Abstract
Non-traumatic intracerebral hemorrhage (ICH) is the most common type of hemorrhagic stroke, most often occurring between the ages of 45 and 60. Hypertension is most often the cause of ICH. Less often, atherosclerosis, blood diseases, inflammatory changes in cerebral vessels, intoxication, vitamin deficiencies, and other reasons cause hemorrhages. Cerebral hemorrhage can occur by diapedesis or as a result of a ruptured vessel. This very dangerous disease is difficult to treat, requires surgery and can lead to disability or death. MicroRNAs (miRNAs) are a class of non-coding RNAs (about 18-22 nucleotides) that are involved in a variety of biological processes including cell differentiation, proliferation, apoptosis, etc., through gene repression. A growing number of studies have demonstrated miRNAs deregulation in various cardiovascular diseases, including ICH. In addition, given that computed tomography (CT) and/or magnetic resonance imaging (MRI) are either not available or do not show clear signs of possible vessel rupture, accurate and reliable analysis of circulating miRNAs in biological fluids can help in early diagnosis for prevention of ICH and prognosis patient outcome after hemorrhage. In this review, we highlight the up-to-date findings on the deregulated miRNAs in ICH, and the potential use of miRNAs in clinical settings, such as therapeutic targets and non-invasive diagnostic/prognostic biomarker tools.
Similar content being viewed by others
Introduction
Non-traumatic intracerebral hemorrhage (ICH) is local bleeding into the parenchyma of the brain, which occurs because of rupture of cerebral blood vessels [1]. ICH usually develops when an atherosclerotic cerebral artery ruptures, the wall of which has undergone changes because of a prolonged existing increase in blood pressure [1]. The problem of early diagnosis, prognosis and treatment of ICH is one of the most important in modern medicine. Despite the vast experience of modern neurosurgery and neurology in the treatment of patients with ICH, the tactics of managing patients are still controversial, and indications for various methods of therapy need to be clarified [2]. Until now, neither conservative nor surgical methods of treatment have a clear advantage. Therefore, the study of the molecular mechanisms of the pathogenesis of ICH will deepen the understanding of the course of ICH and clarify some issues of diagnosis and treatment tactics. The mechanisms of ICH development include impaired blood-brain barrier (BBB) function and cerebral edema, cell apoptosis, inflammation, oxidative stress, activation of signaling pathways that regulate angiogenesis, endothelial disfunction, and suppression of signaling pathways responsible for maintaining the phenotype of vascular smooth muscle cells (VSMCs) [3]. MicroRNAs (miRNAs) are short, on average 18-22 nucleotides, single-stranded non-coding RNAs that post-transcriptional regulate gene expression by binding to the 3'-untranslated region (3'-UTR) of the messenger RNA (mRNA) target, which ultimately leads to decreasing protein expression by blocking translation and/or promoting degradation of the target mRNA [3] (Fig. 1). Many studies have shown that miRNAs play an important regulatory role in the occurrence and development of cerebrovascular diseases (CVDs). The mechanisms by which miRNAs play a role in the development of CVD are very complex (Table 1) [21]. Therefore, to understand the function of miRNAs and their role more clearly in diseases, including ICH, research on miRNA biology must be conducted in the context of a protein interaction network rather than isolated target genes. Moreover, proteins mediated by the same miRNA have a high propensity to interact with each other. These specific characteristics imply that miRNAs may exert their regulatory effects on protein complexes and pathways through a network of protein interactions [22]. Thus, based on the analysis of the network of protein interactions mediated by miRNAs, it is not possible to fully understand the function of miRNAs, but to more accurately identify miRNAs associated with ICH.
In addition, miRNA expression is controlled through DNA modification, such as methylation and transcription factors through the signal transduction pathway [23]. DNA methylation refers to the formation of a covalent bond between the 5' cytosine carbon of a DNA CpG dinucleotide (5'-cytosine-guanine-3') and a methyl group by DNA methyltransferase, forming 5-methylcytosine [23]. DNA hypermethylation can directly inhibit transcription or indirectly inhibit gene expression through transcriptional repression [24]. Changes in DNA methylation is associated with CVDs such as ischemic stroke (IS) and ICH [25, 26]. The hypermethylation of the CpG-rich promoter regions of vascular-specific miRNAs usually leads to their silencing through DNA methyltransferase (DNA MTase, DNMT) [27]. The abnormal DNA methylation of miRNA usually leads to the downregulation of miRNA, which is significantly related to the phenotypic switching of VSMCs or endothelial cells (ECs) disfunction [26]. In turn, miRNA could regulate the expression level of the target gene after transcription, which affects DNA methylation in ECs and VSMCs through the 3′-UTR of the RNA-induced silencing complex (RISC)-targeted DNMT mRNA [27]. In conclusion, this demonstrates the existence of a regulatory loop between miRNA expression and epigenetic modifications.
In recent years, the attention of researchers has been attracted by the study of changes in the expression levels of circulating miRNAs in various biological fluids of the human body for their potential consideration as non-invasive diagnostic and prognostic biomarkers of vascular dysfunction or damage brain tissue in CVDs (Table 2) [28,29,30,31,32,33,34,35,36]. Importantly, the specific expression signatures of circulating miRNAs in biological fluids reflect not only the existence of early-stage diseases, but also the dynamic development of late-stage diseases, disease prognosis, and treatment monitoring [19]. To date, a growing number of circulating miRNAs have been reported as potential noninvasive biomarkers in ICH, but the prospects for their practical application are still unclear. Thus, a summary of the circulating miRNA expression profile is of great importance for the identification of new promising biomarkers in ICH.
This review summarizes the brief data on miRNAs that have been proven to be involved in the pathogenesis of ICH, their targets, and mechanisms of action, and summarizes the latest advances in the context of the potential application of miRNAs in clinical practice, particularly, the possibility of their use in therapy and for early diagnosis, prognosis, and therapy monitoring for ICH. In addition, we will discuss important general questions about the possibilities of using as therapy drugs and biomarkers in ICH, uncovering the benefits of circulating miRNAs.
MiRNAs and the main pathogenetic elements of ICH
ICH is a multifactorial disease with many established causes. A key role in the pathogenesis of ICH is played by endothelial dysfunction and pronounced changes in the walls of cerebral vessels under the influence of chronically elevated blood pressure and atherosclerosis [1, 2]. The molecular basis for the development of ICH is complex and includes a whole range of disorders, among which the apoptosis of ECs of cerebral vessels and neurons, the inflammatory process and the response of the immune system, oxidative stress, impaired BBB function, and cerebral edema occupy a central place [1, 2]. It is widely known that miRNAs are powerful regulators involved in all molecular and cellular processes, both in normal conditions and in various diseases [21, 22]. Understanding the regulation of miRNAs and their targets makes it possible to determine the causes of the occurrence of ICH caused by changes in gene expression. Several in vitro and in vivo studies have provided clear evidence of the involvement of miRNAs in the pathogenesis of the development and progression of ICH, which will be described in this chapter (Table 3) [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73].
Vascular integrity and extracellular matrix remodeling
Maintaining vascular integrity and preserving the extracellular matrix (ECM) are crucial for preventing ICH. Disruptions in the BBB and abnormalities in the ECM contribute to increased vascular permeability and vessel fragility, leading to hemorrhage. The BBB, consisting of ECs, tight junctions, pericytes, and astrocytes, regulates the passage of molecules and cells between the bloodstream and the brain parenchyma [74]. Dysfunction of the BBB is associated with increased permeability, allowing blood components to enter the brain parenchyma, and triggering neuroinflammatory responses. Participation of miRNAs in the regulation of the BBB function occurs largely due to the influence on the cytoskeleton of ECs and on the functional state of cell adhesion proteins [75, 76].
The main cause of ICH is hypertension and associated microangiopathy. Prolonged arterial hypertension contributes to the formation of lipogyalinosis, and subsequently fibrinoid necrosis of the walls of perforating arteries, characterized by the absence of anastomoses with other vessels. Under these conditions, the structure and organization of cell adhesion proteins in the outer membrane of the ECs leads to disruption of BBB permeability (reduced levels of cell adhesion proteins such as claudin 5, occludin and zonula occludens-1 (ZO-1) have been shown) [74, 75]. ZO-1 is an important tight junction protein involved in the formation of the BBB. It is involved in the barrier function, in the regulation of cell transport, cell polarity, cell proliferation and differentiation [77]. An increase or decrease in ZO-1 expression in ECs of the brain microvascular network also significantly affects apoptosis and proliferation of ECs [78]. Hu et al. found that ZO-1 is a potential target for miR-23a-3p. Their data showed that proliferation and apoptosis of hCMEC/D3 cells are regulated by via miR-23a-3p/ZO-1 axis, demonstrating also that aberrant expression of miR -23a-3p promotes the formation of perihematomal edema [59]. In addition, the degradation of cell adhesion proteins by matrix metalloproteinases (MMPs) is also directly regulated by miRNAs [79]. Some miRNAs can protect the integrity of the BBB by reducing immune cell adhesion and the expression of pro-inflammatory cytokines. Activation of microglia in the process of BBB permeability impairment, in turn, exacerbates ICH-induced damage to brain tissue and, thereby, significantly worsens the prognosis and neurological deficit [80, 163]. This requires the identification of a more specific biomarker associated with damage to the vascular elements of the cerebral arteries, in addition, these biomarkers do not reflect cellular function. There is already ample evidence that miRNAs play an important role in the processes underlying the normal function of ECs and VSMCs and their dysfunction [164,165,166,167,168,169]. And the fact that some miRNAs are found freely circulating in biological fluids indicates an early manifestation of damage to the vascular wall of cerebral arteries. Due to their specificity and ability to be detected in the early stages of disease development, circulating miRNAs can undoubtedly be considered as predictors of the onset of ICH (Fig. 6).
