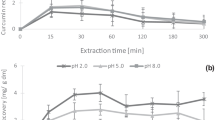
Abstract
Background
In this study, turmeric (Curcuma longa L.) powder was prepared with various extraction and drying methods. Extractions were performed using three solvents (water, 50% ethanol, and 70% ethanol) and three drying methods, namely, spray drying (SD), freeze drying (FD), and spray-freeze drying (SFD).
Results
Levels of the curcuminoids, curcumin, demethoxycurcumin, and bisdemethoxycurcumin, in turmeric powder preparations were analysed using HPLC, and scanning electron microscopy and laser particle size analysis were used to observe and estimate the surface and size distribution of particles. The total amount of curcuminoids ranged from 0.59–14.35 mg/g turmeric powder, and as the concentration of ethanol increased, the level of curcuminoids, antioxidant activity, and average particle size significantly increased (all p < 0.05). In addition, the abundance of curcuminoids significantly increased in the order of SFD, FD, and SD (p < 0.05), whereas the average particle size significantly increased in the order of FD, SFD, and SD (p < 0.05).
Conclusions
The highest TPC and TFC were detected in SFD turmeric powder preparations, whereas the average particle size significantly increased in the order of FD, SFD, and SD. Based on this study, we also showed that the level of curcuminoids, antioxidant activity, TPC, TFC, and average particle size were most affected by the extraction solvent.
Graphical Abstract

Similar content being viewed by others
Introduction
Turmeric (Curcuma longa L.), which is a member of the Zingiberaceae family, is a perennial herb that is widely cultivated throughout tropical and subtropical regions of the world, such as India and China [1]. Turmeric powder is used as a spice and is an essential component of curries. It also adds flavour and colour to rice, pasta, meat and vegetable dishes, and salads [2].
Turmeric powder is a well-known spices containing natural antioxidants and especially yellow pigment of turmeric powder is a phenolic pigment, which is an antioxidant that can scavenge superoxide radicals, hydrogen peroxide and nitric oxide [3]. In addition, Turmeric powder has numerous health benefits, including anti-protozoal, anti-inflammatory, anti-venom activities and anti-tumour activity, and is used widely in the cosmetics and pharmaceutical industry [4]. Major bioactive components of turmeric are curcuminoids, such as curcumin (CUR), demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC). Curcuminoids are hydrophobic and readily soluble in organic solvents, such as methanol, ethanol, and acetone. Among organic solvents, ethanol was reported to have the highest extraction yield [5].
In food processing, freeze drying (FD) and spray drying (SD) are commonly used. Each of various drying methods has its own strength and weakness, and these methods have their own particular characteristics. Although FD can help maintain product quality, such as nutrition, color, and flavor, it entails high production costs, long times, and high energy consumption. Compared to other drying methods, the particles of FD are larger and more uniform [6]. SD is a technology providing with a dry powder product that rapidly evaporates water from the liquid. It is supplied to the spray chamber and has been used in many industries for decades. Hot air is drawn into the chamber to evaporate water from the atomized particles and convert the liquid to a dry powder. SD is advantageous in that it produces free flowing powders in a controlled particle size range from fast drying. Despite these positive aspects, this method can suffer from significant loss of volatiles and thermal degradation of heat-sensitive materials due to high inlet temperature [7]. Whereas spray freeze drying (SFD), which combines SD and FD, is a new method for producing powder formulations. It is profitable to have a fast freezing rate, because small, dried particulates and fine, microporous structures develop [8,9,10,11,12].
In this study, turmeric powder was dried with three drying methods (SD, FD, and SFD) using three solvents (water, 50% ethanol, and 70% ethanol). To investigate the physicochemical properties of these different turmeric powder preparations, levels of curcuminoids, antioxidant activity, total phenolic compounds, total flavonoids compounds, and particle size distribution were analysed.
