Abstract
Alzheimer’s disease (AD) poses an ever-increasing public health concern as the population ages, affecting more than 6 million Americans. AD patients present with mood and sleep changes in the prodromal stages that may be partly driven by loss of monoaminergic neurons in the brainstem, but a causal relationship has not been firmly established. This is due in part to a dearth of animal models that recapitulate early AD neuropathology and symptoms. The goal of the present study was to evaluate depressive and anxiety-like behaviors in a mouse model of AD that overexpresses human wild-type tau (htau) prior to the onset of cognitive impairments and assess these behavior changes in relationship to tau pathology, neuroinflammation, and monoaminergic dysregulation in the dorsal raphe nucleus (DRN) and locus coeruleus (LC). We observed depressive-like behaviors at 4 months in both sexes and hyperlocomotion in male htau mice. Deficits in social interaction persisted at 6 months and were accompanied by an increase in anxiety-like behavior in males. The behavioral changes at 4 months coincided with a lower density of serotonergic (5-HT) neurons, downregulation of 5-HT markers, reduced excitability of 5-HT neurons, and hyperphosphorylated tau in the DRN. Inflammatory markers were also upregulated in the DRN along with protein kinases and transglutaminase 2, which may promote tau phosphorylation and aggregation. Loss of 5-HT innervation to the entorhinal cortex and dentate gyrus of the hippocampus was also observed and may have contributed to depressive-like behaviors. There was also reduced expression of noradrenergic markers in the LC along with elevated phospho-tau expression, but this did not translate to a functional change in neuronal excitability. In total, these results suggest that tau pathology in brainstem monoaminergic nuclei and the resulting loss of serotonergic and/or noradrenergic drive may underpin depressive- and anxiety-like behaviors in the early stages of AD.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a devastating age-related neurodegenerative disease that afflicts a large proportion of individuals aged 65 and older[1]. Recent evidence suggests that neurofibrillary tangles (NFT) develop in brainstem nuclei including the dorsal raphe nucleus (DRN) and locus coeruleus (LC) before the hippocampus and cortex, which may lead to loss of monoaminergic neurons and neuropsychiatric symptoms (NPS) in the prodromal stages of AD [2,3,4,5,6,7,8,9,10,11,12,13]. The DRN contains a large population of serotonin (5-HT) neurons that project to the forebrain and regulate mood, sleep, and reward-seeking behaviors, all of which are perturbed in AD [14,15,16,17,18,19]. The LC is also associated with depressive and anxiety-like behaviors in both humans and rodent models [20, 21], with studies suggesting that even a minimal loss of noradrenergic (NA) neurons can lead to depressive behavior [22].
Perturbations in brainstem monoaminergic nuclei may drive prodromal neuropsychiatric symptoms in AD, but direct evidence linking brainstem neuropathology and monoaminergic depletion to specific behavioral changes in early AD is currently lacking. AD is usually diagnosed at later stages when significant neurodegeneration has already taken place, so there is a critical need to develop and characterize model systems that recapitulate the early stages of AD to help identify new biomarkers and therapeutic targets. The htau mouse model is a genetic cross between a mouse microtubule-associated protein tau (MAPT) tau knockout line [23] and the 8c line [24] that contains a wild-type human MAPT transgene under the tau promoter that results in expression of all six isoforms of human tau. These mice develop tau pathology in a more naturalistic fashion such that hippocampal dysfunction and memory deficits occur relatively late in life around 12 months of age [25]. These mice are cognitively intact at 4 months of age, but there is evidence of hyperphosphorylated tau in the DRN [26] which may be associated with altered serotonergic function and behavioral dysregulation reminiscent of prodromal AD. The goal of the present study was to develop a behavioral and neurochemical profile of htau mice at 4–6 months of age and determine whether they might be a useful model of prodromal AD. We first assessed htau mice for depressive and anxiety-like behaviors using a battery of tests including the elevated plus maze (EPM) and elevated zero maze (EZM), open field, social interaction test, sucrose preference test, and forced swim test. Monoaminergic neurons and glia in the DRN and LC were examined by immunohistochemistry, and ex vivo electrophysiology was used to assess functional changes in 5-HT and NA neurons. Expression of genes involved in monoamine biosynthesis and signaling, neuroinflammation, and proteostasis were also examined in brainstem tissues. Finally, serotonergic inputs to the entorhinal cortex (EC) and hippocampus may also be impacted by brainstem tau pathology and have been implicated in affective and cognitive changes in AD [27, 28]. We examined 5-HT immunoreactivity in the EC and serotonin transporter (SERT) immunoreactivity in the hippocampus, as well as mRNA expression of 5-HT receptors, tau-related genes, and inflammatory markers in these regions. Overall, our results suggest that tau accumulation in the brainstem coincides with monoaminergic dysfunction and NPS in the early stages of AD. Additionally, htau mice may be a useful model for testing therapeutic interventions aimed at ameliorating tau pathology in the brainstem and arresting neurodegeneration in early AD.
Materials and methods
Animals
Male and female C57BL/6J mice (Jackson Labs #000664) and htau +/- mice (Jackson Labs #005491) containing a transgene that encodes the human MAPT gene were used in this experiment. MAPT -/- (global tau knockout; Mapt < tm1(EGFP)Klt) mice were used as negative controls in RT-PCR and Western blot validation of tau splice isoforms. Mice were housed in a temperature-and humidity-controlled, AALAC-approved vivarium at the University of Iowa with ad libitum access to food and water.
Behavior
Anxiety- and depressive-like behaviors in C57BL/6J and htau +/- mice were evaluated at 4 and 6 months of age. The order in which behavioral tests were performed is depicted in Fig. 1A. All behavior tests were performed during the light phase of the light/dark cycle (9 am–3 pm) and were recorded with an overhead or side-view camera integrated with Media Recorder or Ethovision video tracking software (Noldus Information Tech, Inc.). Videos were scored by an experimenter blinded to animal genotypes. Unless otherwise noted, all behavior tests were scored using Ethovision.
