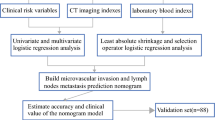
Abstract
Background and aim
The presence of microvascular invasion (MVI) will impair the surgical outcome of hepatocellular carcinoma (HCC). Adipose and muscle tissues have been confirmed to be associated with the prognosis of HCC. We aimed to develop and validate a nomogram based on adipose and muscle related-variables for preoperative prediction of MVI in HCC.
Methods
One hundred fifty-eight HCC patients from institution A (training cohort) and 53 HCC patients from institution B (validation cohort) were included, all of whom underwent preoperative CT scan and curative resection with confirmed pathological diagnoses. Least absolute shrinkage and selection operator (LASSO) logistic regression was applied to data dimensionality reduction and screening. Nomogram was constructed based on the independent variables, and evaluated by external validation, calibration curve, receiver operating characteristic (ROC) curve and decision curve analysis (DCA).
Results
Histopathologically identified MVI was found in 101 of 211 patients (47.9%). The preoperative imaging and clinical variables associated with MVI were visceral adipose tissue (VAT) density, intramuscular adipose tissue index (IMATI), skeletal muscle (SM) area, age, tumor size and cirrhosis. Incorporating these 6 factors, the nomogram achieved good concordance index of 0.79 (95%CI: 0.72–0.86) and 0.75 (95%CI: 0.62–0.89) in training and validation cohorts, respectively. In addition, calibration curve exhibited good consistency between predicted and actual MVI probabilities. ROC curve and DCA of the nomogram showed superior performance than that of models only depended on clinical or imaging variables. Based on the nomogram score, patients were divided into high (> 273.8) and low (< = 273.8) risk of MVI presence groups. For patients with high MVI risk, wide-margin resection or anatomical resection could significantly improve the 2-year recurrence free survival.
Conclusion
By combining 6 preoperative independently predictive factors of MVI, a nomogram was constructed. This model provides an optimal preoperative estimation of MVI risk in HCC patients, and may help to stratify high-risk individuals and optimize clinical decision making.
Similar content being viewed by others
Introduction
Hepatocellular carcinoma (HCC) represents the most frequent type of primary liver cancer and ranks as the third leading cause of cancer-related death worldwide [1]. Surgical resection is the backbone of curative treatment for HCC. Although the surgical operation of HCC has been extremely developed, the prognosis of HCC is still very poor [2]. The recurrence after operation remains a major obstacle to improve survival and the 5-year recurrence rates are up to 70%, even in patients with single small HCC (≤ 2 cm) [3]. One of the main reasons is microvascular invasion (MVI). MVI, a histopathological feature of micro-metastases, can be observed in 15.0%-57.1% of surgical specimens and stands as a key predictor for early recurrence and poor prognosis [4, 5]. In addition, the presence of MVI has been an essential factor influencing the long-term prognosis of HCC patients underwent liver transplantation [6]. However, the impact of MVI has largely been disregarded by clinicians as it can only be found by histologic examination of postoperative specimens. As MVI represents an aggressive behavior of HCC [5], individualized surgical management for patients should be formulated according to the risk–benefit assessment of MVI before surgery. For patients with high risk of MVI, anatomical subsegmentectomy or partial hepatectomy with wide margin is recommended under better liver function, which could decrease the risk of recurrence [7]. Therefore, establishing a highly accurate risk model for predicting MVI can help to stratify high-risk individuals, optimize clinical decision making and improve prognosis.