Materials and methods
Chemical reagents and materials
Commercial grinded turmeric (Curcuma longa L.) was provided by **do Nonghyup in Korea. HPLC-grade water, methanol, acetic acid, chloroform, and acetonitrile were purchased from J. T. Baker (Philipsburg, NJ, USA). CUR (98% purity), DMC (95% purity), and BDMC (95% purity) were purchased from Supelco Corp. (Bellefonte, PA, USA). Primary secondary amine (PSA) was purchased from Agilent (Santa Clara, CA, USA), whereas Folin–Ciocalteu reagent and the antioxidant activity reagents 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All chemicals and reagents used in the study were analytical grade.
The extraction process
The extraction process was modified from the previous study [13]. Ground turmeric (50 g) was extracted with 500 mL of water, 50% ethanol, or 70% ethanol for 2 h at 100 °C. The extract was centrifuged at 6500 ×g and 4 °C for 10 min, and the supernatant was filtered through filter paper (No. 3, 110 mm, Whatman). The filtered solution was concentrated using a rotary evaporator under reduced pressure at 60 °C to remove ethanol.
The drying process
SD, FD, and SFD process were followed by the previous reports [9,10,11,12]. SD was performed in a B-290 spray dryer (Buchi, Flawil, Switzerland). The extract was fed into the chamber using a peristaltic pump, controlling the feed flow rate using the pump rotation speed. The air and feed flow rates were 5 bars and 10 mL/min, respectively. The inlet and outlet temperatures were 150 °C and 85 °C, respectively.
For FD, the extract was frozen in an SE-45 deep freezer (Tefcold, Viborg, Denmark) at − 80 °C for more than 24 h and then dried using a FD 8508 freeze dryer (Ilsin Lab Co., Seoul, Korea) at− 70 °C and under 5 mTorr pressure for 7 days.
For SFD, the solution was sprayed with a feed rate of 43 mL/min and immediately frozen in liquid nitrogen. Drying was performed at a pressure of 0.05 mbar. The shelf temperature was kept at− 20 °C for 28 h and was then increased to 20 °C for 16 h. Before analysis, dried powders were stored at− 80 °C in a deep freezer.
Analysis of curcuminoids
Sample and ethanol were added to a 50 mL teflon centrifuge tube, and ultrasonic extraction was performed for 3 h at 50 °C. After the addition of PSA (0.2 g), the extract was then centrifuged at 14,000 × g and 4 °C for 15 min. The supernatant was filtered through a 0.45 µm nylon filter.
Curcuminoid qualification was performed using thin layer chromatography (TLC) and HPLC. For TLC, 20 μL of sample was manually spotted on a pre-coated silica gel 60-F254 aluminium plate. The distance between each spot was 1 cm. The plate was dried at room temperature and then developed in a TLC chamber previously saturated with chloroform: methanol (47:3, v/v). Bands were scanned at 365 nm. The conditions of HPLC was optimized based on [14]. HPLC was performed using a 1200 Series Gradient system (Agilent Technologies, Santa Clara, CA, USA) equipped with a UV–VIS detector (UVD). The separation of curcuminoids was performed using octadecyl-silica (C18) (4.6 × 250 mm, 5 μm). The column temperature was set at 30 °C, and 2% acetic acid (45% acetonitrile) was used as the mobile phase. The flow rate was constant at 1 mL/min, and the injection volume was 20 μL. The detection wavelength was 425 nm.
Analysis of antioxidant activity
The antioxidant activity of turmeric powder preparations was determined using DPPH and ABTS assays.
DPPH assay
The DPPH free radical scavenging activity of extracted samples was determined according to [15]. DPPH reagent (0.1 mmol L−1) was diluted with methanol to obtain an absorbance of 1.0 ± 0.02 at 517 nm using a spectrophotometer. The DPPH solution (2.9 mL) was mixed with 0.1 mL of turmeric powder dissolved in methanol and then incubated for 30 min in the dark. The absorbance at 517 nm was determined using an UV-1800 spectrophotometer (Shimadzu, Kyoto, Japan). Ascorbic acid was used as a positive control. The percentage of DPPH inhibition was calculated using the following equation:
% DPPH radical scavenging activity = [(Absblank–Abssample)/Absblank] × 100where Absblank and Abssample are the absorbance values of methanol and sample, respectively.