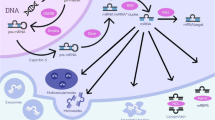
Schematic illustration of the regulatory role of microRNAs (miRNAs) in vascular wall cells (endothelial cells (ECs) and vascular smooth muscle cells (VSMCs)) and their secretion into the bloodstream. Vascularly enriched miRNAs regulate cellular responses to various factors both in normal and pathological conditions by acting on various receptors and signaling pathways: miR-15a - decreases blood-brain barrier (BBB) disruption and protection of cerebral ECs apoptosis; miR-17-92, miR-210 and miR-132 - angiogenesis; miR-23a - inhibition of ECs growth; miR-26a - increase VSMCs proliferation and control VSMC phenotype shifting; miR -143/145 and miR-146a - activation of VSMCs proliferation and neointimal hyperplasia; miR-24 - inhibition of erythropoiesis and control VSMC phenotype shifting; miR-221/miR-222 - pro-proliferative effect on ECs and angiogenesis. In addition, it is known from recent sources that these vascular-enriched miRNAs can occur in the bloodstream and may be biomarkers of early damage to the vascular wall. Note: Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; CTGF, connective tissue growth factor; EGF, epidermal growth factor; Elk-1, ETS domain-containing protein 1; ERK, extracellular signal-regulated kinase; FGF, fibroblast growth factor; GAX, homeobox protein; KLF4, krüppel-like factor 4; KLF5; krüppel-like factor 5; MEK, mitogen-activated protein kinase kinase; PI3K, Phosphatidyl-inositol 3-kinase; p120RasGAP, p21, cyclin-dependent kinase inhibitor 1; Rb, Retinoblastoma; p, Hpophosphorylated; Raf, rapidly growing fibrosarcoma; Ras, rat sarcoma; RhoA, Ras homolog gene family member A; Smurf1, Smad ubiquitin-regulatory factor 1; SRF, serum response factor; TGF-b, transforming growth factor beta; Trb-3, Tribbles-like protein-3; TSR1, thrombospondin-1; VEGF, vascular endothelial growth factor; ZEB2, zinc finger Ebox- binding homeobox 2.
Circulating miRNAs as biomarkers of neuroinflammation
As discussed above, neuroinflammation after ICH is a complex process of interaction between the immune and nervous systems, which occurs in response to damage to the nervous tissue from the hematoma mass effect and can continue for a long period after the hemorrhage [86]. In addition, the lysis of erythrocytes and the reaction of the brain tissue to decay products leads to the death of neurons, in response to which the process of neuroinflammation is triggered as a neuroprotective mechanism that provides protection for intact tissue: microglia are activated, infiltration by granulocytes and lymphocytes of the brain tissue, secretion by immunocompetent and glial pro- and anti-inflammatory cytokines, chemokines, and ROS cells [86, 99]. Adverse consequences and complications of ICH are associated with secondary damage and the development of neuroinflammation. Therefore, recognizing and determining the extent of the inflammatory process at an early stage is essential for optimizing immune-targeted treatment. On the other hand, diagnostic difficulties are due to nonspecific changes according to neuroimaging data. It has been repeatedly shown that miRNAs play a direct role as pro- or anti-inflammatory regulators after ICH, their presence in biological fluids presents the possibility of their use as prognostic biomarkers for the development of neuroinflammation. Hematoma growth and a large hematoma volume are associated with an unfavorable course of the disease, including the activation of inflammatory cascades. To date, there are already successful results from several studies that have shown that circulating miRNAs can be used to predict hematoma growth after ICH [59, 147]. In addition, besides EVs or protein complexes, miRNAs are expressed in erythrocytes, and due to their lysis, circulating miRNAs in apoptotic bodies can enter the bloodstream or CSF (when blood enters the lateral ventricles) and can potentially provide easy and rapid testing in clinical populations, hel** to predict and monitor the degree of development of neuroinflammation [172, 173]. It is important to note that not every laboratory indicator can be called a biomarker. The main thing that distinguishes a biomarker is its statistically substantiated relationship with the disease, complication, and treatment effect, which is expressed in the following characteristics: statistical significance of differences in the selected characteristic, receiver operating characteristic curve (ROC), Kaplan–Meier curves, threshold value with calculated sensitivity and specificity, predictive value of the biomarker, likelihood ratio for the presence and absence of the trait and accuracy index [172, 173]. In most studies, circulating miRNAs are named as potential biomarkers based on their statistically significant increase or decrease in expression in ICH [188, 189]. Dysregulation of miR-124 is associated with the development of CVDs such as ICH. Recently, abnormal expression of miR-124 was shown to be associated with the occurrence and development of ICH via regulating microglia function [58, 69, 195]. Moreover, miR-126 is involved in vascular inflammation and neuroinflammation [195, 196]. Amongst miRNAs, miR-126 is one of the most highly-expressed and highly-studied in ICH [37,38,39,5, circulating miRNAs are associated with a higher incidence of complications or adverse outcomes of ICH. Such an association is not enough to classify circulating as diagnostic or prognostic biomarkers; an in-depth statistical analysis is required, which will allow to identify multifactorial relationships, evaluate the significance, and calculate the diagnostic or prognostic characteristics of the test with the obligatory use of ROC analysis. It is also necessary to increase the number of studies on the use of circulating miRNAs in the differential diagnosis of stroke subtypes (ICH vs SAH or IS). Nevertheless, further study of circulating miRNAs makes it possible to predict the level of damage to the cerebral vessel wall and the likelihood of its rupture, as well as to confirm the fact of an event that occurred in real time and determine the period of ICH (acute, subacute and chronic), distinguish its subtypes and even judge the prognosis.
Availability of data and materials
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Veltkamp R, Purrucker J. Management of Spontaneous Intracerebral Hemorrhage. Curr Neurol Neurosci Rep. 2017;17(10):80. https://doi.org/10.1007/s11910-017-0783-5.
Hostettler IC, Seiffge DJ, Werring DJ. Intracerebral hemorrhage: an update on diagnosis and treatment. Expert Rev Neurother. 2019;19(7):679–94. https://doi.org/10.1080/14737175.2019.1623671.
Giralt-Steinhauer E, Jiménez-Balado J, Fernández-Pérez I, Rey Álvarez L, Rodríguez-Campello A, Ois Á, Cuadrado-Godia E, Jiménez-Conde J, Roquer J. Genetics and Epigenetics of Spontaneous Intracerebral Hemorrhage. Int J Mol Sci. 2022;23(12):6479. https://doi.org/10.3390/ijms23126479.
Zhao Y, Zhu W, Wan T, Zhang X, Li Y, Huang Z, Xu P, Huang K, Ye R, **e Y, Liu X. Vascular endothelium deploys caveolin-1 to regulate oligodendrogenesis after chronic cerebral ischemia in mice. Nat Commun. 2022;13(1):6813. https://doi.org/10.1038/s41467-022-34293-7.
Mao M, Xu Y, Zhang XY, Yang L, An XB, Qu Y, Chai YN, Wang YR, Li TT, Ai J. MicroRNA-195 prevents hippocampal microglial/macrophage polarization towards the M1 phenotype induced by chronic brain hypoperfusion through regulating CX3CL1/CX3CR1 signaling. J Neuroinflamm. 2020;17(1):244. https://doi.org/10.1186/s12974-020-01919-w.
Li Y, Liu Z, Song Y, Pan JJ, Jiang Y, Shi X, Liu C, Ma Y, Luo L, Mamtilahun M, Shi Z, Khan H, **e Q, Wang Y, Tang Y, Zhang Z, Yang GY. M2 microglia-derived extracellular vesicles promote white matter repair and functional recovery via miR-23a-5p after cerebral ischemia in mice. Theranostics. 2022;12(7):3553–73. https://doi.org/10.7150/thno.68895.
Sun J, Tao S, Liu L, Guo D, **a Z, Huang M. miR-140-5p regulates angiogenesis following ischemic stroke by targeting VEGFA. Mol Med Rep. 2016;13(5):4499–505. https://doi.org/10.3892/mmr.2016.5066.
Pan J, Qu M, Li Y, Wang L, Zhang L, Wang Y, Tang Y, Tian HL, Zhang Z, Yang GY. MicroRNA-126-3p/-5p Overexpression Attenuates Blood-Brain Barrier Disruption in a Mouse Model of Middle Cerebral Artery Occlusion. Stroke. 2020;51(2):619–27. https://doi.org/10.1161/STROKEAHA.119.027531.
Li Y, Mao L, Gao Y, Baral S, Zhou Y, Hu B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci Rep. 2015;21(5):13316. https://doi.org/10.1038/srep13316.
