Abstract
Myelin sheath abnormality is the cause of various neurodegenerative diseases (NDDs). G-proteins and their coupled receptors (GPCRs) play the important roles in myelination. Gnao1, encoding the major Gα protein (Gαo) in mammalian nerve system, is required for normal motor function. Here, we show that Gnao1 restricted to Schwann cell (SCs) lineage, but not neurons, negatively regulate SC differentiation, myelination, as well as re-myelination in peripheral nervous system (PNS). Mice lacking Gnao1 expression in SCs exhibit faster re-myelination and motor function recovery after nerve injury. Conversely, mice with Gnao1 overexpression in SCs display the insufficient myelinating capacity and delayed re-myelination. In vitro, Gnao1 deletion in SCs promotes SC differentiation. We found that Gnao1 knockdown in SCs resulting in the elevation of cAMP content and the activation of PI3K/AKT pathway, both associated with SC differentiation. The analysis of RNA sequencing data further evidenced that Gnao1 deletion cause the increased expression of myelin-related molecules and activation of regulatory pathways. Taken together, our data indicate that Gnao1 negatively regulated SC differentiation by reducing cAMP level and inhibiting PI3K-AKT cascade activation, identifying a novel drug target for the treatment of demyelinating diseases.
Similar content being viewed by others
Introduction
In the central and peripheral nervous system (CNS and PNS), most axons are surrounded by the multilayer of specialized membranes known as the myelin sheath, an organelle produced by Schwann cells (SCs) in PNS and oligodendrocytes (OLs) in CNS, which not only accelerates nerve impulse propagation but is essential for the functional integrity and long term health of axons [33, 43]. Loss or disorder of myelin sheath is the cause of a variety of neurodegenerative diseases (NDDs), including multiple sclerosis (MS), inherited leukodystrophies in the central nerve system, peripheral neuropathies such as Guillain-Barré Syndrome, and other demyelinating diseases caused by the external factors (e.g. trauma, infection or poisoning) and various pathological conditions [35, 52]. Moreover, researchers recently found that even subtle myelin abnormalities may also contribute to more complex neurological disorders, such as schizophrenia and epilepsy [7, 11, 34]. Because of the important role of myelin sheath in neurophysiology, discovering of the mechanisms underlying myelin formation and re-myelination could help to identify new targets for the treatment of neurological disorders. Indeed, enhancing endogenous re-myelination has recently emerged as a promising therapeutic approach in the common but complex NDDs [12, 37, 52].
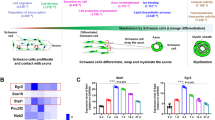
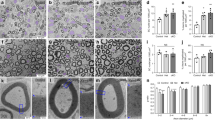
G-proteins and their coupled receptors (GPCRs), the largest intracellular signal molecule superfamily and by far the most successful drug targets, play the important roles in the development of myelin-forming cells (i.e. SCs and OLs), myelination and re-myelination [21, 23, 26]. For example, GPR126 (also known as ADGRG6) is a conserved regulator of SC myelination in the PNS, which initiates myelination by coupling to the Gαs proteins, increasing cyclic AMP (cAMP) levels and activating protein kinase A (PKA), eventually leading to SC differentiation and myelination [25, 27, 29]. GPR126 mutations in humans cause reduced expression of myelin genes leading to lethal congenital contracture syndrome [41]. In addition, GPR44, activated by prostaglandin D2, play an important role in the formation and maintenance of PNS myelin sheath [47]. In the CNS, GPR56 [1, 13] and GPR17 [6, 49] modulate the proliferation and early differentiation of oligodendrocyte precursor cells (OPCs), and mutations of GPR56 cause bilateral frontoparietal polymicrogyria disease in humans [40]. GPR149 [32, 42]. All of these steps are regulated in an orderly manner by multiple factors. In this study, we found that Gnao1 knockdown inhibits SC proliferation and migration, but promotes SC differentiation (Figs. 5, 6, 7), indicating that Gnao1 achieves