ABTS assay
The ABTS cation radical scavenging assay was followed [16]. ABTS reagent (7.4 mmol L−1) and potassium persulfate solution (2.6 mmol L−1) were mixed in equal amounts and reacted in the dark at room temperature for 16 h. Next, ABTS solution (1 mL) was diluted with methanol to obtain an absorbance of 1.0 ± 0.02 at 734 nm using a spectrophotometer. ABTS solution (2850 µL) was mixed with 150 µL of turmeric powder dissolved in methanol and then incubated for 2 h in the dark. The absorbance was measurement at 734 nm, and ascorbic acid was used as a positive control. The percentage of ABTS inhibition was calculated as follows:
% ABTS radical scavenging activity = [(Absblank–Abssample)/Absblank] × 100where Absblank and Abssample are the absorbance values of methanol and sample, respectively.
Analysis of total polyphenol content (TPC) and total flavonoid content (TFC)
Sample extraction for analysis of TPC and TFC
The method of extraction was similar to the method of [17]. Turmeric powder was extracted with 10 mL of methanol: water (80:20) and stirred for 12 h. The extract was then re-extracted with 10 mL of the same solvent and filtered through a 0.45 μm nylon filter.
TPC
Folin–Ciocalteu reagent was used for TPC according to [18]. The extract (300 μL) was mixed with 2250 μL of 10% Folin–Ciocalteu reagent. After 5 min of equilibrium, 6% sodium carbonate solution (2250 μL) was added. After 90 min of reaction, absorbance was measured at 725 nm using a spectrophotometer. A calibration curve of gallic acid was prepared, and the result was expressed as milligram of gallic acid equivalent per gram of turmeric powder (mg GAE/g turmeric powder).
TFC
The total flavonoid content was determined using the colourimetric method described by [19]. For TFC, the extract (1000 μL) was mixed with 400 μL of 5% sodium nitrate. The mixture was allowed to stand for 6 min, followed by the addition of 10% aluminium chloride (400 μL). After 6 min, 4% sodium hydroxide (4000 μL) was added. Methanol was used to adjust the volume to 10 mL. Next, the mixture was incubated for 15 min and absorbance was measured at 510 nm using a spectrophotometer. A calibration curve of quercetin was prepared, and the result was expressed as milligram of quercetin equivalent per gram of turmeric powder (mg QCE/g turmeric powder).
Scanning electron microscopy (SEM)
The particle surface was observed by SEM using an EM-30 scanning electron microscope from COXEM (Daejeon, Korea). Each dried powder was mounted on an aluminium stage using carbon tape and then coated with gold for 3 min.
Analysis of particle size distribution
A laser particle size analyser (Mastersizer 3000, Malvern Panalytical, Malvern, UK) was used to measure the particle size distribution. Turmeric powder (10 mg) was interspersed in 40 mL of water and then analysed.
Statistical analysis
All analyses in this study were performed in triplicate and expressed as mean ± standard deviation (SD). Comparisons were performed by analysis of variance (ANOVA), followed by Duncan’s multiple range test using IBM SPSS Statistics for Windows version 25 (IBM Corp., Armonk, NY, USA). Principal component analysis (PCA) was performed to visualise the distribution of samples according to the various extraction and drying methods using XLSTAT (Addinsoft, New York, NY, USA). A p value less than 0.05 was considered statistically significant.