Cui H, Yang A, Zhou H, Wang Y, Luo J, Zhou J, Liu T, Li P, Zhou J, Hu E, He Z, Hu W, Tang T. Thrombin-induced miRNA-24-1-5p upregulation promotes angiogenesis by targeting prolyl hydroxylase domain 1 in intracerebral hemorrhagic rats. J Neurosurg. 2020;134(5):1515–26. https://doi.org/10.3171/2020.2.JNS193069.
Gu Y, Ampofo E, Menger MD, Laschke MW. miR-191 suppresses angiogenesis by activation of NF-κB signaling. FASEB J. 2017;31(8):3321–33. https://doi.org/10.1096/fj.201601263R.
Feng M, Zhou Q, Tu W, Wang Y, Du Y, Xu K. ATF4 promotes brain vascular smooth muscle cells proliferation, invasion and migration by targeting miR-552-SKI axis. PLoS One. 2022;17(7):e0270880. https://doi.org/10.1371/journal.pone.0270880.
Zhang B, Chen L, Bai YG, Song JB, Cheng JH, Ma HZ, Ma J, **e MJ. miR-137 and its target T-type CaV 3.1 channel modulate dedifferentiation and proliferation of cerebrovascular smooth muscle cells in simulated microgravity rats by regulating calcineurin/NFAT pathway. Cell Prolif. 2020;53(3):e12774. https://doi.org/10.1111/cpr.12774.
Esen N, Vejalla A, Sharma R, Treuttner JS, Dore-Duffy P. Hypoxia-Induced Let-7d Has a Role in Pericyte Differentiation. Adv Exp Med Biol. 2016;923:37–42. https://doi.org/10.1007/978-3-319-38810-6_5.
Ding W, Gu Q, Liu M, Zou J, Sun J, Zhu J. Astrocytes-derived exosomes pre-treated by berberine inhibit neuroinflammation after stroke via miR-182-5p/Rac1 pathway. Int Immunopharmacol. 2023;118:110047. https://doi.org/10.1016/j.intimp.2023.110047.
Li P, Shen M, Gao F, Wu J, Zhang J, Teng F, Zhang C. An Antagomir to MicroRNA-106b-5p Ameliorates Cerebral Ischemia and Reperfusion Injury in Rats Via Inhibiting Apoptosis and Oxidative Stress. Mol Neurobiol. 2017;54(4):2901–21. https://doi.org/10.1007/s12035-016-9842-1.
Teertam SK, Jha S, Prakash Babu P. Up-regulation of Sirt1/miR-149-5p signaling may play a role in resveratrol induced protection against ischemia via p53 in rat brain. J Clin Neurosci. 2020;72:402–11. https://doi.org/10.1016/j.jocn.2019.11.043.
Ge XL, Wang JL, Liu X, Zhang J, Liu C, Guo L. Inhibition of miR-19a protects neurons against ischemic stroke through modulating glucose metabolism and neuronal apoptosis. Cell Mol Biol Lett. 2019;31(24):37. https://doi.org/10.1186/s11658-019-0160-2.
Gareev I, Beylerli O, Yang G, Sun J, Pavlov V, Izmailov A, Shi H, Zhao S. The current state of MiRNAs as biomarkers and therapeutic tools. Clin Exp Med. 2020;20(3):349–59. https://doi.org/10.1007/s10238-020-00627-2.
Dave P, Roth G, Griesbach E, Mateju D, Hochstoeger T, Chao JA. Single-molecule imaging reveals translation-dependent destabilization of mRNAs. Mol Cell. 2023;83(4):589-606.e6. https://doi.org/10.1016/j.molcel.2023.01.013.
Mencia R, Gonzalo L, Tossolini I, Manavella PA. Kee** up with the miRNAs: current paradigms of the biogenesis pathway. J Exp Bot. 2023;74(7):2213–27. https://doi.org/10.1093/jxb/erac322.
Klimentová E, Hejret V, Krčmář J, Grešová K, Giassa IC, Alexiou P. miRBind: A Deep Learning Method for miRNA Binding Classification. Genes (Basel). 2022;13(12):2323. https://doi.org/10.3390/genes13122323.
Zhang L, Lu Q, Chang C. Epigenetics in Health and Disease. Adv Exp Med Biol. 2020;1253:3–55. https://doi.org/10.1007/978-981-15-3449-2_1.
Vohra M, Sharma AR, Prabhu BN, Rai PS. SNPs in sites for DNA methylation, transcription factor binding, and miRNA targets leading to allele-specific gene expression and contributing to complex disease risk: a systematic review. Public Health Genomics. 2020;23(5–6):155–70. https://doi.org/10.1159/000510253.
Zhu MX, Zhao TY, Li Y. Insight into the mechanism of DNA methylation and miRNA-mRNA regulatory network in ischemic stroke. Math Biosci Eng. 2023;20(6):10264–83. https://doi.org/10.3934/mbe.2023450.
Udali S, Guarini P, Moruzzi S, Choi SW, Friso S. Cardiovascular epigenetics: from DNA methylation to microRNAs. Mol Aspects Med. 2013;34(4):883–901. https://doi.org/10.1016/j.mam.2012.08.001.
Cui J, Liu N, Chang Z, Gao Y, Bao M, **e Y, Xu W, Liu X, Jiang S, Liu Y, Shi R, **e W, Jia X, Shi J, Ren C, Gong K, Zhang C, Bade R, Shao G, Ji X. Exosomal MicroRNA-126 from RIPC Serum Is Involved in Hypoxia Tolerance in SH-SY5Y Cells by Downregulating DNMT3B. Mol Ther Nucleic Acids. 2020;5(20):649–60. https://doi.org/10.1016/j.omtn.2020.04.008.
Aldous EK, Toor SM, Parray A, Al-Sarraj Y, Diboun I, Abdelalim EM, Arredouani A, El-Agnaf O, Thornalley PJ, Akhtar N, Pananchikkal SV, Shuaib A, Alajez NM, Albagha OME. Identification of Novel Circulating miRNAs in Patients with Acute Ischemic Stroke. Int J Mol Sci. 2022;23(6):3387. https://doi.org/10.3390/ijms23063387.
Eyileten C, Jakubik D, Shahzadi A, Gasecka A, van der Pol E, De Rosa S, Siwik D, Gajewska M, Mirowska-Guzel D, Kurkowska-Jastrzebska I, Czlonkowska A, Postula M. Diagnostic performance of circulating miRNAs and extracellular vesicles in acute ischemic stroke. Int J Mol Sci. 2022;23(9):4530. https://doi.org/10.3390/ijms23094530.
Chen Y, Song Y, Huang J, Qu M, Zhang Y, Geng J, Zhang Z, Liu J, Yang GY. Increased circulating exosomal miRNA-223 is associated with acute ischemic stroke. Front Neurol. 2017;27(8):57. https://doi.org/10.3389/fneur.2017.00057.
Giordano M, Ciarambino T, D’Amico M, Trotta MC, Di Sette AM, Marfella R, Malatino L, Paolisso G, Adinolfi LE. Circulating MiRNA-195-5p and -451a in transient and acute ischemic stroke patients in an emergency department. J Clin Med. 2019;8(2):130. https://doi.org/10.3390/jcm8020130.
Sheng B, Lai N, Tao T, Chen X, Gao S, Zhu Q, Li W, Zhang Q, Hang C. Diagnosis potential of subarachnoid hemorrhage using miRNA signatures isolated from plasma-derived extracellular vesicles. Front Pharmacol. 2023;13(14):1090389. https://doi.org/10.3389/fphar.2023.1090389.
Su XW, Chan AH, Lu G, Lin M, Sze J, Zhou JY, Poon WS, Liu Q, Zheng VZ, Wong GK. Circulating microRNA 132–3p and 324–3p Profiles in Patients after Acute Aneurysmal Subarachnoid Hemorrhage. PLoS One. 2015;10(12):e0144724. https://doi.org/10.1371/journal.pone.0144724.
Wu J, Gareev I, Beylerli O, Mukhamedzyanov A, Pavlov V, Khasanov D, Khasanova G. Circulating miR-126 as a potential non-invasive biomarker for intracranial aneurysmal rupture: a pilot study. Curr Neurovasc Res. 2021;18(5):525–34. https://doi.org/10.2174/1567202619666211217142116.
Meeuwsen JAL, van T Hof FNG, van Rheenen W, Rinkel GJE, Veldink JH, Ruigrok YM. Circulating microRNAs in patients with intracranial aneurysms. PLoS One. 2017;12(5):e0176558. https://doi.org/10.1371/journal.pone.0176558.
Chen Y, Li Z, Shi Y, Huang G, Chen L, Tan H, Wang Z, Yin C, Hu J. Deep sequencing of small RNAs in Blood of patients with brain arteriovenous malformations. World Neurosurg. 2018;115:e570–9. https://doi.org/10.1016/j.wneu.2018.04.097.
Dong B, Zhou B, Sun Z, Huang S, Han L, Nie H, Chen G, Liu S, Zhang Y, Bao N, Yang X, Feng H. LncRNA-FENDRR mediates VEGFA to promote the apoptosis of brain microvascular endothelial cells via regulating miR-126 in mice with hypertensive intracerebral hemorrhage. Microcirculation. 2018;25(8):e12499. https://doi.org/10.1111/micc.12499.