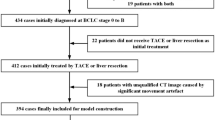
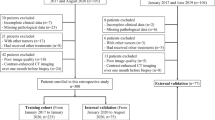
Over the last decades, numerous efforts have been taken to estimate MVI prior to surgery. Cucchetti et al. utilized four clinical common variables, including alpha-fetoprotein (AFP), tumor number, size and volume, to develop an artificial neural network (ANN) model that accurately identified 91% of MVI cases in testing group [8]. However, this ANN model requires specialized computer software, which potentially limits its utility in routine clinical application. In contrast, the nomogram might be a convenient option and provide highly accurate and individualized risk estimate [9]. For hepatitis B virus–related HCC patients, Li et al. constructed a nomogram by incorporating contrast-enhanced magnetic resonance imaging (MRI) data with hematology data, which achieved relatively good predictive accuracy for MVI [ During the study period, 158 HCC patients form institution A and 53 HCC patients form institution B met the inclusion criteria were enrolled. The former was the training cohort with the median follow-up time of 23.9 months, and the latter was the validation cohort with the median follow-up time of 27.1 months. Pathological confirmed MVI was found in 101 (47.9%) individuals of all patients. The detailed clinical, adipose as well as muscle related variables were shown in Table 1, respectively. LASSO regression analysis was performed to identify the most crucial variables linked with the risk of MVI. To guarantee precision, the algorithm's iterations were set to 1000. In addition, cv.glmnet function was used to perform tenfold cross validation to diminish the possibility of overfitting. The distribution of LASSO regression coefficients and cross-validation plots were shown in Fig. 2. Dotted vertical lines indicated the value of lambda.min and lambda.1se. Finally, 18 variables (age, tumor size, CTP, cirrhosis, HBsAg, AFP, TB, resection method, SAT density, VAT area, VAT density, VATI, VSR, IMAT area, IMATI, SM area, SM density, SMI) with nonzero coefficients were selected according to the value of lambda.min (λ = 0.03139931). The above 18 variables were subjected to univariate logistic analysis, and then 8 variables (age, tumor size, cirrhosis, VAT density, IMAT area, IMATI, SM area, SMI) with P-values of less than 0.1 were incorporated into multivariate analysis. Except for IMAT area and SMI, the remaining 6 variables [age (OR = 0.214; 95%CI = 0.088–0.522; P = 0.001), tumor size (OR = 2.359; 95%CI = 1.115–4.989; P = 0.025), cirrhosis (OR = 2.518; 95%CI = 1.077–5.887; P = 0.033), VAT density (OR = 3.468; 95%CI = 1.533–7.847; P = 0.003), IMATI (OR = 4.783; 95%CI = 2.058–11.112; P < 0.001), SM area (OR = 0.295; 95%CI = 0.120–0.725; P = 0.008)] were all significant independent predictors for MVI. The detailed data was shown in Table 2. These Six independent predictors were utilized to form an MVI-predicting nomogram (Fig. 3a). The nomogram provided good accuracy in estimating the risk of MVI, with C index of 0.79 (95%CI: 0.72–0.86), R2 of 0.35, discrimination index (D index) of 0.30, Brier score of 0.18, Emax of 0.05, Evag of 0.02 (Fig. 3b). Following 200 rounds of tenfold internal cross-validation, there was little variation in the values of the above variables (Table 3), with the correct C index of 0.79, R2 of 0.32, D index of 0.24, Brier score of 0.18, Emax of 0.30, Evag of 0.13. In addition, calibration curve exhibited good consistency between predicted MVI probability of nomogram and the actual probability. In validation cohort, the nomogram presented a C index of 0.75 (95%CI: 0.62–0.89) for the evaluation of risk associated with MVI. Moreover, the fitting calibration curve was acceptable (Fig. 3c). The independent predictors of MVI included three clinical variables (age, tumor size and cirrhosis) and three imaging variables (VAT density, IMATI and SM area). These clinical and imaging variables were used separately to construct new clinical and imaging model, which were then compared with this nomogram to verify its clinical utility. The ROC curve indicated that the discriminative ability of the nomogram in predicting MVI was better than that of the imaging and clinical models, with AUC values of 0.791, 0.711, 0.673 and 0.716, 0.654, 0.633 in training and validation cohorts, respectively (Fig. 4a, b). In addition, the DCA of nomogram also exhibited a wider range of threshold probabilities in comparison to the imaging and clinical model (Fig. 4c, d). The optimal cut-off value of the nomogram scores in training cohort was determined to be 273.8, and the sensitivity and specificity for distinguishing the presence from absence of MVI according to this value were 79.7% and 63.3%, respectively (Supplementary Fig. 2). Based on the cut-off value, patients in two cohorts were divided into high (> 273.8) and low (< = 273.8) risk groups. Kaplan–Meier curves showed that in both training and validation cohorts, patients with high-risk of MVI had higher 2-year recurrence rate and shorter OS than patients with low-risk (Fig. 5). For patients with high MVI risk, wide-margin resection or anatomical resection could significantly improve the 2-year RFS, while only wide-margin resection could significantly improve the OS (Figs. 6 and 7). In contrast, for low-risk patients, neither surgical approaches, nor resection methods or surgical margins had impact on recurrence