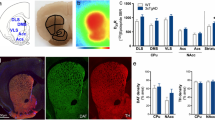
Htau mice exhibit depressive-like behaviors at 4 and 6 months of age. A Experimental timeline of behavioral studies in htau and C57BL/6J mice. Behavior of 4-month-old htau and C57BL/6J mice in the B, C EPM, D, E open field, F social interaction test, G sucrose preference test and H, I forced swim test. Behavior of 6-month-old htau and C57BL/6J mice in the J, K EZM, L, M open field, N social interaction test, O sucrose preference test, P forced swim test and Q Barnes maze. These results indicate depressive-like behaviors in htau mice relative to C57BL/6J mice. *p < 0.05, **p < 0.01, ****p < 0.0001
Elevated plus maze
At 4 months of age, animals were tested in the EPM to evaluate anxiety-like behaviors [29]. Briefly, the maze was 60 cm above the floor and consisted of two open arms, two closed arms (5 × 35 cm), and a neutral starting zone (5 × 5 cm). Overhead LEDs were used to maintain lux in the open arms at 20 lux and < 5 lux in the closed arms. The closed arms had tall dark walls that allowed animals to hide. At the beginning of the test, animals were placed in the neutral zone and allowed to freely explore the maze for 5 min. Distance traveled, and time spent in the open arms vs closed arms were calculated using Ethovision XT14.
Elevated zero maze
At 6 months of age, animals were tested in the EZM to evaluate anxiety-like behaviors in a novel arena [30,31,32]. Briefly, the circular maze (outer diameter: 52 cm) was elevated 60 cm above the ground and consisted of two alternating open and closed corridors (width: 5 cm). Overhead LEDs maintained the lux in the open corridors at 20 lux and < 5 lux in the closed corridors. At the beginning of the test, animals were placed in the open corridor, facing the closed corridor, and allowed to freely explore the arena for 5 min. Behavior was recorded by an overhead camera. Distance traveled, and time spent in the open area vs closed corridors were calculated using Ethovision XT14. The open area preference and probability of entering the open areas were calculated to determine the anxiety-like behavior.
Open field test
Mice were placed in the corner of a 50 × 50 × 25 cm opaque plexiglass arena (20 lux) and allowed to freely explore the arena for 30 min. The open field test was performed at 4 and 6 months of age to evaluate locomotor and exploratory behavior. The total distance traveled (cm), time spent in the center of the arena, and time spent in the corners of the arena were measured through Ethovision XT14. The center of the open field was defined as the central 15% of the arena.
Social interaction test
The social interaction test was performed in 4- and 6-month-old mice, and was performed as previously described [33, 34]. Briefly, the animal was placed in the central chamber of a transparent 3-chamber arena (20 lux) and allowed to explore the environment for 10 min. Following this, a novel C57BL/6J mouse (stranger mouse) of the same sex and approximate age as the experimental mouse was placed in one of the side chambers under a metal cage. An empty metal cage was also placed in the alternate side chamber. The experimental mouse was allowed to explore the environment and interact with the stranger mouse for 10 min. The location of the stranger mouse was alternated between the right and left chamber to control for any side preferences. The total time spent interacting with the stranger mouse and the empty cage was scored.
Sucrose preference test
The sucrose preference test was performed over four days and used to evaluate the degree of anhedonia of htau mice at 4 and 6 months of age [35, 36]. Animals were transferred to PhenoTyper observation cages (Noldus) that were fitted with two sipper bottles and Lickometers (to measure the number of licks the animal makes to a bottle). Animals had ad libitum access to tap water or a 5% sucrose solution (ThermoFisher) for 1 h. The placement of sucrose and water bottles was alternated each day to control for any side preference. The number of approaches to the water bottle and the sucrose bottle was measured through Ethovision XT14.
Barnes maze
Spatial learning and cognitive deficits were evaluated in 6-month-old C57BL/6 J and htau mice as described previously [32, 37, 38]. The Barnes maze was a 5-day protocol [39], with environment habituation on day 1, training sessions on days 2 and 3, rest on day 4, and a probe trial on day 5. The maze was a gray circular arena (diameter: 91 cm), consisting of 20 equally divided holes, and was elevated 93 cm above the ground. The room was well lit and visual cues were present on the walls.
On Day 1, animals were guided to the predetermined ‘goal box’, which had been fitted with an escape chamber. In contrast, the remaining 19 (non-target holes) were not fitted with any chambers, and animals could see the ground below.
Over days 2 and 3, animals underwent a total of five training trials. A buzzer sound (~ 100 dB) was played while animals explored the environment. Once the animal found the goal box and entered the escape chamber, the buzzer was turned off and animals were allowed to rest for 1-min before being returned to a holding cage. If an animal did not find the goal box/escape chamber within a 2-min trial, they were guided to the escape chamber as they had been on Day 1. Inter-trial interval was 30 min. Animals had 3 training trials on day 2, and 2 trials on day 3.
On the probe day, the escape chamber was removed from the goal box. Animals were placed on the maze platform, and the buzzer sound was presented. Animals were allowed to explore the environment for 2 min. Behavior was recorded by an overhead camera.
The number of visits to the goal box & non-target holes was measured along with the latency to approach the goal box and time spent in the target quadrant.
Forced swim test
Depressive-like behavior was evaluated at 4 and 6 months in the forced swim test. Mice were gently placed in a tall cylinder filled with 24–25 °C tap water (32 cm height × 20 cm diameter, water height: 25 cm) for 6 min. After the test, animals were placed in a clean cage under a heating lamp for 5 min to warm them and allow them to dry off. Animal behavior was recorded from a side-view camera and analyzed with Ethovision XT14. The 6-min videos were divided into two bouts: a pretest (first 1–2 min) and a test (last 3–6 min) phase. The latency to the first immobile bout, frequency of immobile bouts, and duration of each immobile bout was evaluated.