Kong F, Zhou J, Zhou W, Guo Y, Li G, Yang L. Protective role of microRNA-126 in intracerebral hemorrhage. Mol Med Rep. 2017;15(3):1419–25. https://doi.org/10.3892/mmr.2017.6134.
Liu Y, Mo C, Mao X, Lu M, Xu L. Increasing miR-126 can prevent brain injury after intracerebral hemorrhage in rats by regulating ZEB1. Contrast Media Mol Imaging. 2022;30(2022):2698773. https://doi.org/10.1155/2022/2698773.
** T, ** F, Zhu Y, Wang J, Tang L, Wang Y, Liebeskind DS, He Z. MicroRNA-126-3p attenuates blood-brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochem Biophys Res Commun. 2017;494(1–2):144–51. https://doi.org/10.1016/j.bbrc.2017.10.064.
Wang MD, Wang Y, **a YP, Dai JW, Gao L, Wang SQ, Wang HJ, Mao L, Li M, Yu SM, Tu Y, He QW, Zhang GP, Wang L, Xu GZ, Xu HB, Zhu LQ, Hu B. High serum MiR-130a levels are associated with severe perihematomal edema and predict adverse outcome in acute ICH. Mol Neurobiol. 2016;53(2):1310–21. https://doi.org/10.1007/s12035-015-9099-0.
Zhang Y, Han B, He Y, Li D, Ma X, Liu Q, Hao J. MicroRNA-132 attenuates neurobehavioral and neuropathological changes associated with intracerebral hemorrhage in mice. Neurochem Int. 2017;107:182–90. https://doi.org/10.1016/j.neuint.2016.11.011.
** T, ** F, Zhu Y, Wang J, Tang L, Wang Y, Liebeskind DS, Scalzo F, He Z. miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. J Biol Chem. 2018;293(52):20041–50. https://doi.org/10.1074/jbc.RA118.001858.
Fu X, Niu T, Li X. MicroRNA-126-3p Attenuates Intracerebral hemorrhage-induced blood-brain barrier disruption by regulating VCAM-1 expression. Front Neurosci. 2019;16(13):866. https://doi.org/10.3389/fnins.2019.00866.
Ouyang Y, Li D, Wang H, Wan Z, Luo Q, Zhong Y, Yin M, Qing Z, Li Z, Bao B, Chen Z, Yin X, Zhu LQ. MiR-21-5p/dual-specificity phosphatase 8 signalling mediates the anti-inflammatory effect of haem oxygenase-1 in aged intracerebral haemorrhage rats. Aging Cell. 2019;18(6):e13022. https://doi.org/10.1111/acel.13022.
Wang C, Cao J, Duan S, Xu R, Yu H, Huo X, Qian Y. Effect of MicroRNA-126a-3p on bone marrow mesenchymal stem cells repairing blood-brain barrier and nerve injury after intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(5):104748. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.104748.
Ren S, Wu G, Huang Y, Wang L, Li Y, Zhang Y. MiR-18a Aggravates Intracranial Hemorrhage by Regulating RUNX1-Occludin/ZO-1 Axis to Increase BBB Permeability. J Stroke Cerebrovasc Dis. 2021;30(8):105878. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105878.
Li Y, Wang J, Chen S, Wu P, Xu S, Wang C, Shi H, Bihl J. miR-137 boosts the neuroprotective effect of endothelial progenitor cell-derived exosomes in oxyhemoglobin-treated SH-SY5Y cells partially via COX2/PGE2 pathway. Stem Cell Res Ther. 2020;11(1):330. https://doi.org/10.1186/s13287-020-01836-y.
Zhao M, Gao J, Zhang Y, Jiang X, Tian Y, Zheng X, Wang K, Cui J. Elevated miR-29a contributes to axonal outgrowth and neurological recovery after intracerebral hemorrhage via targeting PTEN/PI3K/Akt pathway. Cell Mol Neurobiol. 2021;41(8):1759–72. https://doi.org/10.1007/s10571-020-00945-9.
Duan S, Wang F, Cao J, Wang C. Exosomes derived from MicroRNA-146a-5p-enriched bone marrow mesenchymal stem cells alleviate intracerebral hemorrhage by inhibiting neuronal apoptosis and microglial M1 polarization. Drug Des Devel Ther. 2020;5(14):3143–58. https://doi.org/10.2147/DDDT.S255828.
Wang Y, Song Y, Pang Y, Yu Z, Hua W, Gu Y, Qi J, Wu H. miR-183-5p alleviates early injury after intracerebral hemorrhage by inhibiting heme oxygenase-1 expression. Aging (Albany NY). 2020;12(13):12869–95. https://doi.org/10.18632/aging.103343.
Nie H, Hu Y, Guo W, Wang W, Yang Q, Dong Q, Tang Y, Li Q, Tang Z. miR-331-3p Inhibits Inflammatory Response after Intracerebral Hemorrhage by Directly Targeting NLRP6. Biomed Res Int. 2020;7(2020):6182464. https://doi.org/10.1155/2020/6182464.
Wang B, Zhao X, **ao L, Chen Y. FoxO1 silencing facilitates neurological function recovery in intracerebral hemorrhage mice via the lncRNA GAS5/miR-378a-5p/Hspa5 Axis. J Stroke Cerebrovasc Dis. 2022;31(7):106443. https://doi.org/10.1016/j.jstrokecerebrovasdis.2022.106443.
Li D, Zhao Y, Bai P, Li Y, Wan S, Zhu X, Liu M. Baihui (DU20)-penetrating-Qubin (GB7) acupuncture regulates microglia polarization through miR-34a-5p/Klf4 signaling in intracerebral hemorrhage rats. Exp Anim. 2021;70(4):469–78. https://doi.org/10.1538/expanim.21-0034.
Wei M, Li C, Yan Z, Hu Z, Dong L, Zhang J, Wang X, Li Y, Zhang H. Activated microglia exosomes mediated miR-383-3p promotes neuronal necroptosis through inhibiting ATF4 expression in intracerebral hemorrhage. Neurochem Res. 2021;46(6):1337–49. https://doi.org/10.1007/s11064-021-03268-3.
Sun J, Xu G. Mesenchymal stem cell-derived exosomal miR-150-3p affects intracerebral hemorrhage by regulating TRAF6/NF-κB Axis, gut microbiota and metabolism. Stem Cell Rev Rep. 2023. https://doi.org/10.1007/s12015-023-10541-1.
Wang H, Cao X, Wen X, Li D, Ouyang Y, Bao B, Zhong Y, Qin Z, Yin M, Chen Z, Yin X. Transforming growth factor-β1 functions as a competitive endogenous RNA that ameliorates intracranial hemorrhage injury by sponging microRNA-93-5p. Mol Med Rep. 2021;24(1):499. https://doi.org/10.3892/mmr.2021.12138.
Fang Y, Hong X. miR-124-3p inhibits microglial secondary inflammation after basal ganglia hemorrhage by targeting TRAF6 and repressing the activation of NLRP3 inflammasome. Front Neurol. 2021;3(12):653321. https://doi.org/10.3389/fneur.2021.653321.
Hu YL, Wang H, Huang Q, Wang G, Zhang HB. MicroRNA-23a-3p promotes the perihematomal edema formation after intracerebral hemorrhage via ZO-1. Eur Rev Med Pharmacol Sci. 2018;22(9):2809-2816. https://doi.org/10.26355/eurrev_201805_14980
Qu X, Wang N, Cheng W, Xue Y, Chen W, Qi M. MicroRNA-146a protects against intracerebral hemorrhage by inhibiting inflammation and oxidative stress. Exp Ther Med. 2019;18(5):3920–8. https://doi.org/10.3892/etm.2019.8060.
Xu HF, Fang XY, Zhu SH, Xu XH, Zhang ZX, Wang ZF, Zhao ZQ, Ding YJ, Tao LY. Glucocorticoid treatment inhibits intracerebral hemorrhage-induced inflammation by targeting the microRNA-155/SOCS-1 signaling pathway. Mol Med Rep. 2016;14:3798–804. https://doi.org/10.3892/mmr.2016.5716.
Xu W, Li F, Liu Z, Xu Z, Sun B, Cao J, Liu Y. MicroRNA-27b inhibition promotes Nrf2/ARE pathway activation and alleviates intracerebral hemorrhage-induced brain injury. Oncotarget. 2017;8(41):70669–84. https://doi.org/10.18632/oncotarget.19974.
Yang W, Ding N, Luo R, Zhang Q, Li Z, Zhao F, Zhang S, Zhang X, Zhou T, Wang H, Wang L, Hu S, Wang G, Feng H, Hu R. Exosomes from young healthy human plasma promote functional recovery from intracerebral hemorrhage via counteracting ferroptotic injury. Bioact Mater. 2023;23(27):1–14. https://doi.org/10.1016/j.bioactmat.2023.03.007.
Li L, Zhan Y, **a H, Wu Y, Wu X, Chen S. Sevoflurane protects against intracerebral hemorrhage via microRNA-133b/FOXO4/BCL2 axis. Int Immunopharmacol. 2023;114:109453. https://doi.org/10.1016/j.intimp.2022.109453.