and OS (Supplementary Figs. 3, 4). In order to facilitate the clinical use of the nomogram, we have provided an online version accessible via the hyperlink: https://sdmxc.shinyapps.io/MVInom/. By setting variables greater than or equal to the cut-off value to 1 and those less than it to 0, the predicted probability of MVI for patients can be quickly obtained. In the present study, we showed that adipose and muscle tissues-related variables, including VAT density, IMATI and SM area, were capable to serve as independent predictors for MVI. Furthermore, we incorporated the above variables with three common clinical variables (age, tumor size, cirrhosis) to develop an MVI-predicting model which exhibited good consistency and high accuracy. In the process of validating the clinical utility, our nomogram was also superior to the models only based on imaging or clinical variables. Compared with other radiomics nomograms [11, 12], we only need one plain CT image at L3 level for data extraction, without the need to conduct complex three-dimensional tumor target delineation at various phases of enhanced scanning, which will bring heavy computational burden. Recently, some researchers have employed liver biopsy specimens to analyze gene expression or even radio-genomics for predicting MVI [31, 32]. Despite its good predictive performance, tissue biopsy for the diagnosis of HCC is unnecessary for patients with typical imaging findings according to some clinical guidelines [33, 34]. Additionally, invasive procedure may lead to potential risks such as tumor seeding and fatal bleeding, which outweigh the benefits [34, 35]. In the previously published MVI-predicting nomograms, tumor size, cirrhosis and age has been confirmed as risk factors for vascular invasion in HCC. Pawlik et al. reported that the incidence of MVI was almost twice as high in tumors larger than 5 cm (61%) when compared to smaller tumors (32%), and it increased continuously with increasing tumor size [36]. Cirrhosis patients were frequently present with alterations in hemodynamic and blood microenvironment. On one hand, secondary hypersplenism could decrease platelet counts and reduce portal blood flow to the liver, which was conducive to the formation of MVI [10]. On the other hand, the increase of von Willebrand factor and other procoagulants, as well as the decrease in generation of antithrombin in patients with cirrhosis, could cause an imbalance in the coagulation and fibrinolytic systems [37, 38]. The mechanisms mentioned above could both serve as stimuli for thrombosis and vascular invasion. In current study, younger HCC patients had a higher incidence of MVI, which was in consistent with another research [12]. This observation might be attributed to the high aggressiveness of the tumor in young patients; however, the exact underlying mechanism remains to be investigated by future studies [39]. For body composition-related variables, metabolic theory could support our findings. Because most fatty acids in VAT are transported via the portal vein, higher degree of visceral obesity will expose the liver to high concentrations of fatty acids and glycerol. This, in turn, leads to a series of liver dysfunctions, such as reduced hepatic insulin extraction, increased hepatic glucose production, and ultimately results in type 2 diabetes and insulin resistance [40]. In addition, accumulation of visceral adipose is also linked with the upregulation of aquaporin adipose, an adipocyte-specific glycerol channel, leading to the increased glycerol secretion from VAT [41]. The liver-specific aquaporin protein AQP-9 could absorb excess glycerol and convert it into glycerol phosphate through glycerokinase, which is also one of the mechanisms leading to hyperglycemia [42]. Hyperglycemia alters the size and composition of the basement membranes in micro and large vessels, which in turn increase vascular permeability and fragility, and make it easier for tumors to invasion vascular and metastasize [43,44,45]. Furthermore, with continues accumulation of lipids, adipose tissues will undergo complex process of remodeling, and the secretion of adipokines, such as leptin, adiponectin, interleukin, vascular endothelial growth factor (VEGF), and hypoxia-inducible factor (HIF) will significantly increase [46], which could induce angiogenesis by stimulating endothelial cell proliferation, migration, and tube formation [47]. SM area provides information on changes in muscle structure, while IMAT indicates alterations in the content of intra- and inter-myocellular adipose [48]. The former parameter denotes muscle quantity and is served as an assessment metric for sarcopenia, and the latter parameter reflects muscle quality and is served as an evaluation index for myosteatosis [20]. Recent studies have established that sarcopenia is positively associated with evaluated all-cause mortality in HCC patients [49, 50]. Though the detailed mechanism has yet to be fully elucidated, however, there are two generally accepted hypotheses. Depletion of muscle could lead to a reduction in the secretion of specific cytokines, such as insulin-like growth factor (IGF)-1, which is associated with advanced clinicopathological variables of HCC [51, 52]. In addition, sarcopenia could also result in the impairment of certain protein hydrolysis systems, such as the tumor necrosis factor (TNF)-α system, thereby facilitating