Immunofluorescence
A cohort of C57BL/6 J and htau +/- mice were deeply anesthetized with tribromoethanol and transcardially perfused with PBS followed by 4% paraformaldehyde (PFA) to collect brains for immunofluorescence experiments. Brains were cryosectioned at 25 µm using a Leica cryostat (CM3050S, Leica, Germany). Slices were stored at 4 °C in a cryoprotectant solution. The immunofluorescence was performed as described previously [40, 41]. Briefly, for each region of interest (ROI), 3–4 slices were used across the rostral-caudal axis. Slices were washed in PBS and incubated in 0.5% Triton X-100/PBS for 30 min, blocked in 10% normal donkey serum in 0.1% Triton X-100/PBS, and then incubated with the respective primary and secondary antibodies (Table 1). For AT8 IF experiments, mouse-on-mouse blocking reagent (3% final volume; Vector Laboratories) was added to the blocking solution to reduce non-specific binding to endogenous mouse IgG. Slices were subsequently washed in PBS, mounted on glass slides, and coverslipped with Vectashield mounting media (Vector Laboratories, Inc.).
Confocal z-stacks (1 µm) were captured on an Olympus FV3000 laser scanning confocal microscope (20 sections/z-stack) and converted to maximum projection images using Image J software. Images were analyzed by trained researchers blind to experimental conditions to obtain cell counts per unit area, % immunoreactive area, and optical density using ImageJ. The optical density was estimated by first converting images to an 8-bit grayscale image and performing a background correction. Optical density calibration was performed with a 21-step tablet (available from ImageJ) using the Rodbard function. Following optical density calibration, mean grayscale values were recorded from the ROIs with an effort to avoid artifacts. Percent (%) immunoreactive area was performed on the ROIs of thresholded images. For each image, the ROI was drawn according to the shape of the region based on the mouse brain reference atlas of Paxinos & Franklin [42] and the monoaminergic signal. ROIs of the nuclei were drawn based on the reference atlas and IF immunoreactivity for each image, and then applied to all channels (AT8, Iba-1, or GFAP).
Ex vivo electrophysiology
Brain slice preparation
Deeply anesthetized mice were transcardially perfused with ice-cold, oxygenated modified artificial cerebrospinal fluid (aCSF) containing the following (in mm): 110 choline-Cl, 2.5 KCl, 7 MgSO4, 0.5 CaCl2, 1.25 NaH2PO4, 26.2 NaHCO3, 25 glucose, 11.6 Na-ascorbate, 2 thiourea, and 3.1 Na-pyruvate (pH: 7.3–7.4; osmolality: 300–310 mOsmol/kg). Then their brains were quickly dissected and coronal slices containing the DRN or LC (300 μm) were obtained using a vibratome (VT1200S; Leica Biosystems, Wetzlar, Germany). The brain slices recovered at 34 °C for 30 min in a chamber containing the choline-Cl-based aCSF described above and continuously bubbled with 95% O2/5% CO2. After the initial recovery, brain slices were transferred to and held in a different modified aCSF at room temperature, saturated with 95% O2/5% CO2, for at least 1 h before recordings started. The holding aCSF contained the following (in mm): 92 NaCl, 2.5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 Na-ascorbate, 2 thiourea, and 3 Na-pyruvate (pH: 7.3–7.4; 300–310 mOsmol/kg).
Ex vivo electrophysiological recordings
During recordings, slices were continuously perfused (2 ml/min) with standard aCSF containing (in mm): 124 NaCl, 4 KCl, 1.2 MgSO4, 2 CaCl2, 1 NaH2PO4, 26 NaHCO3, and 11 glucose (300–310 mOsmol/kg), saturated with 95% O2/5% CO2 and maintained at 30 ± 1 °C. Patch electrodes (3–5 MΩ) were filled with a solution containing (in mm): 135 K-gluconate, 5 NaCl, 2 MgCl2, 10 HEPES, 0.6 EGTA, 4 Na2-ATP, and 0.4 Na2-GTP (pH: 7.3; 288–292 mOsmol/kg). In order to identify 5-HT or NA neurons post hoc by immunofluorescence, biocytin (2 mg/ml; Tocris Bioscience, Bristol, UK) was added into the internal solution. Only tryptophan hydroxylase 2 (TPH2)-positive neurons from the DRN and tyrosine hydroxylase (TH)-positive neurons from the LC were included in data analysis. Neurons were visualized via an upright microscope (BX51W1; Olympus, Tokyo, Japan) accompanied by a differential interference contrast imaging system. Membrane currents were amplified with a Multiclamp 700 B amplifier (Molecular Devices, San Jose, CA, USA), filtered at 3 kHz, and sampled at 20 kHz with a Digidata 1550B digitizer (Molecular Devices). Data were acquired via the pClamp 11 software (Molecular Devices). Access resistance was monitored online and changes greater than 20% would lead to discontinuation of the recordings.
To examine neuronal intrinsic excitability, recordings were conducted in current clamp mode. The DRN 5-HT neurons we patched were not spontaneously firing, and we performed recordings at resting membrane potential (RMP) and at − 70 mV holding potential to offset the variation of RMP. Input resistance was assessed by the change in membrane potential upon − 100 pA hyperpolarizing current injection. Rheobase reflected the minimal current needed to evoke action potentials (AP). Numbers of evoked APs were recorded after injecting depolarizing currents for 250 ms at 10 pA incremental steps (0–200 pA).
All the LC NA neurons were spontaneously firing, which we continuously recorded for 10 min and analyzed their firing profiles based on the last 5 min: firing frequency, mean amplitude of action potentials, variation of firing timing (assessed by coefficient of variation: CV = mean/standard deviation of inter-firing intervals). Because the NA neurons were spontaneously firing, it was impossible to assess their intrinsic excitability at RMP. Therefore, we slightly hyperpolarized the neurons by holding them at − 55 mV and performed the same recordings as in DRN 5-HT neurons.
AP characteristics were measured using pClamp, version 10 (Clampfit 10.7; Molecular Devices; RRID: SCR_011323). Many AP parameters require a clearly defined baseline in order to be assessed accurately, and the parameters are dependent on current injection magnitude. Due to differences in excitability between neurons, the current injection steps of 120 pA (held at − 70 mV) for the DR and 140 pA (held at − 55 mV) for the LC had the largest sample size for the comparison of AP characteristics that are dependent upon verifiable baseline. The metrics of ‘maximum decay slope’ and ‘time to achieve maximum decay slope’ are not dependent upon the correct identification of the recording baseline, so we examined these metrics simultaneously across all current injection steps.