Results and discussion
Analysis of curcuminoids by TLC and HPLC
Additional file 1: Fig. S1 shows the TLC analysis of each sample’s result using an UV lamp. The chromatogram of curcuminoids using HPLC–UVD is shown in Additional file 1: Fig. S2. The calibration curve of each curcuminoid (CUR, DMC, and BDMC) showed good linearity (all R2 = 1.000) at a concentration range of 0.1 − 25.00 µg/mL. The content of curcuminoids in our turmeric powder preparations is shown in Table 1. The total amount of curcuminoids ranged from 0.945–14.21 mg/g turmeric powder, and the level of CUR, DMC, and BDMC ranged from 0.611–9.443, 0.182–2.704, and 0.151–2.06 mg/g turmeric powder, respectively. We found that the curcuminoid with the highest content was CUR, whereas the lowest content was BDMC. These findings are similar to those reported in a previous study [6]. Furthermore, the abundance of CUR was approximately 4 to 10 times higher than DMC and BDMC in turmeric powder.
Curcuminoid extraction is greater in ethanol than in water. In this study, the content of curcuminoids increased significantly with higher ethanol concentration in the extraction solvent for each drying method (all p < 0.05). Furthermore, the abundance of curcuminoids significantly increased in the order of SFD, FD, and SD (p < 0.05). In the various previous reports, curcuminoids are efficiently extracted in organic solvents [20], whereas heat treatment of turmeric results in a significant loss [21, 22].
Analysis of antioxidant activity, TPC, and TFC
Turmeric is rich in phenolic compounds, and the antioxidant activity of turmeric has been intensively studied [23,24,25]. Both polyphenol compounds and flavonoid compounds have a major influence on determining antioxidant activity [26].
The antioxidant activities of nine turmeric powder preparations were measured by DPPH and ABTS assays using ascorbic acid as a positive control (Table 2). We found that the antioxidant activity differed according to the extraction solvent. For each drying method, the antioxidant activity increased as the ethanol concentration increased. This finding was expected, because curcuminoid abundance increases with increasing ethanol concentration. In vitro study reported previously, curcuminoids such as curcumin have strong antioxidant activity [27]. Because SD occurs at high temperature, the content of curcuminoids is decreased. However, as heat treatment increases free polyphenols, which affect the antioxidant activity, we found no significant difference in antioxidant activity among the three drying methods [28].
For TPC and TFC analysis, calibration curves of gallic acid and quercetin showed linearity (R2 = 0.992 and 0.991, respectively) at a concentration range of 0.1 − 100.0 µg/mL. Based on our analysis, we found that TPC and TFC in turmeric preparations differed according to the extraction solvent (Table 3). For each drying condition, TPC and TFC increased with increasing concentrations of sample extracted using 70% ethanol. Water is not an efficient solvent, because polyphenol compounds are more soluble in less polar solvents than water [29]. Although curcuminoids are not classified as flavonoids, they react similarly as flavonoid compounds to the aluminium chloride (AlCl3) used in the TFC method [30]. The principle in determining TFC is that AlCl3 forms acid-stable complexes with the keto and hydroxyl groups of flavonoids (Additional file 1: Fig. S3). Curcuminoids are natural diarylheptanoids, which consist of two aromatic o-methoxy phenolic groups linked by a seven-carbon chain [30]. They exist in either the enol or keto form; however, the enol configuration is the major component in solution [31]. AlCl3 binds to curcuminoids through the β-diketone group (Additional file 1: Fig. S4) [32].
Evaluation of morphology and particle size distribution
As shown in Fig. 1, we evaluated the particle size distribution of turmeric powder preparations and found that all turmeric extracts subjected to a drying process have a more uniform particle size than the control. Furthermore, the average particle size of turmeric extracted using water was smaller than those using ethanol extraction. In addition, for each extraction solvent, we found that turmeric preparations by FD resulted in the largest average particle size, whereas SD resulted in the smallest (Table 4) [33, 34].
Next, the physical structure of turmeric powder particles was observed using SEM. As shown in Fig. 2, we found that the particle structure of turmeric powder preparations was completely different depending on the drying method. Specifically, the powder from SD samples demonstrated a distorted spherical-like particle shape (Fig. 2b, c), whereas those from FD samples formed a flaky powder (Fig. 2d–f) [35]. In contrast, we found that SFD samples have a spherical particle shape (Fig. 2g–i).