the process of tumor migration and invasion [53]. To date there have no studies that illuminate molecular mechanisms between myosteatosis and tumor angiogenesis. However, pathological changes in muscle have a strong correlation with chronic liver disease [54]. Montano-Loza et al. studied a cohort of cirrhotic patients undergoing liver transplant evaluation, in which the prevalence of muscular steatosis was greater than 50% [55]. In cirrhosis patients, the absorption of lipids and fat-soluble vitamins may be impacted as a result of several physiological changes, such as nausea, anorexia, increased intra-abdominal pressure, and impaired gut motility. Malnutrition promotes the catabolism of muscle proteins, leading to depletion of muscle mass [56, 57]. In addition, chronic hepatic inflammation plays a significant role in promoting systemic inflammation, which in turn also accelerates the catabolism of muscle proteins [58]. There have been studies shown that liver disease not only triggers muscle atrophy and changes its structure, but the muscle in turn promotes the further development of liver disease [54, 59]. Therefore, there may exist an interaction between liver disease and muscle tissue, which exerts an influence on the IMAT, and consequently modulates the expression of downstream adipokines and myokines. Such a dynamic interaction and these cytokines may contribute to the tumor invasion and progression of liver disease. There are several limitations in our study. Firstly, the limited number of patients impose inherent restrictions on our findings, thus larger external datasets are needed to validate and refine it. Secondly, a prospective study is required to further confirm the reliability of the nomogram. Thirdly, MVI grade was not taken into account in the MVI positive group. Fourthly, this nomogram model is constructed to predict the risk of MVI. Since macrovascular invasion is also a common phenomenon in HCC and is known to be an important predictor of poor survival outcomes [60, 61], whether this nomogram model is fitted for the predication of macrovascular invasion in HCC deserves further study. In addition, some baseline characteristics showed difference between the two cohorts, and it’s inevitable as the process of data collection in these two hospitals were conducted independently. However, these differences had minimal impact on our results. Firstly, data selection and model construction were both performed within training cohort. Two hundred rounds of internal tenfold cross-validation method was used to verify the stable of our model, which could reduce overfitting of result and maximize the utilization of the data. Secondly, although split sample approach could create two cohorts with no differences [62], it could lead the model being unstable, that is, the performance evaluation was correlated with the partition of the training and validation cohorts. Different partitions might yield different estimates of the accuracy [14, 63]. Therefore, we chose original cohort distribution to ensure the randomness and independence of the data and increase generalizability of the model. In fact, we also verified our model by propensity score matching method to match the baseline characteristics data of the validation cohort and the training cohort, and it still demonstrated good predictive capability (data not shown). This suggests that our model has good generalizability and can be extrapolated to patients in other hospitals. By combining 6 preoperative independently predictive factors of MVI, a nomogram is constructed. This model provides an optimal preoperative estimation of MVI risk in HCC patients. For patients with higher MVI scores, anatomical hepatectomy with wide margins may be recommend to reduce early recurrence and extend survival.Results
Baseline characteristics of patients
Data dimensionality reduction and screening
Logistic regression analysis, construction, and validation of nomogram
Clinical utility of the nomogram
Risk stratification of MVI based on the nomogram and survival analysis
Discussion
Conclusion
Availability of data and materials
All data and material analyzed during this study are included in this article/supplementary file. Further inquiries can be directed to the corresponding author.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- ANN:
-
Artificial neural network
- BMI:
-
Body mass index
- C-index:
-
Concordance index
- CT:
-
Computed tomography
- DCA:
-
Decision curve analysis
- D index:
-
Discrimination index
- HCC:
-
Hepatocellular carcinoma
- HU:
-
Hounsfield Unit
- IMATI:
-
Intramuscular adipose tissue index
- IGF:
-
Insulin-like growth factor
- KM:
-
Kaplan-Meier
- LASSO:
-
Least absolute shrinkage and selection operator
- L3:
-
Lumbar vertebra
- MRI:
-
Magnetic resonance imaging
- MVI:
-
Microvascular invasion
- RFS:
-
Recurrence free survival
- ROC:
-
Receiver operating characteristic
- SM:
-
Skeletal muscle
- SMI:
-
Skeletal muscle index
- TACE:
-
Transcatheter arterial chemoembolization
- TNF:
-
Tumor necrosis factor
- VAR:
-
Visceral to subcutaneous adipose tissue area ratio
- VAT:
-
Visceral adipose tissue
- VEGF:
-
Vascular endothelial growth factor
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Li X, Han M, Zhang H, Liu F, Pan Y, Zhu J, et al. Structures and biological functions of zinc finger proteins and their roles in hepatocellular carcinoma. Biomark Res. 2022;10(1):2.