Hu LT, Wang BY, Fan YH, He ZY, Zheng WX. Exosomal miR-23b from bone marrow mesenchymal stem cells alleviates oxidative stress and pyroptosis after intracerebral hemorrhage. Neural Regen Res. 2023;18(3):560–7. https://doi.org/10.4103/1673-5374.346551.
Cheng M, Li T, Hu E, Yan Q, Li H, Wang Y, Luo J, Tang T. A novel strategy of integrating network pharmacology and transcriptome reveals antiapoptotic mechanisms of Buyang Huanwu Decoction in treating intracerebral hemorrhage. J Ethnopharmacol. 2024;319(Pt 1):117123. https://doi.org/10.1016/j.jep.2023.117123.
Qi J, Meng C, Mo J, Shou T, Ding L, Zhi T. CircAFF2 Promotes Neuronal Cell Injury in Intracerebral Hemorrhage by Regulating the miR-488/CLSTN3 Axis. Neuroscience. 2023;15(535):75–87. https://doi.org/10.1016/j.neuroscience.2023.10.014.
Chen H, Ren L, Ma W. Mechanism of SOX10 in ferroptosis of hippocampal neurons after intracerebral hemorrhage via the miR-29a-3p/ACSL4 axis. J Neurophysiol. 2023;129(4):862–71. https://doi.org/10.1152/jn.00374.2022.
Wang J, Teng F, Liu S, Pan X, Yang B, Wu W. lncRNA SND1-IT1 delivered via intracerebral hemorrhage-derived exosomes affect the growth of human microglia by regulating the miR-124-3p/MTF1 axis. J Cell Physiol. 2023;238(2):366–78. https://doi.org/10.1002/jcp.30930.
Guo M, Ge X, Wang C, Yin Z, Jia Z, Hu T, Li M, Wang D, Han Z, Wang L, **ong X, Chen F, Lei P. Intranasal delivery of gene-edited microglial exosomes improves neurological outcomes after intracerebral hemorrhage by regulating neuroinflammation. Brain Sci. 2023;13(4):639. https://doi.org/10.3390/brainsci13040639.
Tang J, Yan B, Tang Y, Zhou X, Ji Z, Xu F. Baicalein ameliorates oxidative stress and brain injury after intracerebral hemorrhage by activating the Nrf2/ARE pathway via miR-106a-5p/PHLPP2 axis. Int J Neurosci. 2023;133(12):1380–93. https://doi.org/10.1080/00207454.2022.2080676.
Yu N, Tian W, Liu C, Zhang P, Zhao Y, Nan C, ** Q, Li X, Liu Y. miR-122-5p Promotes Peripheral and Central Nervous System Inflammation in a Mouse Model of Intracerebral Hemorrhage via Disruption of the MLLT1/PI3K/AKT Signaling. Neurochem Res. 2023;48(12):3665–82. https://doi.org/10.1007/s11064-023-04014-7.
Wang BQ, He M, Wang Y, Liu S, Guo ZW, Liu ZL. Hyperbaric oxygen ameliorates neuronal injury and neurological function recovery in rats with intracerebral hemorrhage by silencing microRNA-204-5p-targeted chloride channel protein 3. J Physiol Pharmacol. 2023;74(3). https://doi.org/10.26402/jpp.2023.3.09
Zhao Y, Gan L, Ren L, Lin Y, Ma C, Lin X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022;1(1788):147937. https://doi.org/10.1016/j.brainres.2022.147937.
Almutairi MM, Gong C, Xu YG, Chang Y, Shi H. Factors controlling permeability of the blood-brain barrier. Cell Mol Life Sci. 2016;73(1):57–77. https://doi.org/10.1007/s00018-015-2050-8.
Todoran R, Falcione SR, Clarke M, Joy T, Boghozian R, Jickling GC. microRNA as a therapeutic for ischemic stroke. Neurochem Int. 2023;163:105487. https://doi.org/10.1016/j.neuint.2023.105487.
Zeng X, He G, Yang X, Xu G, Tang Y, Li H, Yu B, Wang Z, Xu W, Song K. Zebularine protects against blood-brain-barrier (BBB) disruption through increasing the expression of zona occludens-1 (ZO-1) and vascular endothelial (VE)-cadherin. Bioengineered. 2022;13(2):4441–54. https://doi.org/10.1080/21655979.2021.2024323.
Ren S, Han S, Wang L, Huang Y, Wu J, Wu G. Minimally invasive surgery for ICH evacuation combined with deferoxamine treatment increased perihematomal claudin-5 and ZO-1 expression levels and decreased BBB permeability in rabbits. Front Neurol. 2022;3(13):835494. https://doi.org/10.3389/fneur.2022.835494.
Wu J, He J, Tian X, Luo Y, Zhong J, Zhang H, Li H, Cen B, Jiang T, Sun X. microRNA-9-5p alleviates blood-brain barrier damage and neuroinflammation after traumatic brain injury. J Neurochem. 2020;153(6):710–26. https://doi.org/10.1111/jnc.14963.
Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H, Moorhouse AJ, Nabekura J, Wake H. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 2019;10(1):5816. https://doi.org/10.1038/s41467-019-13812-z.
Gu L, Sun M, Li R, Zhang X, Tao Y, Yuan Y, Luo X, **e Z. Didymin suppresses microglia pyroptosis and neuroinflammation through the Asc/Caspase-1/GSDMD pathway following experimental intracerebral hemorrhage. Front Immunol. 2022;27(13):810582. https://doi.org/10.3389/fimmu.2022.810582.
Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA, Guerrero-Rodriguez JF, Martinez-Avila N, Martinez-Fierro ML. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int J Mol Sci. 2020;21(24):9739. https://doi.org/10.3390/ijms21249739.
Rempe RG, Hartz AMS, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36(9):1481–507. https://doi.org/10.1177/0271678X16655551.
Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, Sharp FR. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. 2014;34(2):185–99. https://doi.org/10.1038/jcbfm.2013.203.
Wang Y, Wang MD, **a YP, Gao Y, Zhu YY, Chen SC, Mao L, He QW, Yue ZY, Hu B. MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J. 2018;32(2):935–44. https://doi.org/10.1096/fj.201700139RRR.
Simats A, Liesz A. Systemic inflammation after stroke: implications for post-stroke comorbidities. EMBO Mol Med. 2022;14(9):e16269. https://doi.org/10.15252/emmm.202216269.
Kumar V. Toll-like receptors in the pathogenesis of neuroinflammation. J Neuroimmunol. 2019;15(332):16–30. https://doi.org/10.1016/j.jneuroim.2019.03.012.
Tajalli-Nezhad S, Karimian M, Beyer C, Atlasi MA, Azami Tameh A. The regulatory role of Toll-like receptors after ischemic stroke: neurosteroids as TLR modulators with the focus on TLR2/4. Cell Mol Life Sci. 2019;76(3):523–37. https://doi.org/10.1007/s00018-018-2953-2.
Shi K, Zou M, Jia DM, Shi S, Yang X, Liu Q, Dong JF, Sheth KN, Wang X, Shi FD. tPA mobilizes immune cells that exacerbate hemorrhagic transformation in stroke. Circ Res. 2021;128(1):62–75. https://doi.org/10.1161/CIRCRESAHA.120.317596.
Zhang F, Zhang C. Rnf112 deletion protects brain against intracerebral hemorrhage (ICH) in mice by inhibiting TLR-4/NF-κB pathway. Biochem Biophys Res Commun. 2018;507(1–4):43–50. https://doi.org/10.1016/j.bbrc.2018.10.141.
Chen S, Peng J, Sherchan P, Ma Y, **ang S, Yan F, Zhao H, Jiang Y, Wang N, Zhang JH, Zhang H. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J Neuroinflammation. 2020;17(1):168. https://doi.org/10.1186/s12974-020-01853-x.
Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J Stroke. 2020;22(1):29–46. https://doi.org/10.5853/jos.2019.02236.
Stamova B, Ander BP, Jickling G, Hamade F, Durocher M, Zhan X, Liu DZ, Cheng X, Hull H, Yee A, Ng K, Shroff N, Sharp FR. The intracerebral hemorrhage blood transcriptome in humans differs from the ischemic stroke and vascular risk factor control blood transcriptomes. J Cereb Blood Flow Metab. 2019;39(9):1818–35. https://doi.org/10.1177/0271678X18769513.
Zhang L, Chopp M, Liu X, Teng H, Tang T, Kassis H, Zhang ZG. Combination therapy with VELCADE and tissue plasminogen activator is neuroprotective in aged rats after stroke and targets microRNA-146a and the toll-like receptor signaling pathway. Arterioscler Thromb Vasc Biol. 2012;32(8):1856–64. https://doi.org/10.1161/ATVBAHA.112.252619.
Hu J, Huang S, Liu X, Zhang Y, Wei S, Hu X. miR-155: an important role in inflammation response. J Immunol Res. 2022;6(2022):7437281. https://doi.org/10.1155/2022/7437281.