regulation of PNS myelination by coordinating of SC proliferation, migration and differentiation. To explore the molecular mechanisms by which Gnao1 regulates the differentiation of SCs, we sequenced the transcriptome of Gnao1-KD-SCs and control SCs (NCs) before and after differentiation. Surprisingly, we found that inhibiting Gnao1 expression in SCs increased the expression of myelin constitutive proteins (MPZ, MBP, PMP22 and MAG) and some positive regulatory factors such as Olig1, Pou3f2, and Egr2, even before inducing SCs differentiation (Fig. 8A and Additional file 1: Fig. S6, Additional file 3: Table S2), suggesting that the absence of Gnao1 in SCs may lead to spontaneous differentiation of SCs. So how does it work? As well known, that Gαo encoded by Gnao1 usually binds to GDP and forms a heterotrimer with Gβγ. When stimulated by extracellular signals, the GDP bound by Gαo is exchanged with GTP, causing conformational change of Gαo to dissociate the Gβγ from the heterotrimer [16, 44]. On the one hand, the free Gαo inhibits the activity of adenylate cyclase (AC) from converting adenosine triphosphate (ATP) into an important second messenger, cAMP, thereby suppressing its downstream signaling [36]. On the other hand, free Gβγ also regulate cellular function by activating downstream signaling pathways [9, 31, 38]. Therefore, we speculated that the possible mechanism of Gnao1 deficiency triggering spontaneous differentiation of SCs is as follows: (1) The absence of Gnao1 in SCs reduced the content of the Gαo, resulting in the depolymerization of Gαβγ heterotrimers, which increased the number of free Gβγ dimers, promoted the activation of downstream signaling pathways (such as PI3K/AKT and MAPK/ERK), and facilitates the differentiation of SCs. (2) Gnao1 deletion in SCs led to increased synthesis of cAMP, the second messenger in the G protein-coupled receptor signaling pathway, which accelerated SCs differentiation. In fact, we did notice that the increased expression of Adcy2 and Prkaa2 (protein kinase AMP-activated catalytic subunit alpha 2, Prkaa2) in Gnao1-KD-SCs compared to NCs (Additional file 1: Fig. S6, Additional file 3: Table S2). Adcy2, has been found to convert ATP to cAMP, which activates the protein kinase PKA (Prkaa2), leading to activation of the PI3K-AKT pathway. Furthermore, we also found that the functions of up-regulated DEGs between Gnao1-KD-SCs and NCs were related to cAMP and PI3K/AKT signaling pathway by KEGG analysis (Fig. 8A, Additional file 3: Table S2). We found through WB analysis that compared with NCs, the expression of Adcy2 in Gnao1-KD-SCs was indeed increased, and ELISA results also evidenced the elevated cAMP content in Gnao1-KD-SCs (Fig. 8Bb1-2). Next, we used the SC differentiation medium without db-cAMP to culture Gnao1-KD-SCs, and found that Gnao1-KD-SCs had higher expression of myelin-related protein MAG compared with NCs (Fig. 8Bb3), suggesting that knockdown of Gnao1 expression in SCs could promote the formation of cAMP, which compensates for the effect of removed db-cAMP. Moreover, our data showed that Gnao1 knockdown only affected the PI3K/AKT signaling pathway, which is involved in glial differentiation and myelination of the nervous system [18, 19, 46], but had no effect on MAPK/ERK signaling (Fig. 9). Taken together, we believe that down-regulation of Gnao1 expression in SCs can increase cAMP content and the number of free Gβγ dimer, resulting in activation of PI3K/AKT signaling pathway, promoting differentiation of SCs.
Conclusion
Gnao1 is important for myelination in PNS. Gnao1 knockdown in SCs promotes the axonal re-myelination and motor function recovery after nerve injury. Conversely, mice with Gnao1 overexpression in SCs display the insufficient myelinating capacity and delayed re-myelination. Gnao1 deletion in SCs promotes SC differentiation by the elevation of cAMP content and the activation of PI3K/AKT pathway (Fig. 10). In light of the current data, our findings uncover a function of Gnao1 to negatively regulate SC differentiation, identifying a novel candidate drug target for the treatment of demyelinating diseases.
