zibek S, Salvia R, Falabella P. Insect chitin-based nanomaterials for innovative cosmetics and cosmeceuticals. Cosmetics. 2021;8:40. https://doi.org/10.3390/cosmetics8020040.
Moretta A, Salvia R, Scieuzo C, Di Somma A, Vogel H, Pucci P, Sgambato A, Wolff M, Falabella P. A bioinformatic study of antimicrobial peptides identified in the black soldier fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci Rep. 2020;10(1):16875. https://doi.org/10.1038/s41598-020-74017-9.
Moretta A, Scieuzo C, Petrone AM, Salvia R, Manniello MD, Franco A, Lucchetti D, Vassallo A, Vogel H, Sgambato A, Falabella P. Antimicrobial peptides: a new hope in biomedical and pharmaceutical fields. Front Cell Infect Microbiol. 2021. https://doi.org/10.3389/fcimb.2021.668632.
Manniello MD, Moretta A, Salvia R, Scieuzo C, Lucchetti D, Vogel H, Sgambato A, Falabella P. Insect antimicrobial peptides: potential weapons to counteract the antibiotic resistance. Cell Mol Life Sci. 2021;78:4259–82. https://doi.org/10.1007/s00018-021-03784-z.
Di Somma A, Moretta A, Cané C, Scieuzo C, Salvia R, Falabella P, Duilio A. Structural and functional characterization of a novel recombinant antimicrobial peptide from Hermetia illucens. Curr Issues Mol Biol. 2021;44(1):1–13. https://doi.org/10.3390/cimb44010001.
Franco A, Scieuzo C, Salvia R, Mancini IM, Caniani D, Masi S, Falabella P. A mobile black soldier fly farm for on-site disposal of animal dairy manure. Bull Insectol. 2022;75(1):75–82.
Scieuzo C, Franco A, Salvia R, Triunfo M, Addeo NF, Vozzo S, Piccolo G, Bovera F, Ritieni A, Francia AD, Laginestra A, Schmitt E, Falabella P. Enhancement of fruit byproducts through bioconversion by Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2022. https://doi.org/10.1111/1744-7917.13155.
Moretta A, Scieuzo C, Salvia R, Popović ŽD, Sgambato A, Falabella P. Tools in the era of multidrug resistance in bacteria: applications for new antimicrobial peptides discovery. Curr Pharm Des. 2022;28(35):2856–66. https://doi.org/10.2174/1381612828666220817163339.
Scieuzo C, Giglio F, Rinaldi R, Lekka ME, Cozzolino F, Monaco V, Monti M, Salvia R, Falabella P. In vitro evaluation of the antibacterial activity of the peptide fractions extracted from the hemolymph of Hermetia illucens (Diptera: Stratiomyidae). Insects. 2023;14:464. https://doi.org/10.3390/insects14050464.
Brigode C, Hobbi P, Jafari H, Verwilghen F, Baeten E, Shavandi A. Isolation and physicochemical properties of chitin polymer from insect farm side stream as a new source of renewable biopolymer. J Clean Prod. 2020;275:122924. https://doi.org/10.1016/j.jclepro.2020.122924.
Triunfo M, Tafi E, Guarnieri A, Salvia R, Scieuzo C, Hahn T, Zibek S, Gagliardini A, Panariello L, Coltelli MB, De Bonis A, Falabella P. Characterization of chitin and chitosan derived from Hermetia illucens, a further step in a circular economy process. Sci Rep. 2022;12:6613. https://doi.org/10.1038/s41598-022-10423-5.
Hogsette JA. New diets for production of house fliesand stable flies (Diptera: Muscidae) in the laboratory. J Econ Entomol. 1992;85:2291–4.
Jiang X, Chen L, Zhong W. A new linear potentiometric titration method for the determination of deacetylation degree of chitosan. Carbohydr Polym. 2003;54:457–63. https://doi.org/10.1016/j.carbpol.2003.05.004.
Singh A, Benjakul S, Prodpran T. Ultrasound-assisted extraction of chitosan from squid pen: molecular characterization and fat binding capacity. J Food Sci. 2019;84:224–34. https://doi.org/10.1111/1750-3841.14439.
Tafi E, Triunfo M, Guarnieri A, Ianniciello D, Salvia R, Scieuzo C, Ranieri A, Castagna A, Lepuri S, Hahn T, Zibek S, De Bonis A, Falabella P. Preliminary investigation on the effect of insect-based chitosan on preservation of coated fresh cherry tomatoes. Sci Rep. 2023;13(1):7030. https://doi.org/10.1038/s41598-023-33587-0.
International Organization for Standardization. Fruit and vegetable products—Determination of soluble solids - Refractometric method, 2003 (EN ISO Standard No. 2173:2003).