Roayaie S, Obeidat K, Sposito C, Mariani L, Bhoori S, Pellegrinelli A, et al. Resection of hepatocellular cancer ≤2 cm: results from two Western centers. Hepatology (Baltimore, MD). 2013;57(4):1426–35.
Rodríguez-Perálvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20(1):325–39.
Lim KC, Chow PK, Allen JC, Chia GS, Lim M, Cheow PC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg. 2011;254(1):108–13.
Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43.
Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, et al. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol Res. 2014;44(8):846–53.
Cucchetti A, Piscaglia F, Grigioni AD, Ravaioli M, Cescon M, Zanello M, et al. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52(6):880–8.
Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. 2008;14(14):4400–7.
Lei Z, Li J, Wu D, **a Y, Wang Q, Si A, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151(4):356–63.
Yang L, Gu D, Wei J, Yang C, Rao S, Wang W, et al. A radiomics nomogram for preoperative prediction of microvascular invasion in hepatocellular carcinoma. Liver Cancer. 2019;8(5):373–86.
Ma X, Wei J, Gu D, Zhu Y, Feng B, Liang M, et al. Preoperative radiomics nomogram for microvascular invasion prediction in hepatocellular carcinoma using contrast-enhanced CT. Eur Radiol. 2019;29(7):3595–605.
Steyerberg EW, Harrell FE Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774–81.
Simon R. Development and evaluation of therapeutically relevant predictive classifiers using gene expression profiling. J Natl Cancer Inst. 2006;98(17):1169–71.
Yang J, Chen K, Zheng C, Chen K, Lin J, Meng Q, et al. Impact of sarcopenia on outcomes of patients undergoing liver resection for hepatocellular carcinoma. J Cachexia Sarcopenia Muscle. 2022;13(5):2383–92.
Ebadi M, Dunichand-Hoedl AR, Rider E, Kneteman NM, Shapiro J, Bigam D, et al. Higher subcutaneous adipose tissue radiodensity is associated with increased mortality in patients with cirrhosis. JHEP Rep. 2022;4(7): 100495.
Radu P, Ebadi M, Montano-Loza AJ, Dufour JF. What is the role of body composition assessment in HCC management? Cancers. 2022;14(21):5290.
von Hessen L, Roumet M, Maurer MH, Lange N, Reeves H, Dufour JF, et al. High subcutaneous adipose tissue density correlates negatively with survival in patients with hepatocellular carcinoma. Liver Int. 2021;41(4):828–36.
Ohki T, Tateishi R, Shiina S, Goto E, Sato T, Nakagawa H, et al. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 2009;58(6):839–44.
Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131–40.
Gallo P, Silletta M, De Vincentis A, Lo Prinzi F, Terracciani F, Di Fazio G, et al. Sarcopenia in hepatocellular carcinoma: pathogenesis and management. Chemotherapy. 2022;67(3):152–63.
Ebadi M, Bhanji RA, Mazurak VC, Montano-Loza AJ. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54(10):845–59.
Arsenii N, Piardi T, Diébold MD, Chenard MP, Drame M, De Mestier L, et al. Impact of visceral obesity on microvascular invasion in hepatocellular carcinoma. Cancer Invest. 2016;34(6):271–8.
Wu Z, Lu H, **e Q, Cheng J, Ma K, Hu X, et al. Preoperative assessment of abdominal adipose tissue to predict microvascular invasion in small hepatocellular carcinoma. J Clin Transl Hepatol. 2022;10(2):184–9.