Scanning electron microscopy (SEM) image of various extraction solvents and drying methods. a Control; (b) WSD: water extract spray drying; (c) 50ESD: 50% ethanol extract spray drying; (d) WFD: water extract freeze drying; (e) 50EFD: 50% ethanol extract freeze drying; (f) 70EFD: 70% ethanol extract freeze drying; (g) WSFD: water extract spray freeze drying; (h) 50ESFD: 50% ethanol extract spray freeze drying; (i) 70ESFD: 70% ethanol extract spray freeze drying
These findings are mainly caused by the atomisation during SD and SFD that results in smaller droplets, and these atomised droplets are often spherical in shape. In the previous reports, the low temperature involved in FD and a lack of strength to break the frozen drops or alterations to the surface during drying may be reasons for the larger particles and flake-like structures [33, 36].
PCA
In general, the applicability of simple statistical descriptors, such as mean, variance, and covariance, decrease with increasing number of variables. As the size and complexity of data increase, such methods fail to accurately explain trends in data. PCA is a well-suited technique for describing large data sets, using a covariance matrix system to do what univariate methods cannot [37].
Quantitative data obtained from curcuminoid abundance, antioxidant activity, TPC, TFC, and particle size were analysed using PCA. Two principal components explained 82.17% and 15.86% of data variance (F1 and F2, respectively). We identified three separate groups according to extraction solvent (Additional file 1: Fig. S5). As a result of measuring curcuminoids, antioxidant activity, TPC, and TFC, we found that turmeric powder extracted from 70% ethanol was the closest.
Conclusions
In this study, we investigated the characteristics of turmeric powder prepared with three solvents (water, 50% ethanol, and 70% ethanol) and drying methods (SD, FD, and SFD). We determined the level of curcuminoids, antioxidant activity, TPC, TFC, and particle size in nine different samples, including control, and discovered, as the concentration of ethanol increased, the level of curcuminoids, antioxidant activity, TPC, TFC, and average particle size significantly increased (all p < 0.05). In addition, SFD turmeric powder had higher levels of curcuminoids than SD and FD turmeric powder. However, it was confirmed that the antioxidant activity was not affected by drying method. Furthermore, the highest TPC and TFC were detected in SFD turmeric powder preparations, whereas the average particle size significantly increased in the order of FD, SFD, and SD. Based on PCA, we also showed that the level of curcuminoids, antioxidant activity, TPC, TFC, and average particle size were most affected by the extraction solvent.
Availability of data and materials
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Araújo C, Leon L. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96:723–8.
Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by turmeric, the golden spice: From kitchen to clinic. Mol Nutr Food Res. 2013;57(9):1510–28.
Lim HS, Park SH, Ghafoor K, Hwang SY, Park J. Quality and antioxidant properties of bread containing turmeric (Curcuma longa L.) cultivated in South Korea. Food Chem. 2011;124(4):1577–82.
Zhang L, Yang Z, Chen F, Su P, Chen D, Pan W, et al. Composition and bioactivity assessment of essential oils of Curcuma longa L. collected in China. Ind Crops Prod. 2017;109:60–73.
Kwon H-L, Chu M-S. Pilot-scale subcritical solvent extraction of curcuminoids from Curcuma long L. Food Chem. 2015;185:58–64.
Chumroenphat T, Somboonwatthanakul I, Saensouk S, Siriamornpun S. Changes in curcuminoids and chemical components of turmeric (Curcuma longa L.) under freeze-drying and low-temperature drying methods. Food Chem. 2021;339:128121.
Ishwarya SP, Anandharamakrishnan C, Stapley AG. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci Technol. 2015;41(2):161–81.
Wang ZL, Finlay WH, Peppler MS, Sweeney LG. Powder formation by atmospheric spray-freeze-drying. Powder Technol. 2006;170(1):45–52.
