Abstract
Background
Accumulating evidence from human trials and rodent studies has indicated that modulation of gut microbiota affects host physiological homeostasis and behavioral characteristics. Similarly, alterations in gut microbiota could be a feasible strategy for reducing aggressive behavior and improving health in chickens. The study was conducted to determine the effects of early-life cecal microbiota transplantation (CMT) on cecal microbial composition, brain serotonergic activity, and aggressive behavior of recipient chickens.
Methods
Chicken lines 63 and 72 with nonaggressive and aggressive behavior, respectively, were used as donors and a commercial strain Dekalb XL was used as recipients for CMT. Eighty-four 1-d-old male chicks were randomly assigned to 1 of 3 treatments with 7 cages per treatment and 4 chickens per cage (n = 7): saline (control, CTRL), cecal solution of line 63 (63-CMT), and cecal solution of line 72 (72-CMT). Transplantation was conducted via oral gavage once daily from d 1 to 10, and then boosted once weekly from week 3 to 5. At weeks 5 and 16, home-cage behavior was recorded, and chickens with similar body weights were assigned to paired aggression tests between the treatments. Samples of blood, brain, and cecal content were collected from the post-tested chickens to detect CMT-induced biological and microbiota changes.
Results
63-CMT chickens displayed less aggressive behavior with a higher hypothalamic serotonergic activity at week 5. Correspondingly, two amplicon sequence variants (ASVs) belonging to Lachnospiraceae and one Ruminococcaceae UCG-005 ASV were positively correlated with the levels of brain tryptophan and serotonin, respectively. 72-CMT chickens had lower levels of brain norepinephrine and dopamine at week 5 with higher levels of plasma serotonin and tryptophan at week 16. ASVs belonging to Mollicutes RF39 and GCA-900066225 in 72-CMT chickens were negatively correlated with the brain 5-hydroxyindoleacetic acid (5-HIAA) at week 5, and one Bacteroides ASV was negatively correlated with plasma serotonin at week 16.
Conclusion
Results indicate that CMT at an early age could regulate aggressive behavior via modulating the cecal microbial composition, together with central serotonergic and catecholaminergic systems in recipient chickens. The selected CMT could be a novel strategy for reducing aggressive behavior through regulating signaling along the microbiota-gut-brain axis.
Similar content being viewed by others
Introduction
Aggression in psychology refers to a range of behaviors resulting in physical, physiological, and psychological harm within a social group. As an evolutionarily conserved behavior, aggression exists widely across the animal kingdom from humans to various animals including chickens. From the evolutionary perspective, aggression serves as a crucial skill for survival and reproduction enabling an animal to secure resources and adapt to the surrounding environment [1]. In chickens, the origin of aggression can be traced back to their wild ancestors (red junglefowl Gallus gallus) who naturally live within a small group that maintains a dominance hierarchy by showing establishment fights towards individuals, with the memory and recognition of past encounters (pecking order or social rank) through visual cues such as body size, head, appendages, and comb [2, 3]. However, based on the cost–benefit analysis, aggression and related damaging behaviors, such as violence within a group, cause social stress, interfere with societal safety, and damage the health of both dominants and subordinates [4, 5]. In the modern intensive animal production system, some routine management practices, such as mixing and regrou** unfamiliar animals, may disrupt social structural stability and increase aggressive behavior among individuals, compromising animal production, health, and welfare [6]. In chickens, aggression leads to excessive stress, injuries, and even death [7], which results in huge economic losses for the poultry industry. Thus, it is pivotal to develop an approach to control aggression, thereby improving bird health and increasing the economic profits of the poultry industry.
In recent years, many studies have focused on the gut microbiota and their link with host physiological and psychological health via the functional regulation of the gut-brain axis. The first evidence for the existence of the gut-brain axis was reported in the early nineteenth century when researchers found that a person’s emotional state could govern gut health [8]. Correspondingly, the gut microbiota functions as an endocrine organ, influencing both mood stabilization and behavioral exhibition of the host via regulating the biofunctions of neurotransmitters and neurotrophic factors [9], immune process [10], and energy metabolism [11]. In humans, perturbation of the gut microbiota composition has been linked to the pathophysiology of mental disorders [12]. For instance, patients with depression have increased abundances of Bacteroidetes and Proteobacteria as compared to healthy individuals [13]. Microbiota transplantation studies in germ-free (GF) mice have further revealed the causal role of gut microbiota in regulating brain functions. For example, fecal microbiota transplantation (FMT) from patients with depression induces the specific depression-like behavior, together with a decrease in several brain neurotransmitters such as serotonin (5-HT), norepinephrine (NE), and epinephrine (EP) in GF recipients [14]. Similar findings of FMT eliciting the phenotype of the donors in the recipients have been evidenced in several psychiatric disorders including autism [15], schizophrenia [16], and Alzheimer’s disease [17], which further indicates the functional link between the gut microbiota and the brain, i.e., the microbiota-gut-brain axis. Based on these findings, we hypothesized that the gut microbiota may present a similar function in regulating aggressive behavior in chickens.