Reverse transcriptase-quantitative PCR
DRN, LC, EC, dorsal hippocampus (DHP) and ventral hippocampus (VHP) tissues were micro-punched from C57BL/6J and htau +/- brains, and total RNA was isolated as described previously [41]. Briefly, the RNA was extracted from the tissue using the TRIzol reagent method. The DNA contaminants from the extracted RNA were eliminated using a DNA-free™ DNA Removal Kit (Life Technologies, USA). The concentration and purity of RNA were checked using a NanoDrop 1000 spectrophotometer and the RNA was reverse transcribed to cDNA. RT-qPCR for the target genes was performed using SYBR green qPCR master mix (Bio-Rad Laboratories, USA) and specified primers (Table 3) on a CFX96™ Real-time-PCR System (Bio-Rad Laboratories, USA). The thermal profile used for RT-qPCR was 95 °C for 10 min, 40 cycles of 95 °C for 30 s, 60 °C for 30 s, followed by a melt curve analysis profile (60 °C to 95 °C in 0.5 °C increments at a rate of 5 s/step). Fold changes in the mRNA levels were determined for each gene after normalizing with β-actin Ct values using the fold change 2−ΔΔCT method [43]. Results are represented as fold changes in the mRNA levels (± SEM).
Western blot
A separate group of C57BL/6J and htau +/- mice were decapitated under isoflurane anesthesia to collect brains for Western blot experiments as reported [41]. Briefly, DRN and LC brain regions were dissected for Western blots of ptau (AH36; pSer202/pThr205), total tau (HT7), TPH2, TH, indoleamine 2,3 dioxygenase 1 (IDO1), transglutaminase 2 (TGM2), β-actin, and GAPDH. Total proteins were isolated using RIPA buffer supplemented with protease and phosphatase inhibitors. Proteins were quantified using the BCA method and an equal amount of protein was resolved in 10% SDS-PAGE gel and transferred to a PVDF membrane (0.2 µm; Millipore) for immunoblotting. The blots were blocked with Starting Block T20 (TBS) Blocking Buffer (ThermoFisher Scientific) for 20 min at 37 °C and incubated overnight with primary antibodies specific to AH36, HT7, TPH2, TH, IDO1, TGM2, β-actin, and GAPDH at 4 °C (Table 2). Further, the blots were incubated with fluorescent or HRP-conjugated secondary antibodies (Table 2) as per the manufacturer's instructions. The blots were imaged and acquired using the LI-COR Odyssey Imager (LI-COR Inc.) at 700 and 800 nm. Protein bands were quantified using ImageJ software (National Institutes of Health) and the average relative density of the TPH2, TH, IDO1, and TGM2 was determined after normalization to β-actin or GAPDH. Results are represented as a mean relative density of the protein levels (± SEM).
Statistical analysis
All data were analyzed with GraphPad Prism version 9 (GraphPad Software Inc, La Jolla, CA). Outliers were identified and removed using a ROUT test (Q = 1%). Experiments comparing two groups were analyzed with a Student’s t-test, with p < 0.05. Where variance between groups was unequal, a Welch’s t-test was performed (p < 0.05). Behavior experiments were analyzed using a two-way ANOVA and Bonferroni corrections for post hoc analyses. Data are reported and graphically represented as means ± standard error of the mean (SEM).
Results
Htau mice exhibit depressive-like behaviors by 4 months and anxiety-like behaviors by 6 months of age
In this study, we used htau +/- mice (Jackson Labs strain # 005491) that had been backcrossed to a mouse tau knockout line (Mapt < tm1(EGFP)Klt). These mice are on a C57BL/6J background, so C57BL/6J mice were used as controls throughout this study as in previous reports [25, 26, 44]. Htau mice express all six isoforms of human tau including the 3R and 4R isoforms and are thought to represent a more naturalistic model of AD with late-onset cognitive impairment [25] (Additional file 1: Fig. S1). We asked whether htau mice exhibit behavioral phenotypes reminiscent of prodromal AD by 4 months of age, which coincides with the appearance of hyperphosphorylated tau in the DRN but prior to the onset of cognitive impairments [25, 26]. Male and female htau and C57BL/6J mice were tested in the EPM, open field, social interaction test, sucrose preference test, and forced swim test at this time point (Fig. 1A). We found a significant interaction between sex and genotype on time spent in the open arms of the EPM at 4 months (F1,53 = 4.92, p < 0.05), but post-hoc comparisons between htau and C57BL/6J mice were non-significant in both males and females. No group differences in locomotor activity were observed in this assay (Fig. 1B, C). There was a main effect of sex in time spent in the center of the open field (F1,55 = 12.25, p < 0.001) but no sex x genotype interaction, suggesting that female mice generally exhibited more anxiety-like behavior in this test. Htau mice also exhibited hyperlocomotion in the open field (Main effect genotype: F1,55 = 8.32, p < 0.01) although Bonferroni post-tests were only significant in males (t55 = 2.77, p < 0.05) (Fig. 1D, E). Interestingly, both sexes exhibited significant depressive-like behaviors in the social interaction test (Main effect of genotype: F1,57 = 74.37, p < 0.0001; Bonferroni post-tests t57 = 7.32, p < 0.01 for males and t57 = 5.06, p < 0.0001 for females) (Fig. 1F), whereas sucrose preference was only reduced in male mice (Main effect genotype: F1,56 = 5.89, p < 0.05; Bonferroni post-test: t56 = 2.65, p < 0.05) (Fig. 1G). Overall, there was no effect of genotype on immobility time or latency to immobility in the forced swim test, but female mice spent more time immobile than males (Main effect of sex: F1,57 = 4.22, p < 0.05) (Fig. 1H, I).