Availability of data and materials
Microarray expression data for SCs at various stages of myelination (i.e., immature, pre-myelination, and myelination) was supplied in NCBI Gene Expression Omnibus (Accession number: GSE163132). The other datasets that support the findings of the current study are available from the corresponding author upon reasonable request.
Abbreviations
- NDDs:
-
Neurodegenerative diseases
- PNS:
-
Peripheral nervous system
- SCs:
-
Schwann cells
- DRG:
-
Dorsal root ganglion
- Gnao1-NKD mice:
-
Mice with Gnao1 knockdown in the sciatic nerve
- Gnao1-NOE mice:
-
Mice with Gnao1 overexpression in the sciatic nerve
- Gnao1-SKD mice:
-
Mice with Gnao1 knockdown in spinal cords
- db-cAMP:
-
Dibutyryl cyclic AMP
- Ara-C:
-
Cytosine arabinoside
- PDL:
-
Poly-D-lysine
- Nrg1:
-
Neuregulin 1
- EdU:
-
5-Ethynyl-2'-deoxyuridine
- AAV:
-
Adeno-associated virus
- IHC:
-
Immunohistochemistry
- ICC:
-
Immunocytochemistry
- RT-qPCR:
-
Real-time quantitative PCR
- WB:
-
Western blotting
- TEM:
-
Transmission electron microscopy
- GPR:
-
G-protein coupled receptors
- MAG:
-
Myelin associated glycoprotein
- P0 (MPZ):
-
Myelin protein zero
- MBP:
-
Myelin basic protein
- PMP22:
-
Peripheral myelin protein-22
- PI3K:
-
Phosphatidylinositol 3-kinase
- TuJ1:
-
β-III tubulin
- RNA-seq:
-
RNA sequencing
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
References
Ackerman SD, Garcia C, Piao X, Gutmann DH, Monk KR (2015) The adhesion GPCR Gpr56 regulates oligodendrocyte development via interactions with Galpha12/13 and RhoA. Nat Commun 6:6122. https://doi.org/10.1038/ncomms7122
Blugeon C, Le Crom S, Richard L, Vallat JM, Charnay P, Decker L (2011) Dok4 is involved in Schwann cell myelination and axonal interaction in vitro. Glia 59:351–362. https://doi.org/10.1002/glia.21106
Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE (2003) Insights into G protein structure, function, and regulation. Endocr Rev 24:765–781. https://doi.org/10.1210/er.2000-0026
Cha HL, Choi JM, Oh HH, Bashyal N, Kim SS, Birnbaumer L, Suh-Kim H (2019) Deletion of the alpha subunit of the heterotrimeric Go protein impairs cerebellar cortical development in mice. Mol Brain 12:57. https://doi.org/10.1186/s13041-019-0477-9
Chamero P, Katsoulidou V, Hendrix P, Bufe B, Roberts R, Matsunami H, Abramowitz J, Birnbaumer L, Zufall F, Leinders-Zufall T (2011) G protein G(alpha)o is essential for vomeronasal function and aggressive behavior in mice. Proc Natl Acad Sci USA 108:12898–12903. https://doi.org/10.1073/pnas.1107770108
Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA et al (2009) The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat Neurosci 12:1398–1406. https://doi.org/10.1038/nn.2410
de Curtis M, Garbelli R, Uva L (2021) A hypothesis for the role of axon demyelination in seizure generation. Epilepsia 62:583–595. https://doi.org/10.1111/epi.16824
Feldmann A, Amphornrat J, Schonherr M, Winterstein C, Mobius W, Ruhwedel T, Danglot L, Nave KA, Galli T, Bruns D et al (2011) Transport of the major myelin proteolipid protein is directed by VAMP3 and VAMP7. J Neurosci 31:5659–5672. https://doi.org/10.1523/Jneurosci.6638-10.2011
Feng H, Khalil S, Neubig RR, Sidiropoulos C (2018) A mechanistic review on GNAO1-associated movement disorder. Neurobiol Dis 116:131–141. https://doi.org/10.1016/j.nbd.2018.05.005
Feng H, Sjogren B, Karaj B, Shaw V, Gezer A, Neubig RR (2017) Movement disorder in GNAO1 encephalopathy associated with gain-of-function mutations. Neurology 89:762–770. https://doi.org/10.1212/WNL.0000000000004262