Cong WM, Bu H, Chen J, Dong H, Zhu YY, Feng LH, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol. 2016;22(42):9279–87.
Wang D, Zhao J, Zhang R, Yan Q, Zhou L, Han X, et al. The value of CT radiomic in differentiating mycoplasma pneumoniae pneumonia from streptococcus pneumoniae pneumonia with similar consolidation in children under 5 years. Front Pediatr. 2022;10: 953399.
Pang G, Shao G, Zhao F, Liu C, Zhong H, Guo W. CT virtual endoscopy for analyzing variations in the hepatic portal vein. Surg Radiol Anat. 2015;37(5):457–62.
Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (Bethesda, Md: 1985). 2004;97(6):2333–8.
Miller KD, Jones E, Yanovski JA, Shankar R, Feuerstein I, Falloon J. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet (London, England). 1998;351(9106):871–5.
Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (Bethesda, Md : 1985). 1998;85(1):115–22.
Banerjee S, Wang DS, Kim HJ, Sirlin CB, Chan MG, Korn RL, et al. A computed tomography radiogenomic biomarker predicts microvascular invasion and clinical outcomes in hepatocellular carcinoma. Hepatology (Baltimore, MD). 2015;62(3):792–800.
Beaufrère A, Caruso S, Calderaro J, Poté N, Bijot JC, Couchy G, et al. Gene expression signature as a surrogate marker of microvascular invasion on routine hepatocellular carcinoma biopsies. J Hepatol. 2022;76(2):343–52.
Marrero JA, Ahn J, Rajender Reddy K. ACG clinical guideline: the diagnosis and management of focal liver lesions. Am J Gastroenterol. 2014;109(9):1328–47.
Tapper EB, Lok AS. Use of liver imaging and biopsy in clinical practice. N Engl J Med. 2017;377(8):756–68.
Ao H, **n Z, Jian Z. Liquid biopsy to identify biomarkers for immunotherapy in hepatocellular carcinoma. Biomark Res. 2021;9(1):91.
Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–92.
Ferro D, Quintarelli C, Lattuada A, Leo R, Alessandroni M, Mannucci PM, et al. High plasma levels of von Willebrand factor as a marker of endothelial perturbation in cirrhosis: relationship to endotoxemia. Hepatology (Baltimore, MD). 1996;23(6):1377–83.
Carmassi F, Morale M, De Negri F, Carrai M. Modulation of hemostatic balance with antithrombin III replacement therapy in a case of liver cirrhosis associated with recurrent venous thrombosis. J Mol Med (Berl). 1995;73(2):89–93.
Wallington M, Saxon EB, Bomb M, Smittenaar R, Wickenden M, McPhail S, et al. 30-day mortality after systemic anticancer treatment for breast and lung cancer in England: a population-based, observational study. Lancet Oncol. 2016;17(9):1203–16.
Neeland IJ, Hughes C, Ayers CR, Malloy CR, ** ES. Effects of visceral adiposity on glycerol pathways in gluconeogenesis. Metabolism. 2017;67:80–9.
Kishida K, Kuriyama H, Funahashi T, Shimomura I, Kihara S, Ouchi N, et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J Biol Chem. 2000;275(27):20896–902.
Neeland IJ, Ross R, Després JP, Matsuzawa Y, Yamashita S, Shai I, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–25.
Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Götting C, et al. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85(5):1236–43.
Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, et al. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy. Soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97(1):238–43.
Morss AS, Edelman ER. Glucose modulates basement membrane fibroblast growth factor-2 via alterations in endothelial cell permeability. J Biol Chem. 2007;282(19):14635–44.
Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Investig. 2011;121(6):2094–101.
Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–64.
Meister FA, Lurje G, Verhoeven S, Wiltberger G, Heij L, Liu WJ, et al. The role of sarcopenia and myosteatosis in short- and long-term outcomes following curative-intent surgery for hepatocellular carcinoma in a European cohort. Cancers. 2022;14(3):720.
Chang KV, Chen JD, Wu WT, Huang KC, Hsu CT, Han DS. Association between loss of skeletal muscle mass and mortality and tumor recurrence in hepatocellular carcinoma: a systematic review and meta-analysis. Liver Cancer. 2018;7(1):90–103.