We then examined anxiety and depressive-like behaviors in these mice at 6 months of age. Here we found a significant effect of genotype in time spent in the open area of the EZM (F1,56 = 12.21, p < 0.001), with male htau mice exhibiting a significant reduction in open area time (Bonferroni post-tests: t56 = 3.11, p < 0.01) (Fig. 1J). Locomotor activity did not significantly differ between groups in this test (Fig. 1K). There was also a significant effect of genotype in time spent in the center of the open field (F1,55 = 5.15, p < 0.05) that is suggestive of enhanced anxiety-like behavior, although post-hoc comparisons were non-significant in both sexes (Fig. 1L). Locomotor activity in the open field did not differ significantly between groups, which contrasted with the hyperlocomotive phenotype observed at 4 months (Fig. 1M). Depressive-like behaviors in the social interaction test persisted at 6 months in both sexes (Main effect of genotype: F1,50 = 94.73, p < 0.0001; Bonferroni post-tests: t50 = 8.23 for males and t50 = 5.70 for females) (Fig. 1N), although the decrease in the sucrose preference noted at 4 months had resolved by this time point (Fig. 1O). Similar to what we observed at 4 months, there was no effect of genotype in the forced swim test at 6 months, although female mice continued to spend more time inactive than males (Main effect of sex: F1,57 = 5.76, p < 0.05) (Fig. 1P). There was also no effect of sex or genotype on spatial memory in the Barnes Maze at 6 months (Fig. 1Q), which is consistent with previous reports that memory impairments in htau mice only become apparent at 12 months of age [25].
Monoaminergic depletion and hyperphosphorylated tau in the brainstem at 4 months
Next, we investigated whether loss of monoaminergic neurons in the DRN or LC at the 4-month mark might account for these behavioral phenotypes. We focused on males in these experiments as they had the most robust behavioral phenotypes at 4 months of age. All histological experiments were performed in separate cohorts of mice to avoid any confounds of behavioral testing on monoamine levels. The DRN was subdivided into rostral, mid and caudal subregions, which were previously found to have distinct forebrain projections and behavioral outputs [19, 45,46, Tau accumulation in the DRN and subsequent loss of monoaminergic drive may promote depressive-like behaviors in the prodromal phase of AD prior to the onset of cognitive decline. Further studies in AD mouse models are needed to define a causal relationship between DRN or LC neuropathology and specific AD symptoms.Conclusion
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional file 1.
References
Alzheimer’s Association (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12:459–509
Grinberg LT, Rüb U, Ferretti REL, Nitrini R, Farfel JM, Polichiso L et al (2009) The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol 35:406–416
Theofilas P, Ehrenberg AJ, Nguy A, Thackrey JM, Dunlop S, Mejia MB et al (2018) Probing the correlation of neuronal loss, neurofibrillary tangles, and cell death markers across the Alzheimer’s disease Braak stages: a quantitative study in humans. Neurobiol Aging 61:1–12
Michelsen KA, Prickaerts J, Steinbusch HWM (2008) The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog Brain Res 172:233–264
Modrego PJ (2010) Depression in Alzheimer’s disease. Pathophysiology, diagnosis, and treatment. J Alzheimer’s Dis 21:1077–1087
Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J et al (2011) Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer’s & Dement 7:532–539
Steffens DC, McQuoid DR, Potter GG (2014) Amnestic mild cognitive impairment and incident dementia and Alzheimer’s disease in geriatric depression. Int Psychogeriatr 26:2029–2036
Tao P, Yang S-N, Tung Y-C, Yang M-C (2019) Development of Alzheimer disease in old major depressive patients based upon their health status: a retrospective study in Taiwan. Medicine 98:e15527
Oh J, Eser RA, Ehrenberg AJ, Morales D, Petersen C, Kudlacek J et al (2019) Profound degeneration of wake-promoting neurons in Alzheimer’s disease. Alzheimers Dement. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31416793
Hendricksen M, Thomas AJ, Ferrier IN, Ince P, O’Brien JT (2004) Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am J Psychiatry 161:1096–1102
Bondareff W, Mountjoy CQ, Roth M (1982) Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 32:164–168
Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ et al (1988) The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol 24:233–242
Chen CP, Eastwood SL, Hope T, McDonald B, Francis PT, Esiri MM (2000) Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioural changes. Neuropathol Appl Neurobiol 26:347–355
Cohen JY, Amoroso MW, Uchida N (2015) Serotonergic neurons signal reward and punishment on multiple timescales. Elife 4:e06346
Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D et al (2015) Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41:1404–1415
Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF et al (2016) Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537:97–101
Oikonomou G, Altermatt M, Zhang R-W, Coughlin GM, Montz C, Gradinaru V et al (2019) The serotonergic raphe promote sleep in zebrafish and mice. Neuron 103:686-701.e8
Challis C, Beck SG, Berton O (2014) Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front Behav Neurosci 8:43
Paul ED, Lowry CA (2013) Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. J Psychopharmacol 27:1090–1106
Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD et al (1994) Depression and anxiety: role of the locus coeruleus and corticotropin-releasing factor. Brain Res Bull 35:561–572
Du X, Yin M, Yuan L, Zhang G, Fan Y, Li Z et al (2020) Reduction of depression-like behavior in rat model induced by ShRNA targeting norepinephrine transporter in locus coeruleus. Transl Psychiatry 10:130
Szot P, Franklin A, Miguelez C, Wang Y, Vidaurrazaga I, Ugedo L et al (2016) Depressive-like behavior observed with a minimal loss of locus coeruleus (LC) neurons following administration of 6-hydroxydopamine is associated with electrophysiological changes and reversed with precursors of norepinephrine. Neuropharmacology 101:76–86