Zheng Y, **ong S, Jiang P, Liu R, Liu X, Qian J, Zheng X, Chu Y. Glucocorticoids inhibit lipopolysaccharide-mediated inflammatory response by downregulating microRNA-155: a novel anti-inflammation mechanism. Free Radic Biol Med. 2012;52(8):1307–17. https://doi.org/10.1016/j.freeradbiomed.2012.01.031.
Chinenov Y, Coppo M, Gupte R, Sacta MA, Rogatsky I. Glucocorticoid receptor coordinates transcription factor-dominated regulatory network in macrophages. BMC Genomics. 2014;15(1):656. https://doi.org/10.1186/1471-2164-15-656.
Su W, Aloi MS, Garden GA. MicroRNAs mediating CNS inflammation: small regulators with powerful potential. Brain Behav Immun. 2016;52:1–8. https://doi.org/10.1016/j.bbi.2015.07.003.
Magid-Bernstein J, Girard R, Polster S, Srinath A, Romanos S, Awad IA, Sansing LH. Cerebral hemorrhage: pathophysiology, treatment, and future directions. Circ Res. 2022;130(8):1204–29. https://doi.org/10.1161/CIRCRESAHA.121.319949.
Wan J, Ren H, Wang J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc Neurol. 2019;4(2):93–5. https://doi.org/10.1136/svn-2018-000205.
Alkadi H. A review on free radicals and antioxidants. Infect Disord Drug Targets. 2020;20(1):16–26. https://doi.org/10.2174/1871526518666180628124323.
Yao H, Ago T, Kitazono T, Nabika T. NADPH oxidase-related pathophysiology in experimental models of stroke. Int J Mol Sci. 2017;18(10):2123. https://doi.org/10.3390/ijms18102123.
Chen-Roetling J, Regan RF. Targeting the Nrf2-Heme Oxygenase-1 Axis after Intracerebral Hemorrhage. Curr Pharm Des. 2017;23(15):2226–37. https://doi.org/10.2174/1381612822666161027150616.
Huang L, Ma Q, Li Y, Li B, Zhang L. Inhibition of microRNA-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp Neurol. 2018;300:41–50. https://doi.org/10.1016/j.expneurol.2017.10.024.
Modak JM, Roy-O’Reilly M, Zhu L, Staff I, McCullough LD. Differential MicroRibonucleic acid expression in cardioembolic stroke. J Stroke Cerebrovasc Dis. 2019;28(1):121–4. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.09.018.
Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release. 2013;172(3):962–74. https://doi.org/10.1016/j.jconrel.2013.09.015.
Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–65. https://doi.org/10.1002/jcp.27486.
Vishnoi A, Rani S. miRNA biogenesis and regulation of diseases: an updated overview. Methods Mol Biol. 2023;2595:1–12. https://doi.org/10.1007/978-1-0716-2823-2_1.
Agaverdiev M, Shamsov B, Mirzoev S, Vardikyan A, Ramirez ME, Nurmukhametov R, Beilerli A, Zhang B, Gareev I, Pavlov V. MiRNA regulated therapeutic potential of the stromal vascular fraction: Current clinical applications - a systematic review. Noncoding RNA Res. 2022;8(2):146–54. https://doi.org/10.1016/j.ncrna.2022.12.003.
Wang P, Zhou Y, Richards AM. Effective tools for RNA-derived therapeutics: siRNA interference or miRNA mimicry. Theranostics. 2021;11(18):8771–96. https://doi.org/10.7150/thno.62642.
Beylerli O, Sufianova G, Shumadalova A, Zhang D, Gareev I. MicroRNAs-mediated regulation of glucose transporter (GLUT) expression in glioblastoma. Noncoding RNA Res. 2022;7(4):205–11. https://doi.org/10.1016/j.ncrna.2022.09.001.
Henning RJ. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. J Cardiovasc Transl Res. 2021;14(2):195–212. https://doi.org/10.1007/s12265-020-10040-5.
Sørensen SS, Nygaard AB, Nielsen MY, Jensen K, Christensen T. miRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl Stroke Res. 2014;5(6):711–8. https://doi.org/10.1007/s12975-014-0364-8.
Bulygin KV, Beeraka NM, Saitgareeva AR, Nikolenko VN, Gareev I, Beylerli O, Akhmadeeva LR, Mikhaleva LM, Torres Solis LF, Solís Herrera A, Avila-Rodriguez MF, Somasundaram SG, Kirkland CE, Aliev G. Can miRNAs be considered as diagnostic and therapeutic molecules in ischemic stroke pathogenesis?-Current status. Int J Mol Sci. 2020;21(18):6728. https://doi.org/10.3390/ijms21186728.
Menon A, Abd-Aziz N, Khalid K, Poh CL, Naidu R. miRNA: a promising therapeutic target in cancer. Int J Mol Sci. 2022;23(19):11502. https://doi.org/10.3390/ijms231911502.
Ban E, Kwon TH, Kim A. Delivery of therapeutic miRNA using polymer-based formulation. Drug Deliv Transl Res. 2019;9(6):1043–56. https://doi.org/10.1007/s13346-019-00645-y.
Kara G, Calin GA, Ozpolat B. RNAi-based therapeutics and tumor targeted delivery in cancer. Adv Drug Deliv Rev. 2022;182:114113. https://doi.org/10.1016/j.addr.2022.114113.
Asakiya C, Zhu L, Yuhan J, Zhu L, Huang K, Xu W. Current progress of miRNA-derivative nucleotide drugs: modifications, delivery systems, applications. Expert Opin Drug Deliv. 2022;19(4):435–50. https://doi.org/10.1080/17425247.2022.2063835.
Traber GM, Yu AM. RNAi-based therapeutics and novel RNA bioengineering technologies. J Pharmacol Exp Ther. 2023;384(1):133–54. https://doi.org/10.1124/jpet.122.001234.
Bajan S, Hutvagner G. RNA-based therapeutics: from antisense oligonucleotides to miRNAs. Cells. 2020;9(1):137. https://doi.org/10.3390/cells9010137.
Munir J, Yoon JK, Ryu S. Therapeutic miRNA-enriched extracellular vesicles: current approaches and future prospects. Cells. 2020;9(10):2271. https://doi.org/10.3390/cells9102271.
Kamtchum-Tatuene J, Jickling GC. Blood biomarkers for stroke diagnosis and management. Neuromolecular Med. 2019;21(4):344–68. https://doi.org/10.1007/s12017-019-08530-0.
Dias A, Silva L, Moura J, Gabriel D, Maia LF. Fluid biomarkers in stroke: From animal models to clinical care. Acta Neurol Scand. 2022;146(4):332–47. https://doi.org/10.1111/ane.13668.
Ng GJL, Quek AML, Cheung C, Arumugam TV, Seet RCS. Stroke biomarkers in clinical practice: a critical appraisal. Neurochem Int. 2017;107:11–22. https://doi.org/10.1016/j.neuint.2017.01.005.
Karceski S. Biomarkers and stroke: can we determine who is at risk? Neurology. 2022;98(17):e1794–7. https://doi.org/10.1212/WNL.0000000000200381.
Aronson JK, Ferner RE. Biomarkers-A General Review. Curr Protoc Pharmacol. 2017;76:9.23.1-9.23.17. https://doi.org/10.1002/cpph.19.
Bhatia R, Warrier AR, Sreenivas V, Bali P, Sisodia P, Gupta A, Singh N, Padma Srivastava MV, Prasad K. Role of Blood Biomarkers in Differentiating Ischemic Stroke and Intracerebral Hemorrhage. Neurol India. 2020;68(4):824–9. https://doi.org/10.4103/0028-3886.293467.
Gareev I, Beylerli O, Yang G, Izmailov A, Shi H, Sun J, Zhao B, Liu B, Zhao S. Diagnostic and prognostic potential of circulating miRNAs for intracranial aneurysms. Neurosurg Rev. 2021;44(4):2025–39. https://doi.org/10.1007/s10143-020-01427-8.
Gareev I, Pavlov V, Du W, Yang B. MiRNAs and their role in venous thromboembolic complications. Diagnostics (Basel). 2023;13(21):3383. https://doi.org/10.3390/diagnostics13213383.
Theofilatos K, Korfiati A, Mavroudi S, Cowperthwaite MC, Shpak M. Discovery of stroke-related blood biomarkers from gene expression network models. BMC Med Genomics. 2019;12(1):118. https://doi.org/10.1186/s12920-019-0566-8.
Chang JJ, Emanuel BA, Mack WJ, Tsivgoulis G, Alexandrov AV. Matrix metalloproteinase-9: dual role and temporal profile in intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2014;23(10):2498–505. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.07.005.
Gyldenholm T, Hvas CL, Hvas AM, Hviid CVB. Serum glial fibrillary acidic protein (GFAP) predicts outcome after intracerebral and subarachnoid hemorrhage. Neurol Sci. 2022;43(10):6011–9. https://doi.org/10.1007/s10072-022-06274-7.
Zhou Q, Zhang D, Chen X, Yang Z, Liu Z, Wei B, ** M, Feng K, Guo C, Sun J, Chen S, Zhang R, Piao X, Gareev I, Sun Z, Wang X, Li L, Zhao S, Yang G. Plasma D-dimer predicts poor outcome and mortality after spontaneous intracerebral hemorrhage. Brain Behav. 2021;11(1):462–8. https://doi.org/10.1002/brb3.1946.