Kumirska J, Czerwicka M, Kaczyński Z, Bychowska A, Brzozowski K, Thöming J, Stepnowski P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar Drugs. 2010;8(5):1567–636. https://doi.org/10.3390/md8051567.
Peng T, Yao KD, Yuan C, Goosen MF. Structural changes of pH-sensitive chitosan/polyether hydrogels in different pH solution. J Polym Sci Part A Polym Chem. 1994;32(3):591–6. https://doi.org/10.1002/pola.1994.080320322.
Sucharitha KV, Beulah AM, Ravikiran K. Effect of chitosan coating on storage stability of tomatoes (Lycopersicon esculentum Mill). Int Food Res J. 2018;25(1):93–9.
Dotto GL, Vieira ML, Pinto LA. Use of chitosan solutions for the microbiological shelf-life extension of papaya fruits during storage at room temperature. LWT-Food Sci Technol. 2015;64(1):126–30. https://doi.org/10.1016/j.lwt.2015.05.042.
Meng X, Yang L, Kennedy JF, Tian S. Effects of chitosan and oligochitosan on growth of two fungal pathogens and physiological properties in pear fruit. Carbohydr Polym. 2010;81(1):70–5. https://doi.org/10.1016/j.carbpol.2010.01.057.
Jitareerat P, Paumchai S, Kanlayanarat S, Sangchote S. Effect of chitosan on ripening, enzymatic activity, and disease development in mango (Mangifera indica) fruit. N Z J Crop Hortic Sci. 2007;35(2):211–8. https://doi.org/10.1080/01140670709510187.
Jiang H, Sun Z, Jia R, Wang X, Huang J. Effect of chitosan as an antifungal and preservative agent on postharvest blueberry. J Food Qual. 2016;39(5):516–23. https://doi.org/10.1111/jfq.12211.
Hernández-Muñoz P, Almenar E, Ocio MJ, Gavara R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biol Technol. 2006;39(3):247–53. https://doi.org/10.1016/j.postharvbio.2005.11.006.
Petriccione M, Mastrobuoni F, Pasquariello MS, Zampella L, Nobis E, Capriolo G, Scortichini M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods. 2015;4(4):501–23. https://doi.org/10.3390/foods4040501.
Marvdashti L, Abdulmajid Ayatollahi S, Salehi B, Sharifi-Rad J, Abdolshahi A, Sharifi-Rad R, Maggi F. Optimization of edible Alyssum homalocarpum seed gum-chitosan coating formulation to improve the postharvest storage potential and quality of apricot (Prunus armeniaca L.). J Food Saf. 2020;40(4):e12805. https://doi.org/10.1111/jfs.12805.
Morsy NE, Rayan AM. Effect of different edible coatings on biochemical quality and shelf life of apricots (Prunus armenica L. cv Canino). J Food Meas Charact. 2019;13(4):3173–82. https://doi.org/10.1007/s11694-019-00240-2.
Ziaolhagh SH, Kanani S. Extending the shelf life of apricots by using gum tragacanth-chitosan edible coating. J Agric Sci Technol. 2021;23(2):319–31.
Chiabrando V, Giacalone G. Effect of chitosan and sodium alginate edible coatings on the postharvest quality of fresh-cut nectarines during storage. Fruits. 2016;71(2):79–85. https://doi.org/10.1051/fruits/2015049.
Zhang W, Zhao H, Zhang J, Sheng Z, Cao J, Jiang W. Different molecular weights chitosan coatings delay the senescence of postharvest nectarine fruit in relation to changes of redox state and respiratory pathway metabolism. Food Chem. 2019;289:160–8. https://doi.org/10.1016/j.foodchem.2019.03.047.
Baswal AK, Dhaliwal HS, Singh Z, Mahajan BVC, Kalia A, Gill KS. Influence of carboxy methylcellulose, chitosan and beeswax coatings on cold storage life and quality of Kinnow mandarin fruit. Sci Hortic. 2020;260:108887. https://doi.org/10.1016/j.scienta.2019.108887.
Chen F, Zhang J, Chen C, Kowaleguet MG, Banù Z, Fei L, Xu C. Chitosan-based layer-by-layer assembly: towards application on quality maintenance of lemon fruits. Adv Polym Technol. 2020. https://doi.org/10.1155/2020/7320137.
Andrijanto E, Reksa T, Maheda J, Diani R, Wahyu E. Synthesis and utilization of chitosan as edible coating material for natural fruit preservation. IOP Conf Ser Mater Sci Eng. 2020;830(2):022009. https://doi.org/10.1088/1757-899X/830/2/022009.