Tucker KL, Meyer M, Barde YA (2001) Neurotrophins are required for nerve growth during development. Nat Neurosci 4:29–37
Duff K, Knight H, Refolo LM, Sanders S, Yu X, Picciano M et al (2000) Characterization of pathology in transgenic mice over-expressing human genomic and cDNA tau transgenes. Neurobiol Dis 7:87–98
Polydoro M, Acker CM, Duff K, Castillo PE, Davies P (2009) Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci 29:10741–10749
Dengler-Crish CM, Smith MA, Wilson GN (2017) Early evidence of low bone density and decreased serotonergic synthesis in the dorsal raphe of a tauopathy model of Alzheimer’s disease. J Alzheimers Dis 55:1605–1619
Yun H-M, Park K-R, Kim E-C, Kim S, Hong JT (2015) Serotonin 6 receptor controls Alzheimer’s disease and depression. Oncotarget 6:26716–26728
Garcia-Alloza M, Hirst WD, Chen CPL-H, Lasheras B, Francis PT, Ramírez MJ (2004) Differential involvement of 5-HT(1B/1D) and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology 29:410–416
Shoji H, Miyakawa T (2021) Effects of test experience, closed-arm wall color, and illumination level on behavior and plasma corticosterone response in an elevated plus maze in male C57BL/6J mice: a challenge against conventional interpretation of the test. Mol Brain 14:34
Coutellier L, Beraki S, Ardestani PM, Saw NL, Shamloo M (2012) Npas4: a neuronal transcription factor with a key role in social and cognitive functions relevant to developmental disorders. PLoS ONE 7:e46604
Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT (1994) Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology 116:56–64
Gonçalves RA, Wijesekara N, Fraser PE, De Felice FG (2020) Behavioral abnormalities in knockout and humanized tau mice. Front Endocrinol (Lausanne) 11:1–13
Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR (2010) Assessment of social interaction behaviors. J Vis Exp 5:1–6
Yang M, Silverman JL, Crawley JN (2011) Automated three-chambered social approach task for mice. Curr Protoc Neurosci Chapter 8:Unit 8.26
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX et al (2018) Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc 13:1686–1698
Romano A, Pace L, Tempesta B, Lavecchia AM, Macheda T, Bedse G et al (2014) Depressive-like behavior is paired to monoaminergic alteration in a murine model of Alzheimer’s disease. Int J Neuropsychopharmacol 18:1–12
Flinn JM, Lorenzo Bozzelli P, Adlard PA, Railey AM (2014) Spatial memory deficits in a mouse model of late-onset Alzheimer’s disease are caused by zinc supplementation and correlate with amyloid-beta levels. Front Aging Neurosci 6:1–10
Lippi SLP, Smith ML, Flinn JM (2018) A novel hAPP/htau mouse model of Alzheimer’s disease: inclusion of APP with tau exacerbates behavioral deficits and zinc administration heightens tangle pathology. Front Aging Neurosci 10:382
Attar A, Liu T, Chan W-TC, Hayes J, Nejad M, Lei K, et al. A shortened barnes maze protocol reveals memory deficits at 4-months of age in the triple-transgenic mouse model of Alzheimer’s disease. Coulson EJ, editor. PLoS One 2013;8:e80355.
Khan KM, Bierlein-De La Rosa G, Biggerstaff N, Pushpavathi Selvakumar G, Wang R, Mason S et al (2022) Adolescent ethanol drinking promotes hyperalgesia, neuroinflammation and serotonergic deficits in mice that persist into adulthood. Brain Behav Immun 107:419–431
Balasubramanian N, James TD, Selvakumar GP, Reinhardt J, Marcinkiewcz CA (2022) Repeated ethanol exposure and withdrawal alters angiotensin-converting enzyme 2 expression in discrete brain regions: implications for SARS-CoV-2 neuroinvasion. Alcohol Clin Exp Res 47:219–239
Paxinos G, Franklin KB (2001) The mouse brain in stereotaxic coordinates. Elsevier Academic Press, San Diego
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108
Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde Y-A et al (2003) Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 86:582–590
Graeff FG, Guimarães FS, de Andrade TG, Deakin JF (1996) Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 54:129–141
Ren J, Isakova A, Friedmann D, Zeng J, Grutzner SM, Pun A et al (2019) Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. Elife 8. Available from: https://elifesciences.org/articles/49424
Ren J, Friedmann D, **ong J, Liu CD, Ferguson BR, Weerakkody T et al (2018) anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30146164
Int’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T et al (2001) Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med 345:1515–1521
Heppner FL, Ransohoff RM, Becher B (2015) Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci 16:358–372
Condello C, Yuan P, Schain A, Grutzendler J (2015) Microglia constitute a barrier that prevents neurotoxic protofibrillar Aβ42 hotspots around plaques. Nat Commun 6:6176
Hansen DV, Hanson JE, Sheng M (2018) Microglia in Alzheimer’s disease. J Cell Biol 217:459–472
Carrero I, Gonzalo MR, Martin B, Sanz-Anquela JM, Arévalo-Serrano J, Gonzalo-Ruiz A (2012) Oligomers of β-amyloid protein (Aβ1-42) induce the activation of cyclooxygenase-2 in astrocytes via an interaction with interleukin-1β, tumour necrosis factor-α, and a nuclear factor κ-B mechanism in the rat brain. Exp Neurol 236:215–227
Navarro V, Sanchez-Mejias E, Jimenez S, Muñoz-Castro C, Sanchez-Varo R, Davila JC et al (2018) Microglia in Alzheimer’s disease: activated, dysfunctional or degenerative. Front Aging Neurosci 10:140
Heneka MT, Nadrigny F, Regen T, Martinez-Hernandez A, Dumitrescu-Ozimek L, Terwel D et al (2010) Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc Natl Acad Sci U S A 107:6058–6063
Matthews GA, Nieh EH, vander Weele CM, Halbert SA, Pradhan RV, Yosafat AS et al (2016) Dorsal raphe dopamine neurons represent the experience of social isolation. Cell 164:617–631
Spoida K, Masseck OA, Deneris ES, Herlitze S (2014) Gq/5-HT2c receptor signals activate a local GABAergic inhibitory feedback circuit to modulate serotonergic firing and anxiety in mice. Proc Natl Acad Sci U S A 111:6479–6484
Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C (2011) Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience 184:128–138
Lee G, Thangavel R, Sharma VM, Litersky JM, Bhaskar K, Fang SM et al (2004) Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J Neurosci 24:2304–2312