Sahu A, Jha PK, Prabhakar A, Singh HD, Gupta N, Chatterjee T, Tyagi T, Sharma S, Kumari B, Singh S, Nair V, Goel S, Ashraf MZ. MicroRNA-145 impedes thrombus formation via targeting tissue factor in venous thrombosis. EBioMedicine. 2017;26:175–86. https://doi.org/10.1016/j.ebiom.2017.11.022.
Tobieson L, Gard A, Ruscher K, Marklund N. Intracerebral proinflammatory cytokine increase in surgically evacuated intracerebral hemorrhage: a microdialysis study. Neurocrit Care. 2022;36(3):876–87. https://doi.org/10.1007/s12028-021-01389-9.
Branyan TE, Selvamani A, Park MJ, Korula KE, Kosel KF, Srinivasan R, Sohrabji F. Functional assessment of stroke-induced regulation of miR-20a-3p and its role as a neuroprotectant. Transl Stroke Res. 2022;13(3):432–48. https://doi.org/10.1007/s12975-021-00945-x.
Xue WS, Wang N, Wang NY, Ying YF, Xu GH. miR-145 protects the function of neuronal stem cells through targeting MAPK pathway in the treatment of cerebral ischemic stroke rat. Brain Res Bull. 2019;144:28–38. https://doi.org/10.1016/j.brainresbull.2018.08.023.
Shan Y, Hu J, Lv H, Cui X, Di W. miR-221 Exerts neuroprotective effects in ischemic stroke by inhibiting the proinflammatory response. J Stroke Cerebrovasc Dis. 2021;30(2):105489. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105489.
Pavlov V, Beylerli O, Gareev I, Torres Solis LF, Solís Herrera A, Aliev G. COVID-19-Related Intracerebral Hemorrhage. Front Aging Neurosci. 2020;22(12):600172. https://doi.org/10.3389/fnagi.2020.600172.
Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, ** G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology. 2018;134(Pt B):240–8. https://doi.org/10.1016/j.neuropharm.2017.09.033.
Nobleza COS. Intracerebral Hemorrhage. Continuum (Minneap Minn). 2021;27(5):1246–77. https://doi.org/10.1212/CON.0000000000001018.
Egashira Y, Hua Y, Keep RF, ** G. Intercellular cross-talk in intracerebral hemorrhage. Brain Res. 2015;14(1623):97–109. https://doi.org/10.1016/j.brainres.2015.04.003.
Laso-García F, Piniella D, Gómez-de Frutos MC, Casado-Fernández L, Pérez-Mato M, Alonso-López E, Otero-Ortega L, Bravo SB, Chantada-Vázquez MDP, Trilla-Fuertes L, Fresno-Vara JÁ, Fuentes B, Díez-Tejedor E, Gutiérrez-Fernández M, Alonso De Leciñana M. Protein content of blood-derived extracellular vesicles: An approach to the pathophysiology of cerebral hemorrhage. Front Cell Neurosci. 2023;16:1058546. https://doi.org/10.3389/fncel.2022.1058546.
Yi X, Tang X. Exosomes from miR-19b-3p-modified ADSCs inhibit ferroptosis in intracerebral hemorrhage mice. Front Cell Dev Biol. 2021;7(9):661317. https://doi.org/10.3389/fcell.2021.661317.
Rufino-Ramos D, Albuquerque PR, Carmona V, Perfeito R, Nobre RJ, Pereira de Almeida L. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J Control Release. 2017;262:247-258. https://doi.org/10.1016/j.jconrel.2017.07.001.
Bahram Sangani N, Gomes AR, Curfs LMG, Reutelingsperger CP. The role of Extracellular Vesicles during CNS development. Prog Neurobiol. 2021;205:102124. https://doi.org/10.1016/j.pneurobio.2021.102124.
Zhu Y, Wang JL, He ZY, ** F, Tang L. Association of Altered Serum MicroRNAs with Perihematomal Edema after Acute Intracerebral Hemorrhage. PLoS One. 2015;10(7):e0133783. https://doi.org/10.1371/journal.pone.0133783.
Gareev I, Yang G, Sun J, Beylerli O, Chen X, Zhang D, Zhao B, Zhang R, Sun Z, Yang Q, Li L, Pavlov V, Safin S, Zhao S. Circulating MicroRNAs as potential noninvasive biomarkers of spontaneous intracerebral hemorrhage. World Neurosurg. 2020;133:e369–75. https://doi.org/10.1016/j.wneu.2019.09.016.
Guo D, Liu J, Wang W, Hao F, Sun X, Wu X, Bu P, Zhang Y, Liu Y, Liu F, Zhang Q, Jiang F. Alteration in abundance and compartmentalization of inflammation-related miRNAs in plasma after intracerebral hemorrhage. Stroke. 2013;44(6):1739–42. https://doi.org/10.1161/STROKEAHA.111.000835.
Zheng HW, Wang YL, Lin JX, Li N, Zhao XQ, Liu GF, Liu LP, Jiao Y, Gu WK, Wang DZ, Wang YJ. Circulating MicroRNAs as potential risk biomarkers for hematoma enlargement after intracerebral hemorrhage. CNS Neurosci Ther. 2012;18(12):1003–11. https://doi.org/10.1111/cns.12019.
Wang Z, Lu G, Sze J, Liu Y, Lin S, Yao H, Zhang J, **e D, Liu Q, Kung HF, Lin MC, Poon WS. Plasma miR-124 Is a Promising Candidate Biomarker for Human Intracerebral Hemorrhage Stroke. Mol Neurobiol. 2018;55(7):5879–88. https://doi.org/10.1007/s12035-017-0808-8.
Robles D, Guo DH, Watson N, Asante D, Sukumari-Ramesh S. Dysregulation of Serum MicroRNA after Intracerebral Hemorrhage in Aged Mice. Biomedicines. 2023;11(3):822. https://doi.org/10.3390/biomedicines11030822.
Cheng X, Ander BP, Jickling GC, Zhan X, Hull H, Sharp FR, Stamova B. MicroRNA and their target mRNAs change expression in whole blood of patients after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2020;40(4):775–86. https://doi.org/10.1177/0271678X19839501.
Iwuchukwu I, Nguyen D, Beavers M, Tran V, Sulaiman W, Fannin E, Lasseigne L, Ramsay E, Wilson J, Bazan NG. MicroRNA Regulatory Network as Biomarkers of Late Seizure in Patients with Spontaneous Intracerebral Hemorrhage. Mol Neurobiol. 2020;57(5):2346–57. https://doi.org/10.1007/s12035-020-01872-y.
Hao Y, Xu X, Wang Y, ** F, Tang L, Zheng W, Zhang H, He Z. Comprehensive analysis of immune-related biomarkers and pathways in intracerebral hemorrhage using weighted gene co-expression network analysis and competing endogenous ribonucleic acid. Front Mol Neurosci. 2022;26(15):955818. https://doi.org/10.3389/fnmol.2022.955818.
Kalani MYS, Alsop E, Meechoovet B, Beecroft T, Agrawal K, Whitsett TG, Huentelman MJ, Spetzler RF, Nakaji P, Kim S, Van Keuren-Jensen K. Extracellular microRNAs in blood differentiate between ischaemic and haemorrhagic stroke subtypes. J Extracell Vesicles. 2020;9(1):1713540. https://doi.org/10.1080/20013078.2020.1713540.
Cepparulo P, Cuomo O, Vinciguerra A, Torelli M, Annunziato L, Pignataro G. Hemorrhagic Stroke Induces a Time-Dependent Upregulation of miR-150-5p and miR-181b-5p in the Bloodstream. Front Neurol. 2021;27(12):736474. https://doi.org/10.3389/fneur.2021.736474.
Fejes Z, Erdei J, Pócsi M, Takai J, Jeney V, Nagy A, Varga A, Bácsi A, Bognár L, Novák L, Kappelmayer J, Nagy B Jr. Elevated Pro-Inflammatory Cell-Free MicroRNA Levels in Cerebrospinal Fluid of Premature Infants after Intraventricular Hemorrhage. Int J Mol Sci. 2020;21(18):6870. https://doi.org/10.3390/ijms21186870.
Wang J, Zhu Y, ** F, Tang L, He Z, He Z. Differential expression of circulating microRNAs in blood and haematoma samples from patients with intracerebral haemorrhage. J Int Med Res. 2016;44(3):419–32. https://doi.org/10.1177/0300060516630852.
Leung LY, Chan CP, Leung YK, Jiang HL, Abrigo JM, de Wang F, Chung JS, Rainer TH, Graham CA. Comparison of miR-124-3p and miR-16 for early diagnosis of hemorrhagic and ischemic stroke. Clin Chim Acta. 2014;10(433):139–44. https://doi.org/10.1016/j.cca.2014.03.007.
Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14(4):300–6. https://doi.org/10.1007/s11883-012-0252-1.
Keep RF, Zhou N, **ang J, Andjelkovic AV, Hua Y, ** G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;10(11):18. https://doi.org/10.1186/2045-8118-11-18.
Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. 2014;41(1):47–67. https://doi.org/10.1016/j.clp.2013.09.007.