Fields RD (2008) White matter in learning, cognition and psychiatric disorders. Trends Neurosci 31:361–370. https://doi.org/10.1016/j.tins.2008.04.001
Franklin RJM, Ffrench-Constant C (2017) Regenerating CNS myelin—from mechanisms to experimental medicines. Nat Rev Neurosci 18:753–769. https://doi.org/10.1038/nrn.2017.136
Giera S, Deng Y, Luo R, Ackerman SD, Mogha A, Monk KR, Ying Y, Jeong SJ, Makinodan M, Bialas AR et al (2015) The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development. Nat Commun 6:6121. https://doi.org/10.1038/ncomms7121
Goldstein LB (2006) Neurotransmitters and motor activity: effects on functional recovery after brain injury. NeuroRx 3:451–457. https://doi.org/10.1016/j.nurx.2006.07.010
Gomis-Coloma C, Velasco-Aviles S, Gomez-Sanchez JA, Casillas-Bajo A, Backs J, Cabedo H (2018) Class IIa histone deacetylases link cAMP signaling to the myelin transcriptional program of Schwann cells. J Cell Biol 217:1249–1268. https://doi.org/10.1083/jcb.201611150
He JC, Neves SR, Jordan JD, Iyengar R (2006) Role of the Go/i signaling network in the regulation of neurite outgrowth. Can J Physiol Pharmacol 84:687–694. https://doi.org/10.1139/y06-025
Hildebrand MS, Jackson VE, Scerri TS, Van Reyk O, Coleman M, Braden RO, Turner S, Rigbye KA, Boys A, Barton S et al (2020) Severe childhood speech disorder: gene discovery highlights transcriptional dysregulation. Neurology 94:e2148–e2167. https://doi.org/10.1212/WNL.0000000000009441
Ishii A, Furusho M, Bansal R (2021) Mek/ERK1/2-MAPK and PI3K/Akt/mTOR signaling plays both independent and cooperative roles in Schwann cell differentiation, myelination and dysmyelination. Glia 69:2429–2446. https://doi.org/10.1002/glia.24049
Ishii A, Furusho M, Macklin W, Bansal R (2019) Independent and cooperative roles of the Mek/ERK1/2-MAPK and PI3K/Akt/mTOR pathways during developmental myelination and in adulthood. Glia 67:1277–1295. https://doi.org/10.1002/glia.23602
Kagiava A, Richter J, Tryfonos C, Leal-Julia M, Sargiannidou I, Christodoulou C, Bosch A, Kleopa KA (2021) Efficacy of AAV serotypes to target Schwann cells after intrathecal and intravenous delivery. Sci Rep 11:23358. https://doi.org/10.1038/s41598-021-02694-1
Lecca D, Raffaele S, Abbracchio MP, Fumagalli M (2020) Regulation and signaling of the GPR17 receptor in oligodendroglial cells. Glia 68:1957–1967. https://doi.org/10.1002/glia.23807
Liu B, **n W, Tan JR, Zhu RP, Li T, Wang D, Kan SS, **ong DK, Li HH, Zhang MM et al (2019) Myelin sheath structure and regeneration in peripheral nerve injury repair. P Natl Acad Sci USA 116:22347–22352. https://doi.org/10.1073/pnas.1910292116
Mehta P, Piao X (2017) Adhesion G-protein coupled receptors and extracellular matrix proteins: roles in myelination and glial cell development. Dev Dyn 246:275–284. https://doi.org/10.1002/dvdy.24473
Mercimek-Mahmutoglu S, Sidky S, Hyland K, Patel J, Donner EJ, Logan W, Mendoza-Londono R, Moharir M, Raiman J, Schulze A et al (2015) Prevalence of inherited neurotransmitter disorders in patients with movement disorders and epilepsy: a retrospective cohort study. Orphanet J Rare Dis 10:12. https://doi.org/10.1186/s13023-015-0234-9
Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR (2013) Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. J Neurosci 33:17976–17985. https://doi.org/10.1523/JNEUROSCI.1809-13.2013