Tantai X, Liu Y, Yeo YH, Praktiknjo M, Mauro E, Hamaguchi Y, et al. Effect of sarcopenia on survival in patients with cirrhosis: a meta-analysis. J Hepatol. 2022;76(3):588–99.
Peake JM, Della Gatta P, Suzuki K, Nieman DC. Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev. 2015;21:8–25.
Elmashad N, Ibrahim WS, Mayah WW, Farouk M, Ali LA, Taha A, et al. Predictive value of serum insulin-like growth factor-1 in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2015;16(2):613–9.
Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol. 2015;7(17):2080–90.
Nachit M, Leclercq IA. Emerging awareness on the importance of skeletal muscle in liver diseases: time to dig deeper into mechanisms! Clin Sci (Lond). 2019;133(3):465–81.
Montano-Loza AJ, Angulo P, Meza-Junco J, Prado CM, Sawyer MB, Beaumont C, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126–35.
Thandassery RB, Montano-Loza AJ. Role of Nutrition and Muscle in Cirrhosis. Curr Treat Options Gastroenterol. 2016;14(2):257–73.
Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232–44.
Pérez-Baos S, Prieto-Potin I, Román-Blas JA, Sánchez-Pernaute O, Largo R, Herrero-Beaumont G. Mediators and patterns of muscle loss in chronic systemic inflammation. Front Physiol. 2018;9:409.
Eslamparast T, Montano-Loza AJ, Raman M, Tandon P. Sarcopenic obesity in cirrhosis-The confluence of 2 prognostic titans. Liver Int. 2018;38(10):1706–17.
Costentin CE, Ferrone CR, Arellano RS, Ganguli S, Hong TS, Zhu AX. Hepatocellular carcinoma with macrovascular invasion: defining the optimal treatment strategy. Liver Cancer. 2017;6(4):360–74.
Huang C, Zhu XD, Shen YH, Wu D, Ji Y, Ge NL, et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomarker Res. 2021;9(1):19.
Shen J, Zhou Y, Yu B, Zhao K, Ding Y. Construction and validation of a nomogram for patients with multiple hepatocellular carcinoma: A SEER-based study. Eur J Surg Oncol. 2023;49(10):106966.
Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245–7.
Acknowledgements
Not applicable.
Funding
This work was supported by the grants from the Taishan Scholars Program of Shandong Province (Grant No. tstp20221158), National Natural Science Foundation of China (Grant No. 82073200 & 81874178), Major basic research of Shandong Provincial Natural Science Foundation (Grant No. ZR2021ZD26) and funds for Independent Cultivation of Innovative Team from Universities in **an (Grant No. 2020GXRC023).
Author information
Authors and Affiliations
Contributions
XCM: methodology, clinical data collection, image segmentation, data analysis, results interpretation, manuscript writing and revision; SS: image segmentation, data analysis; LJY and HCW: clinical data collection, data analysis; ZND, HL and GQP: software and code, results interpretation; XZ, CLH and BWT: clinical data collection, manuscript revision; DXW and SYT: software, manuscript revision; ZRD and YCY: conceptualization, supervision; TL: conceptualization, supervision, funding acquisition, manuscript revision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was reviewed and approved by Institutional Review Board of Qilu hospital of Shandong university [approval number: 2021(222)].
Consent for publication
All authors have read and approved the manuscript and agree with submission to Biomarker Research.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Figure 1. Examples of muscle and adipose tissue measurements: (a) subcutaneous adipose area (b) skeletal muscle area; (c) visceral adipose area; (d) intra-muscular adipose tissue. Supplementary Figure 2. The optimal cut-off values of the nomogram scores. Supplementary Figure 3. Kaplan-Meier curves of OS for low-risk patients under different surgical approaches, resection methods and surgical margins in training cohort (a-c) and validation cohort (d-f). OS = overall survival. Supplementary Figure 4. Kaplan-Meier curves of 2-RFS for low-risk patients under different surgical approaches, resection methods and surgical margins in training cohort (a-c) and validation cohort (d-f). RFS = recurrence free survival. Supplementary Table 1. Cut-off values of body composition for male and female.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mao, XC., Shi, S., Yan, LJ. et al. A model based on adipose and muscle-related indicators evaluated by CT images for predicting microvascular invasion in HCC patients. Biomark Res 11, 87 (2023). https://doi.org/10.1186/s40364-023-00527-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40364-023-00527-z