Nishiguchi T, Imanishi T, Akasaka T. MicroRNAs and cardiovascular diseases. Biomed Res Int. 2015;2015:682857. https://doi.org/10.1155/2015/682857.
Tiwari A, Mukherjee B, Dixit M. MicroRNA Key to Angiogenesis Regulation: MiRNA Biology and Therapy. Curr Cancer Drug Targets. 2018;18(3):266–77. https://doi.org/10.2174/1568009617666170630142725.
Song Z, Li G. Role of specific microRNAs in regulation of vascular smooth muscle cell differentiation and the response to injury. J Cardiovasc Transl Res. 2010;3(3):246–50. https://doi.org/10.1007/s12265-010-9163-0.
Hartmann D, Thum T. MicroRNAs and vascular (dys)function. Vascul Pharmacol. 2011;55(4):92–105. https://doi.org/10.1016/j.vph.2011.07.005.
Fernández-Hernando C, Suárez Y. MicroRNAs in endothelial cell homeostasis and vascular disease. Curr Opin Hematol. 2018;25(3):227–36. https://doi.org/10.1097/MOH.0000000000000424.
Schulte C, Karakas M, Zeller T. microRNAs in cardiovascular disease - clinical application. Clin Chem Lab Med. 2017;55(5):687–704. https://doi.org/10.1515/cclm-2016-0576.
Chen Y, Chen S, Chang J, Wei J, Feng M, Wang R. Perihematomal edema after intracerebral hemorrhage: an update on pathogenesis, risk factors, and therapeutic advances. Front Immunol. 2021;19(12):740632. https://doi.org/10.3389/fimmu.2021.740632.
Zille M, Farr TD, Keep RF, Römer C, ** G, Boltze J. Novel targets, treatments, and advanced models for intracerebral haemorrhage. EBioMedicine. 2022;76:103880. https://doi.org/10.1016/j.ebiom.2022.103880.
Huber C, Friede T, Stingl J, Benda N. Classification of companion diagnostics: a new framework for biomarker-driven patient selection. Ther Innov Regul Sci. 2022;56(2):244–54. https://doi.org/10.1007/s43441-021-00352-2.
Wu J, Al-Zahrani A, Beylerli O, Sufianov R, Talybov R, Meshcheryakova S, Sufianova G, Gareev I, Sufianov A. Circulating miRNAs as diagnostic and prognostic biomarkers in high-grade gliomas. Front Oncol. 2022;12(12):898537. https://doi.org/10.3389/fonc.2022.898537.
Gareev I, Beylerli O, Liang Y, Lu E, Ilyasova T, Sufianov A, Sufianova G, Shi H, Ahmad A, Yang G. The role of mitochondria-targeting miRNAs in intracerebral hemorrhage. Curr Neuropharmacol. 2023;21(5):1065–80. https://doi.org/10.2174/1570159X20666220507021445.
Gu X, Chen A, Su Y, You M, Guo H, Tan S, He Q, Hu B. Extracellular vesicles: a new communication paradigm of complement in neurological diseases. Brain Res Bull. 2023;199:110667. https://doi.org/10.1016/j.brainresbull.2023.110667.
Xu M, Feng T, Liu B, Qiu F, Xu Y, Zhao Y, Zheng Y. Engineered exosomes: desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics. 2021;11(18):8926–44. https://doi.org/10.7150/thno.62330.
Lu Z, Tang H, Li S, Zhu S, Li S, Huang Q. Role of circulating exosomes in cerebrovascular diseases: a comprehensive review. Curr Neuropharmacol. 2023;21(7):1575–93. https://doi.org/10.2174/1570159X21666230214112408.
Aslani M, Mortazavi-Jahromi SS, Mirshafiey A. Efficient roles of miR-146a in cellular and molecular mechanisms of neuroinflammatory disorders: an effectual review in neuroimmunology. Immunol Lett. 2021;238:1–20. https://doi.org/10.1016/j.imlet.2021.07.004.
Slota JA, Booth SA. MicroRNAs in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Noncoding RNA. 2019;5(2):35. https://doi.org/10.3390/ncrna5020035.
Zhao J, He Z, Wang J. MicroRNA-124: a key player in microglia-mediated inflammation in neurological diseases. Front Cell Neurosci. 2021;2(15):771898. https://doi.org/10.3389/fncel.2021.771898.
Cardoso AL, Guedes JR, de Lima MC. Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions. Curr Opin Pharmacol. 2016;26:1–9. https://doi.org/10.1016/j.coph.2015.09.001.
Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. https://doi.org/10.1016/j.redox.2018.11.017.
Wu D, Cerutti C, Lopez-Ramirez MA, Pryce G, King-Robson J, Simpson JE, van der Pol SM, Hirst MC, de Vries HE, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA. Brain endothelial miR-146a negatively modulates T-cell adhesion through repressing multiple targets to inhibit NF-κB activation. J Cereb Blood Flow Metab. 2015;35(3):412–23. https://doi.org/10.1038/jcbfm.2014.207.
Kijima C, Inaba T, Hira K, Miyamoto N, Yamashiro K, Urabe T, Hattori N, Ueno Y. Astrocytic extracellular vesicles regulated by microglial inflammatory responses improve stroke recovery. Mol Neurobiol. 2023. https://doi.org/10.1007/s12035-023-03629-9.
Chu B, Zhou Y, Zhai H, Li L, Sun L, Li Y. The role of microRNA-146a in regulating the expression of IRAK1 in cerebral ischemia-reperfusion injury. Can J Physiol Pharmacol. 2018;96(6):611–7. https://doi.org/10.1139/cjpp-2017-0586.
Iyer A, Zurolo E, Prabowo A, Fluiter K, Spliet WG, van Rijen PC, Gorter JA, Aronica E. MicroRNA-146a: a key regulator of astrocyte-mediated inflammatory response. PLoS One. 2012;7(9):e44789. https://doi.org/10.1371/journal.pone.0044789.
Lang F, Guelinckx I, Lemetais G, Melander O. Two liters a day keep the doctor away? Considerations on the pathophysiology of suboptimal fluid intake in the common population. Kidney Blood Press Res. 2017;42(3):483–94. https://doi.org/10.1159/000479640.
Mavroudis I, Balmus IM, Ciobica A, Nicoara MN, Luca AC, Palade DO. The role of microglial exosomes and miR-124-3p in neuroinflammation and neuronal repair after traumatic brain injury. Life (Basel). 2023;13(9):1924. https://doi.org/10.3390/life13091924.
Bao WD, Zhou XT, Zhou LT, Wang F, Yin X, Lu Y, Zhu LQ, Liu D. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020;19(11):e13235. https://doi.org/10.1111/acel.13235.
Sun M, Hou X, Ren G, Zhang Y, Cheng H. Dynamic changes in miR-124 levels in patients with acute cerebral infarction. Int J Neurosci. 2019;129(7):649–53. https://doi.org/10.1080/00207454.2018.1513931.
Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao H, Xu T, Chen L, Xu Y. Increased brain-Specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One. 2016;11(9):e0163645. https://doi.org/10.1371/journal.pone.0163645.].
Zhou X, Qi L. miR-124 is downregulated in serum of acute cerebral infarct patients and shows diagnostic and prognostic value. Clin Appl Thromb Hemost. 2021;27:10760296211035446. https://doi.org/10.1177/10760296211035446.
Welten SM, Goossens EA, Quax PH, Nossent AY. The multifactorial nature of microRNAs in vascular remodelling. Cardiovasc Res. 2016;110(1):6–22. https://doi.org/10.1093/cvr/cvw039.
Yu B, Jiang Y, Wang X, Wang S. An integrated hypothesis for miR-126 in vascular disease. Med Res Arch. 2020;8(5):2133. https://doi.org/10.18103/mra.v8i5.2133.
Zhuang Y, Peng H, Mastej V, Chen W. MicroRNA regulation of endothelial junction proteins and clinical consequence. Mediators Inflamm. 2016;2016:5078627. https://doi.org/10.1155/2016/5078627.
Tang Y, Chen Y, Guo Q, Zhang L, Liu H, Wang S, Wu X, Shen X, Tao L. MiR-126-loaded immunoliposomes against vascular endothelial inflammation in vitro and vivo evaluation. Pharmaceutics. 2023;15(5):1379. https://doi.org/10.3390/pharmaceutics15051379.
Acknowledgements
We sincerely appreciate the enormous amount of time and effort expended by editorial board and peer reviewers.
Funding
This work was supported by the Innovative Scientific Research Foundation of Harbin Medical University, Project No. 2021-KYYWF-0222 and by the Bashkir State Medical University Strategic Academic Leadership Program (PRIORITY-2030)
Author information
Authors and Affiliations
Contributions
Ilgiz Gareev: conceptualization, writing – original draft, and project administration. Boxian Zhao and Ozal Beylerli: writing – review and editing, and investigation. Ilgiz Gareev and Ozal Beylerli: resources and data curation. Boxian Zhao: validation and visualization. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gareev, I., Beylerli, O. & Zhao, B. MiRNAs as potential therapeutic targets and biomarkers for non-traumatic intracerebral hemorrhage. Biomark Res 12, 17 (2024). https://doi.org/10.1186/s40364-024-00568-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-024-00568-y