Mogha A, D’Rozario M, Monk KR (2016) G Protein-coupled receptors in myelinating glia. Trends Pharmacol Sci 37:977–987. https://doi.org/10.1016/j.tips.2016.09.002
Mogha A, Harty BL, Carlin D, Joseph J, Sanchez NE, Suter U, Piao X, Cavalli V, Monk KR (2016) Gpr126/Adgrg6 has Schwann cell autonomous and nonautonomous functions in peripheral nerve injury and repair. J Neurosci 36:12351–12367. https://doi.org/10.1523/JNEUROSCI.3854-15.2016
Monje PV, Soto J, Bacallao K, Wood PM (2010) Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state. J Biol Chem 285:31024–31036. https://doi.org/10.1074/jbc.M110.116970
Monk KR, Oshima K, Jors S, Heller S, Talbot WS (2011) Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 138:2673–2680. https://doi.org/10.1242/dev.062224
Morgan L et al (1991) The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. J Cell Biol 112:457
Muntean BS, Masuho I, Dao M, Sutton LP, Zucca S, Iwamoto H, Patil DN, Wang D, Birnbaumer L, Blakely RD et al (2021) Galphao is a major determinant of cAMP signaling in the pathophysiology of movement disorders. Cell Rep 34:108718. https://doi.org/10.1016/j.celrep.2021.108718
Nave K-A, Trapp BD (2008) Axon-glial signaling and the glial support of axon function. Annu Rev Neurosci 31:535–561. https://doi.org/10.1146/annurev.neuro.30.051606.094309
Nave KA (2010) Myelination and support of axonal integrity by glia. Nature 468:244–252. https://doi.org/10.1038/nature09614
Nave KA (2010) Myelination and the trophic support of long axons. Nat Rev Neurosci 11:275–283. https://doi.org/10.1038/nrn2797
Nave KA, Werner HB (2014) Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Bi 30:503. https://doi.org/10.1146/annurev-cellbio-100913-013101
Neves SR, Ram PT, Iyengar R (2002) G protein pathways. Science 296:1636–1639. https://doi.org/10.1126/science.1071550
Pan S, Chan JR (2021) Clinical applications of myelin plasticity for remyelinating therapies in multiple sclerosis. Ann Neurol 90:558–567. https://doi.org/10.1002/ana.26196
Pearson TS, Helbig I (2017) Epileptic encephalopathy, movement disorder, and the yin and yang of GNAO1 function. Neurology 89:754–755. https://doi.org/10.1212/WNL.0000000000004277
Pereira JA, Lebrun-Julien F, Suter U (2012) Molecular mechanisms regulating myelination in the peripheral nervous system. Trends Neurosci 35:123–134. https://doi.org/10.1016/j.tins.2011.11.006
Piao X, Chang BS, Bodell A, Woods K, Benzeev B, Topcu M, Guerrini R, Goldberg-Stern H, Sztriha L, Dobyns WB et al (2005) Genotype-phenotype analysis of human frontoparietal polymicrogyria syndromes. Ann Neurol 58:680–687. https://doi.org/10.1002/ana.20616
Ravenscroft G, Nolent F, Rajagopalan S, Meireles AM, Paavola KJ, Gaillard D, Alanio E, Buckland M, Arbuckle S, Krivanek M et al (2015) Mutations of GPR126 are responsible for severe arthrogryposis multiplex congenita. Am J Hum Genet 96:955–961. https://doi.org/10.1016/j.ajhg.2015.04.014
Salzer JL, Brophy PJ, Peles E (2008) Molecular domains of myelinated axons in the peripheral nervous system. Glia 56:1532–1540. https://doi.org/10.1002/glia.20750
Simons M, Trotter J (2007) Wrap** it up: the cell biology of myelination. Curr Opin Neurobiol 17:533–540. https://doi.org/10.1016/j.conb.2007.08.003
Slep KC, Kercher MA, Wieland T, Chen CK, Simon MI, Sigler PB (2008) Molecular architecture of Galphao and the structural basis for RGS16-mediated deactivation. Proc Natl Acad Sci USA 105:6243–6248. https://doi.org/10.1073/pnas.0801569105