Her J-Y, Kim MS, Lee K-G. Preparation of probiotic powder by the spray freeze-drying method. J Food Eng. 2015;150:70–4.
Her J-Y, Song C-S, Lee SJ, Lee K-G. Preparation of kanamycin powder by an optimized spray freeze-drying method. Powder Technol. 2010;199(2):159–64.
Her J-Y, Lee K-GJDT. Effect of the solvent composition and annealing process on the preparation of spray freeze-dried acetaminophen powder. Drying Technol. 2017;35(5):625–30.
Her JY, Kim MS, Kim MK, Lee KGJIJoFS, Technology. Development of a spray freeze-drying method for preparation of volatile shiitake mushroom (Lentinus edodes) powder. Int J Food Sci Technol. 2015;50(10):2222–8.
**ong J, Chan YH, Rathinasabapathy T, Grace MH, Komarnytsky S, Lila MA. Enhanced stability of berry pomace polyphenols delivered in protein-polyphenol aggregate particles to an in vitro gastrointestinal digestion model. Food Chem. 2020;331: 127279.
Syed HK, Liew KB, Loh GOK, Peh KK. Stability indicating HPLC–UV method for detection of curcumin in Curcuma longa extract and emulsion formulation. Food Chem. 2015;170:321–6.
Siriamornpun S, Tangkhawanit E, Kaewseejan N. Reducing retrogradation and lipid oxidation of normal and glutinous rice flours by adding mango peel powder. Food Chem. 2016;201:160–7.
Jorjong S, Butkhup L, Samappito S. Phytochemicals and antioxidant capacities of mao-luang (Antidesma bunius L.) cultivars from Northeastern Thailand. Food Chem. 2015;181:248–55.
Jelled A, Fernandes Â, Barros L, Chahdoura H, Achour L, Ferreira IC, et al. Chemical and antioxidant parameters of dried forms of ginger rhizomes. Ind Crops Prod. 2015;77:30–5.
Ratseewo J, Warren F, Siriamornpun S. The influence of starch structure and anthocyanin content on the digestibility of Thai pigmented rice. Food Chem. 2019;298: 124949.
Siriamornpun S, Kaewseejan N. Quality, bioactive compounds and antioxidant capacity of selected climacteric fruits with relation to their maturity. Sci Hortic. 2017;221:33–42.
Popuri AK, Pagala B. Extraction of curcumin from turmeric roots. Int J Innov Res Stud. 2013;2:289–99.
Srinivasan K, Sambaiah K, Chandrasekhara N. Loss of active principles of common spices during domestic cooking. Food Chem. 1992;43(4):271–4.
Suresh D, Manjunatha H, Srinivasan K. Effect of heat processing of spices on the concentrations of their bioactive principles: Turmeric (Curcuma longa), red pepper (Capsicum annuum) and black pepper (Piper nigrum). J Food Compos Anal. 2007;20(3–4):346–51.
Park CY, Lee K-Y, Gul K, Rahman MS, Kim A-N, Chun J, et al. Phenolics and antioxidant activity of aqueous turmeric extracts as affected by heating temperature and time. LWT. 2019;105:149–55.
Braga MEM, Leal PF, Carvalho JE, Meireles MAA. Comparison of yield, composition and antioxidant activity of turmeric (Curcuma longa L) extracts obtained using various techniques. J Agric Food Chem. 2003;51(22):6604–11.
Prathapan A, Lukhman M, Arumughan C, Sundaresan A, Raghu K. Effect of heat treatment on curcuminoid, colour value and total polyphenols of fresh turmeric rhizome. Int J Food Sci Technol. 2009;44(7):1438–44.
Velioglu Y, Mazza G, Gao L, Oomah B. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46(10):4113–7.
Ak T, Gülçin İ. Antioxidant and radical scavenging properties of curcumin. Chemico-Biol Interact. 2008;174(1):27–37.