Liu G, Fiock KL, Levites Y, Golde TE, Hefti MM, Lee G (2020) Fyn depletion ameliorates tauP301L-induced neuropathology. Acta Neuropathol Commun 8:108
Norlund MA, Lee JM, Zainelli GM, Muma NA (1999) Elevated transglutaminase-induced bonds in PHF tau in Alzheimer’s disease. Brain Res 851:154–163
Zemaitaitis MO, Lee JM, Troncoso JC, Muma NA (2000) Transglutaminase-induced cross-linking of tau proteins in progressive supranuclear palsy. J Neuropathol Exp Neurol 59:983–989
Halverson RA, Lewis J, Frausto S, Hutton M, Muma NA (2005) Tau protein is cross-linked by transglutaminase in P301L tau transgenic mice. J Neurosci 25:1226–1233
Grierson AJ, Johnson GV, Miller CC (2001) Three different human tau isoforms and rat neurofilament light, middle and heavy chain proteins are cellular substrates for transglutaminase. Neurosci Lett 298:9–12
Tucholski J, Kuret J, Johnson GV (1999) Tau is modified by tissue transglutaminase in situ: possible functional and metabolic effects of polyamination. J Neurochem 73:1871–1880
Das S, Ooi FK, Cruz Corchado J, Fuller LC, Weiner JA, Prahlad V (2020) Serotonin signaling by maternal neurons upon stress ensures progeny survival. Elife 9:e55246
Cruz-Corchado J, Ooi FK, Das S, Prahlad V (2020) Global transcriptome changes that accompany alterations in serotonin levels in Caenorhabditis elegans. G3 (Bethesda) 10:1225–1246
Ortega JE, Mendiguren A, Pineda J, Meana JJ (2012) Regulation of central noradrenergic activity by 5-HT(3) receptors located in the locus coeruleus of the rat. Neuropharmacology 62:2472–2479
Deng P-Y, Lei S (2008) Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci 39:273–284
Glikmann-Johnston Y, Saling MM, Reutens DC, Stout JC (2015) Hippocampal 5-HT1A receptor and spatial learning and memory. Front Pharmacol 6:289
Sant’Ana AB, Vilela-Costa HH, Vicente MA, Hernandes PM, de Andrade TGCS, Zangrossi H (2019) Role of 5-HT2C receptors of the dorsal hippocampus in the modulation of anxiety- and panic-related defensive responses in rats. Neuropharmacology 148:311–319
Luo J, Feng Q, Wei L, Luo M (2017) Optogenetic activation of dorsal raphe neurons rescues the autistic-like social deficits in Shank3 knockout mice. Cell Res 27:950–953
Luo M, Zhou J, Liu Z (2015) Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learn Mem 22:452–460
Dölen G, Darvishzadeh A, Huang KW, Malenka RC (2013) Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501:179–184
Baumann B, Bielau H, Krell D, Agelink MW, Diekmann S, Wurthmann C et al (2002) Circumscribed numerical deficit of dorsal raphe neurons in mood disorders. Psychol Med 32:93–103
O’Shea DM, Dotson VM, Woods AJ, Porges EC, Williamson JB, O’Shea A et al (2018) Depressive symptom dimensions and their association with hippocampal and entorhinal cortex volumes in community dwelling older adults. Front Aging Neurosci 10:40
Touron E, Moulinet I, Kuhn E, Sherif S, Ourry V, Landeau B et al (2022) Depressive symptoms in cognitively unimpaired older adults are associated with lower structural and functional integrity in a frontolimbic network. Mol Psychiatry 27:5086–5095
Bijata M, Bączyńska E, Müller FE, Bijata K, Masternak J, Krzystyniak A et al (2022) Activation of the 5-HT7 receptor and MMP-9 signaling module in the hippocampal CA1 region is necessary for the development of depressive-like behavior. Cell Rep 38:110532
Hoogendijk WJ, Feenstra MG, Botterblom MH, Gilhuis J, Sommer IE, Kamphorst W et al (1999) Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann Neurol 45:82–91
Iannitelli AF, Kelberman MA, Lustberg DJ, Korukonda A, McCann KE, Mulvey B et al (2023) The neurotoxin DSP-4 dysregulates the locus coeruleus-norepinephrine system and recapitulates molecular and behavioral aspects of prodromal neurodegenerative disease. eNeuro 10(1):ENEURO.0483-22.2022
Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA (2005) Sex Differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry 62:685
Irvine K, Laws KR, Gale TM, Kondel TK (2012) Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J Clin Exp Neuropsychol 34:989–998
Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80:1778–1783
Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM et al (2015) Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 1:103–110
Wood H (2022) Menopause influences tau pathology. Nat Rev Neurol 18:317
Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM et al (2007) Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27:13357–13365
Flurkey K, Mcurrer J, Harrison D (2007) Mouse models in aging research. In: The Mouse in Biomedical Research, pp 637–72, Elsevier
Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S (2002) Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 288:1475–1483
Howerton AR, Roland AV, Bale TL (2014) Dorsal raphe neuroinflammation promotes dramatic behavioral stress dysregulation. J Neurosci 34:7113–7123
Aloisi F, Borsellino G, Caré A, Testa U, Gallo P, Russo G et al (1995) Cytokine regulation of astrocyte function: in-vitro studies using cells from the human brain. Int J Dev Neurosci 13:265–274
Staurenghi E, Cerrato V, Gamba P, Testa G, Giannelli S, Leoni V et al (2021) Oxysterols present in Alzheimer’s disease brain induce synaptotoxicity by activating astrocytes: a major role for lipocalin-2. Redox Biol 39:101837
Reid MJ, Beltran-Lobo P, Johnson L, Perez-Nievas BG, Noble W (2020) Astrocytes in tauopathies. Front Neurol 11:572850
Aitta-Aho T, Maksimovic M, Dahl K, Sprengel R, Korpi ER (2019) Attenuation of novelty-induced hyperactivity of Gria1-/-Mice by cannabidiol and hippocampal inhibitory chemogenetics. Front Pharmacol 10:309
Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N et al (2007) Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55:697–711
Busche MA, Konnerth A (2015) Neuronal hyperactivity–a key defect in Alzheimer’s disease? BioEssays 37:624–632
Acknowledgements
We would like to thank Gloria Lee for providing RD3 and RD4 antibodies for Western blot analysis and Gabrielle Bierlein-De La Rosa for technical assistance with behavioral studies.
Funding
This work was supported by R01 AA028931, R01 AG070841, R00 AA024215, and K99 AA024215 to C.A.M. We also thank the Carver Trust and the Williams-Cannon Foundation for their support. K.K. was supported by T32 DK112751, S.P. was supported by T32 GM067795, and L.K. was supported by T32 NS045549.