Suo N, He B, Cui S, Yang Y, Wang M, Yuan Q, **e X (2022) The orphan G protein-coupled receptor GPR149 is a negative regulator of myelination and remyelination. Glia 70:1992–2008. https://doi.org/10.1002/glia.24233
Taveggia C, Feltri ML, Wrabetz L (2010) Signals to promote myelin formation and repair. Nat Rev Neurol 6:276–287. https://doi.org/10.1038/nrneurol.2010.37
Trimarco A, Forese MG, Alfieri V, Lucente A, Brambilla P, Dina G, Pieragostino D, Sacchetta P, Urade Y, Boizet-Bonhoure B et al (2014) Prostaglandin D2 synthase/GPR44: a signaling axis in PNS myelination. Nat Neurosci 17:1682–1692. https://doi.org/10.1038/nn.3857
Wang D, Dao M, Muntean BS, Giles AC, Martemyanov KA, Grill B (2022) Genetic modeling of GNAO1 disorder delineates mechanisms of Galphao dysfunction. Hum Mol Genet 31:510–522. https://doi.org/10.1093/hmg/ddab235
Wang J, He X, Meng H, Li Y, Dmitriev P, Tian F, Page JC, Lu QR, He Z (2020) Robust myelination of regenerated axons induced by combined manipulations of GPR17 and microglia. Neuron 108(876–886):e874. https://doi.org/10.1016/j.neuron.2020.09.016
Yang HJ, Vainshtein A, Maik-Rachline G, Peles E (2016) G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat Commun 7:10884. https://doi.org/10.1038/ncomms10884
Zhang B, Su W, Hu J, Xu J, Askar P, Bao S, Zhou S, Chen G, Gu Y (2022) Transcriptome analysis of Schwann cells at various stages of myelination implicates chromatin regulator Sin3A in control of myelination identity. Neurosci Bull 38:720–740. https://doi.org/10.1007/s12264-022-00850-9
Zhou Y, Notterpek L (2016) Promoting peripheral myelin repair. Exp Neurol 283:573–580. https://doi.org/10.1016/j.expneurol.2016.04.007
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (82272169, 32271418 and 82001296), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
YG and CLZ conceived and designed the study. JHX, QQP, JYZ and GHSG performed the experiments including cell culture, cell biology experiments, molecular biology experiments, animal behavior experiments and morphology experiments. WFS assisted with the technical details and data analysis. CG and HLS assisted for data acquisition and data analysis. JHX and QQP prepared figures and wrote the manuscript. YG and CLZ revised and approved the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal use and studies were conducted in accordance with relevant ethical regulations and were reviewed and approved by the Nantong University Administration Committee of Experimental Animals (approval number S20210037).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Fig. S1. Gnao1 expression in nerve tissue (sciatic nerves and spinal cords) and myelination-related cells (neurons and SCs) by RT-qPCR (A) and WB analysis (B). Fig. S2 Validation of Gnao1-shRNAs interference efficiency. A RT-qPCR comparing the mRNA levels of Gnao1 in SCs treated with Gnao1-shRNAs or Scramble (NC-shRNA) for 48 h. T-test, n = 3, **, p < 0.01 and ***, p < 0.001 vs NC-shRNA. B WB comparing the protein levels of Gnao1 in SCs treated with Gnao1-shRNAs or Scramble for 48 h. T-test, n = 3, *, p < 0.05 and **, p < 0.01 vs NC-shRNA. Fig. S3 Knockdown or overexpression of Gnao1 level in Schwann cells of mouse sciatic nerve by injection of virus carried with Gnao1-shRNA or mouse Gnao1 coding sequence, respectively. A Schematic diagram illustrates the experimental process, that is, by respectively injecting the viruses carrying the Gnao1-shRNA or coding sequences into sciatic nerve, to generate mice with Gnao1 knockdown or overexpression in the sciatic nerve (referred to as Gnao1-NKD and