Choi Y, Lee SM, Chun J, Lee HB, Lee J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006;99(2):381–7.
Pinelo M, Rubilar M, Jerez M, Sineiro J, Núñez MJ. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J Agric Food Chem. 2005;53(6):2111–7.
Sepahpour S, Selamat J, Manap MYA, Khatib A, Razis AFA. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules. 2018;23(2):402.
Priyadarsini KI. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19(12):20091–112.
Indira PK. Chemical and structural features influencing the biological activity of curcumin. Curr Pharm Des. 2013;19(11):2093–100.
Pellicer JA, Fortea MI, Trabal J, Rodríguez-López MI, Gabaldón JA, Núñez-Delicado E. Stability of microencapsulated strawberry flavour by spray drying, freeze drying and fluid bed. Powder Technol. 2019;347:179–85.
Di A, Zhang S, Liu X, Tong Z, Sun S, Tang Z, et al. Microfluidic spray dried and spray freeze dried uniform microparticles potentially for intranasal drug delivery and controlled release. Powder Technol. 2021;379:144–53.
Wu G, Hui X, Liang J, Liu H, Chen H, Gong X, et al. Combination of rehydrated whey protein isolate aqueous solution with blackcurrant concentrate and the formation of encapsulates via spray-drying and freeze-drying: Alterations to the functional properties of protein and their anticancer properties. Food Chem. 2021;355: 129620.
Chen C, Chi Y-J, Xu W. Comparisons on the functional properties and antioxidant activity of spray-dried and freeze-dried egg white protein hydrolysate. Food Bioprocess Technol. 2012;5(6):2342–52.
Perera KDC, Weragoda GK, Haputhanthri R, Rodrigo SK. Study of concentration dependent curcumin interaction with serum biomolecules using ATR-FTIR spectroscopy combined with principal component analysis (PCA) and partial least square regression (PLS-R). Vib Spectrosc. 2021;116: 103288.
Chamorro S, Cueva-Mestanza R, Pascual-Teresa Sd. Effect of spray drying on the polyphenolic compounds present in purple sweet potato roots: Identification of new cinnamoylquinic acids. Food Chem. 2021;345:128679.
Acknowledgements
Not applicable.
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea [grant number NRF, 2021R1A2B5B01002296] and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries [IPET, No. 320110–01].
Author information
Authors and Affiliations
Contributions
[38] JP: formal analysis, investigation, and methodology; SD: formal analysis; ML: validation; SH: methodology; KGL: supervision, validation, investigation, and project administration. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
TLC of turmeric powder according to the various extract and drying methods. STD: Standard; WSD: Water extract Spray Drying; 50ESD: 50% Ethanol extract Spray Drying; WFD: Water extract Freeze Drying; 50EFD: 50% Ethanol extract Freeze Drying; 70EFD: 70% Ethanol extract Freeze Drying; WSFD: Water extract Spray Freeze Drying; 50ESFD: 50% Ethanol extract Spray Freeze Drying; 70ESFD: 70% Ethanol extract Spray Freeze Drying. Figure S2 HPLC–UV chromatogram of curcuminoids (Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin). Figure S3 Formation of flavonoid complex with AlCl3. Figure S4 Formation of curcumin complex with AlCl3. Figure S5 PCA of curcuminoids, antioxidant activity, TPC, TFC, and particle size. WSD: water extract spray drying; 50ESD: 50% ethanol extract spray drying; WFD: water extract freeze drying; 50EFD: 50% ethanol extract freeze drying; 70EFD: 70% ethanol extract freeze drying; WSFD: water extract spray-freeze drying; 50ESFD: 50% ethanol extract spray-freeze drying; 70ESFD: 70% ethanol extract spray-freeze drying.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Park, J., Do, S., Lee, M. et al. Preparation of turmeric powder with various extraction and drying methods. Chem. Biol. Technol. Agric. 9, 39 (2022). https://doi.org/10.1186/s40538-022-00307-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40538-022-00307-1