Author information
Authors and Affiliations
Contributions
CAM, KK and NB designed the studies and wrote the manuscript with editorial help from MH and TA. KK performed behavioral and histological studies and analysis. NB also performed tissue collection and RT-qPCR analysis of gene expression in the DRN and LC and assisted with Western blot experiments. GG performed histological experiments, confocal microscopy and analysis of tau pathology in the DRN, LC, EC, and hippocampus. RW performed slice electrophysiology experiments and analysis. GPS performed Western blot experiments and analysis for histology studies. LK provided additional analysis of electrophysiological data. SP provided conceptual input into the design of histological studies and assisted with imaging and analysis. SMT performed RNA isolation and RT-qPCR analysis in EC and hippocampal tissues. MH and TA also provided intellectual input into experimental design and interpreting the results. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures on mice in this study were approved by the Institutional Care and Use Committee at the University of Iowa.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Methods
: RT-PCR quantification and tau splice isoforms; Detection of tau isoforms in whole brain lysates. Supplementary Figure 1: Validation of htau mice showing presence of insoluble 3R tau isoform in whole brain lysates at 12 months of age. (A) Mouse and human tau splice isoforms in C57BL/6J, htau +/- and MAPT -/- mice. The human-specific primers only amplified products in htau +/- while mouse-specific primers only amplified products in C57BL/6J mice. (B) (i) Protein expression of human tau (HT7) in total protein extracts and (ii) 3R and 4R tau isoforms in Sarkosyl soluble and insoluble fractions from C57BL/6J, htau +/- and MAPT -/- mice. Only htau +/- mice contained human-specific tau (HT7) and the 3R tau isoform in detergent insoluble fractions. Both htau +/- and C57 mice contained the 4R isoform in soluble fractions. Supplementary Figure 2: Orthogonal images confirming hyperphosphorylated tau in 5-HT neurons in the DRN. (A) Atlas plate depicting the mid region of the DRN that was used to obtain orthogonal planes. (B-D) Representative orthogonal planes showing colocalization between 5-HT (cyan) and ptau (red) in the DRN. (E) Atlas plate depicting the mid region of the LC that was used to obtain orthogonal planes. (F-H) Representative orthogonal planes showing colocalization between TH (cyan) and ptau (red) in the LC. Supplementary Figure 3: Hyperphosphorylated tau and monoaminergic depletion in the brainstem of female htau mice at 4 months. (A) Representative confocal images of 5-HT immunostaining (20X; scale bar = 200 µm) and 5-HT/AT8 co-staining (60X; scale bar = 50 µm) in the DRN of C57BL/6J and htau +/- mice. (B) Atlas plate depicting one of the DRN regions analyzed (mid DRN) (C) Representative orthogonal image showing colocalizaton of ptau with 5-HT neurons in the DRN (100X; scale bar = 20 µm). (D) Histogram of 5-HT cell counts/mm2, (E) 5-HT immunoreactive area (%), (F) ptau (AT8) optical density in subregions of the DRN and (G) Correlation analysis between 5-HT IR area and AT8 optical density in the DRN (H) Representative confocal images of TH immunostaining (20X; scale bar = 200 µm) and TH/AT8 colocalization (60X; scale bar = 50 µm) in the LC of C57BL/6J and htau +/- mice. (I) Atlas plate depicting one of the LC regions analyzed (mid LC) (J) Representative orthogonal image showing colocalization of ptau with TH neurons in the LC (100X; scale bar = 20 µm). (K) Histogram of TH cell counts/mm2, (L) TH immunoreactive area, (M) ptau (AT8) optical density in subregions of the LC, and (N) Correlation analysis between TH immunoreactive area and AT8 optical density in the mid LC. *p < 0.05, **p < 0.01, ***p < 0.001. Supplementary Figure 4: Glial activation in the DRN and LC in htau mice at 4 months. (A) Representative confocal images of Iba-1 and GFAP immunostaining in the DRN of C57 and htau +/- mice (60X; scale bar = 50 µm). (B) Iba-1+ cell counts/mm2, (C) Iba-1 optical density, and (D) Iba-1 immunoreactive area in subregions of the DRN. (E) GFAP+ cells/mm2, (F) GFAP optical density, and (G) GFAP immunoreactive area in subregions of the DRN. (H) Representative confocal images of Iba-1 and GFAP immunostaining in the LC of C57 and htau +/- mice. (I) Iba-1+ cells/mm2, (J) Iba-1 optical density, and (K) Iba-1 immunoreactive area in subregions of the LC. (L) GFAP+ cells/mm2, (M) GFAP optical density, and (N) GFAP immunoreactive area in subregions of the LC. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01. White arrows denote Iba-1 +or GFAP+ cell bodies. Supplementary Figure 5: Reduced 5-HT innervation of the entorhinal cortex in htau mice at 4 months. Representative confocal images of 5-HT fiber immunostaining (40x; scale bar = 50 um) in C57 and htau mice. (A) Atlas representation of a section of the EC used for analysis (in red). (B) Representative confocal images of 5-HT staining in the EC of C57BL/6J and htau mice at 40X. (C) Histogram of % 5-HT IR area in subregions of the EC. (D) Representative confocal images of 5-HT staining in the EC at 100X. (E) Atlas representation of CA1 region of the hippocampus used in the analysis (in red). (F) Representative confocal image of SERT staining in the CA1 region of the hippocampus in C57BL/6J and htau mice. (G) Histogram of % SERT IR area in subregions of the hippocampus (H) Representative confocal images of SERT staining in the CA1 region of the hippocampus at 100X. Data are represented as mean ± SEM. *p < 0.05, **p < 0.01. Supplementary Table 1: List of fold change values obtained from RT-qPCR of genes tested in the dorsal raphe nucleus, locus coeruleus, dorsal and ventral hippocampus, and entorhinal cortex of C57 and htau (*p < 0.05, **p < 0.01, ***p < 0.001).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khan, K.M., Balasubramanian, N., Gaudencio, G. et al. Human tau-overexpressing mice recapitulate brainstem involvement and neuropsychiatric features of early Alzheimer’s disease. acta neuropathol commun 11, 57 (2023). https://doi.org/10.1186/s40478-023-01546-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40478-023-01546-5