Gnao1-NOE-mice). B and C WB comparing the protein levels of Gnao1 in Gnao1-NKD-mice (B), Gnao1-NOE-mice (C) together with their own controls, showing the lower Gnao1 expression in Gnao1-NKD-mice and higher Gnao1 expression in Gnao1-NOE-mice compared to controls, and IHC showing the Gnao1 knockdown or overexpression occurred mainly in the SCs of the sciatic nerves. Scale bar = 100 μm. T-test, n = 3, **, p < 0.01 and ****, p < 0.0001 vs controls. Fig. S4 Knockdown Gnao1 expression in neurons of mouse spinal cord by injection of virus carried with Gnao1-shRNA. A Schematic diagram illustrates the experimental process, that is, by intrathecally injecting the viruses carrying the Gnao1-shRNA into spinal cord, to generate mice with Gnao1 knockdown in the spinal cord (referred to as Gnao1-SKD-mice). B WB comparing the protein levels of Gnao1 in Gnao1-SKD-mice together with controls, showing the lower Gnao1 expression in Gnao1-SKD-mice compared to controls. T-test, n = 3, **, p < 0.01 vs controls. C IHC showing the Gnao1 knockdown occurred mainly in the spinal cord neurons. Scale bar = 500 μm. Zoomed in is the enlargement of the white box area, scale bar = 200 μm. Fig. S5. Analysis of RNA sequencing data of Gnao1-KD-SCs and control (NC) before and after differentiation. A Bar chart showing the number of differentially expressed genes (DEGs) obtained by pair-to-pair comparison of NC-SCs, Gnao1-KD-SCs, Diff-NC-SCs and Diff-Gnao1-KD-SCs (cutoff: fold change (FC) > 1.5 or < 0.67 plus p-value < 0.05). Bb1-Ee1 Heat map showing representative DEGs in 4 datasets (i.e., 1960 DEGs in Gnao1-KD-SCs versus NCs (b1), 3607 DEGs in Diff-NC-SCs versus NCs (c1), 4672 DEGs in Diff-Gnao1-KD-SCs versus Gnao1-KD-SCs (d1), and 161 DEGs in Diff-Gnao1-KD-SCs versus Diff-NC-SCs (e1)) by cluster heatmap analysis. Bb2-Ee2 Bubble map showing the top 20 functions of upregulated DEGs in 4 datasets (i.e., Gnao1-KD-SCs versus NCs (b2), Diff-NC-SCs versus NCs (c2), Diff-Gnao1-KD-SCs versus Gnao1-KD-SCs (d2), and Diff-Gnao1-KD-SCs versus Diff-NC-SCs (e2)) by KEGG enrichment analysis. Fig. S6. Analyze of myelination-related genes in RNA sequencing data with heat map cluster and qPCR. A Heat map showing representative myelination-related DEGs in 4 datasets (i.e., DEGs in Gnao1-KD-SCs versus NCs, Diff-NC-SCs versus NCs, Diff-Gnao1-KD-SCs versus Gnao1-KD-SCs, and Diff-Gnao1-KD-SCs versus Diff-NC-SCs) by cluster heatmap analysis. B Histogram showing the relative expression of (b1) myelin-forming proteins (MPZ, MBP, PMP22 and MAG), (b2) positive transcriptional regulators of myelination (EGR2, Pou3F1, Pou3F2 and Id2), (b3) the molecules that have been proven to affect myelination (Dok4, Vamp7, Erbb2, Erbb3, Cadm3 and Adcy2), and others (Gnao1 and Adcy2) in NC-SCs, Gnao1-KD-SCs, Diff-NC-SCs and Diff-Gnao1-KD-SCs. Pair t-test, n = 3, Ns, p > 0.05, no statistical difference. *, p < 0.05, **, p < 0.01, and ***, p < 0.001
Additional file 2
: Table S1. The analysis of microarray data (GSE163132).
Additional file 3
: Table S2. The analysis of RNA-seq data of Gnao1-KD-SCs and control (NC) before and after differentiation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Xu, J., Peng, Q., Cai, J. et al. The Schwann cell-specific G-protein Gαo (Gnao1) is a cell-intrinsic controller contributing to the regulation of myelination in peripheral nerve system. acta neuropathol commun 12, 24 (2024). https://doi.org/10.1186/s40478-024-01720-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-024-01720-3