Cosme Silva GM, Silva WB, Medeiros DB, Salvador AR, Cordeiro MHM, da Silva NM, Santana DB, Mizobutsi GP. The chitosan affects severely the carbon metabolism in mango (Mangifera indica L. cv. Palmer) fruit during storage. Food Chem. 2017;237:372–8. https://doi.org/10.1016/j.foodchem.2017.05.123.
Ramirez ME, Timón ML, Petrón MJ, Andrés AI. Effect of chitosan, pectin and sodium caseinate edible coatings on shelf life of fresh-cut Prunus persica var. Nectarine J Food Process Preserv. 2015;39(6):2687–97. https://doi.org/10.1111/jfpp.12519.
Ghasemnezhad M, Shiri MA, Sanavi M. Effect of chitosan coatings on some quality indices of apricot (Prunus armeniaca L.) during cold storage. Casp J Environ Sci. 2010;8(1):25–33.
Ghasemnezhad M, Nezhad MA, Gerailoo S. Changes in postharvest quality of loquat (Eriobotrya japonica) fruits influenced by chitosan. Hortic Environ Biotechnol. 2011;52(1):40–5. https://doi.org/10.1007/s13580-011-0028-5.
Valero D, Serrano M. Growth and ripening stage at harvest modulates postharvest quality and bioactive compounds with antioxidant activity. Stewart Postharvest Rev. 2013. https://doi.org/10.2212/spr.2013.3.7.
Lin Y, Li N, Lin H, Lin M, Chen Y, Wang H, Ritenour MA, Lin Y. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chem. 2020;306:125627. https://doi.org/10.1016/j.foodchem.2019.125627.
Chiabrando V, Giacalone G. Effect of different coatings in preventing deterioration and preserving the quality of fresh-cut nectarines (cv Big Top). CyTA-J Food. 2013;11(3):285–92. https://doi.org/10.1080/19476337.2012.745096.
Pizato S, Cortez-Vega WR, de Souza JTA, Prentice-Hernández C, Borges CD. Effects of different edible coatings in physical, chemical and microbiological characteristics of minimally processed peaches (Prunus persica L. Batsch). J Food Saf. 2013;33(1):30–9. https://doi.org/10.1111/jfs.12020.
Varasteh F, Arzani K, Barzegar M, Zamani Z. Pomegranate (Punica granatum L.) fruit storability improvement using pre-storage chitosan coating. J Agric Sci Technol. 2017;19:389–400.
Etienne A, Génard M, Lobit P, Mbeguié-A-Mbéguié D, Bugaud C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J exp bot. 2013;64(6):1451–69. https://doi.org/10.1093/jxb/ert035.
Bal E. Postharvest application of chitosan and low temperature storage affect respiration rate and quality of plum fruits. J Agric Sci Technol. 2013;15:1219–30.
Jiang Y, Yu L, Hu Y, Zhu Z, Zhuang C, Zhao Y, Zhong Y. The preservation performance of chitosan coating with different molecular weight on strawberry using electrostatic spraying technique. Int J Biol Macromol. 2020;151:278–85. https://doi.org/10.1016/j.ijbiomac.2020.02.169.
Jongsri P, Wangsomboondee T, Rojsitthisak P, Seraypheap K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. Lwt. 2016;73:28–36. https://doi.org/10.1016/j.lwt.2016.05.038.
Acknowledgements
We thank Mangimi Lossasso srl—Balvano (Potenza, Italy), APOFRUIT Italia soc. coop. agricola—Scanzano Jonico, (Matera, Italy) for supporting our work. This study was carried out within the Agritech National Research Center and received funding from the European Union Next-GenerationEU (PIANO NAZIONALE DI RIPRESA E RESILIENZA (PNRR)—MISSIONE 4 COMPONENTE 2, INVESTIMENTO 1.4—D.D. 1032 17/06/2022, CN00000022). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them.
Funding
This work was supported by University of Basilicata, INPS within PhD Program Industry 4.0 and by Basilicata Region within the framework of FSC “Fondo per lo Sviluppo e la Coesione” (Gestione del ciclo di scarti e sottoprodotti della filiera agroalimentare attraverso la loro bio-conversione in prodotti di valore—D.G.R. n. 652 30/09/2022).
Author information
Authors and Affiliations
Contributions
Conceptualization, PF; funding acquisition, PF, data curation, PF, MT, ET, AG, CS; methodology, PF, MT, ET, AG; supervision, PF, CS, RS; writing—original draft, PF, MT, ET, AG, CS; writing—review and editing, PF, MT, ET, AG, DI, CS, RS, TH, SZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Triunfo, M., Tafi, E., Guarnieri, A. et al. Usage of chitosan from Hermetia illucens as a preservative for fresh Prunus species fruits: a preliminary analysis. Chem. Biol. Technol. Agric. 10, 101 (2023). https://doi.org/10.1186/s40538-023-00480-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-023-00480-x