The regulation of aggressive behavior is primarily mediated through altering central neuronal activities [18]. Based on the 5-HT deficiency hypothesis of aggression [19], 5-HT is a primary modulatory neurotransmitter involved in this process. In humans, diminished serotonergic function within the central nervous system (CNS) has been linked with mood and behavioral disorders including interpersonal aggression and impulsive behavior [20]. Similarly, aggressive chickens usually exhibit lower 5-HT concentrations in specific brain regions, such as the hypothalamus [21]. Also, it has been reported that dietary supplementation with tryptophan (TRP), the precursor of 5-HT, reduces fear in quail [22], aggression in broilers [23], and teleost fishes [24].
Highly inbred chicken lines 63 and 72 have been continuously selected for resistance and susceptibility, respectively, to Marek’s disease for more than 60 years [25, 26]. It has also been evidenced line’s difference in neuroendocrine systems, immunity [27, 28], gut microbiome composition [29], and aggressive behavior [30]. In response to social stress, line 72 chickens are more aggressive than line 63 chickens. Consistent with these findings, line 72 roosters exhibit lower levels of brain 5-HT than line 63 roosters [31]. Considering the essential role of brain serotonergic mediation in aggression, the objective of this study was to determine if early-life cecal microbiota transplantation (CMT) from the divergently selected donors (lines 63 and 72) would potentially reduce aggressive behaviors in recipients through modulating the cecal microbial composition and the central serotonergic system via the gut-brain axis.
Methods
Study design and treatment
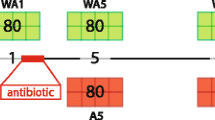
The experimental workflow is summarized in Fig. 1. Inbred chicken lines 63 and 72 selected for Marek’s disease resistance or susceptibility (the Avian Disease and Oncology Laboratory, East Lansing, MI, USA) were used as donors [25]. Cecal contents were randomly collected from 10 sixty-week-old hens per line after 60 generations of selection and then evenly pooled within the line. Five grams of pooled cecal contents were suspended with gut microbiome media at a ratio of 1:10, then kept at −20 °C until oral gavage [32]. A total of 84 day-old Dekalb XL chicks, a commercial strain, was used as recipients and randomly assigned to 1 of 3 treatments with 7 cages per treatment and 4 chickens per cage (n = 7): CTRL (0.1 mL saline, control), 63-CMT (0.1 mL of line 63 cecal content solution), and 72-CMT (0.1 mL of line 72 cecal content solution) for a 16-week trial. Oral gavage was performed daily from d 1 to 10, and then boosted weekly from week 3 to 5. Samples of blood, hypothalamus, and both sides of cecal contents were collected from 1 bird per cage at week 5 (5 d after the boost) and week 16, respectively. A 5-mL blood sample was collected through the brachial vein of each sampled bird using an EDTA-coated tube. The blood samples were centrifuged at 700 × g for 15 min at 4 °C, and then plasma was separated and stored at −80 °C until further analysis. The sampled chickens were then killed by cervical dislocation for tissue collection (i.e., cecal contents and hypothalamic tissues). The hypothalamus was dissected from each sampled bird according to brain anatomical landmarks described previously [33, 34]. After collection, cecal contents and hypothalamic tissues were flash frozen and stored at −80 °C until analysis. Water and feed were provided ad libitum. The general management, including vaccination, dietary formulation and nutrient contents, ambient temperature and humidity, and lighting program was followed the Dekalb White management guideline [35].
Behavioral analysis
Home-cage aggressive behavior
The behavior of chickens in the home cage was recorded for a 24-h period at week 5 and 16 (n = 5) using digital video recording (DVR) systems (Geovision GV-1480) linked to overhead cameras (Panasonic WV-CP254H). The recorded video was viewed using EZViewLog500 in real-time and collected from 1 h after lights on (at 5:58) and 1 h before lights off (at 19:58). The aggressive pecking was determined following the definition described previously: “Occurs when one bird raises head and forcefully stabs beak either once or multiple times at the head or body of another bird. The recipient will usually show avoidance behavior by ducking or moving away from aggressive chickens” [36]. The frequency of aggressive pecking was counted during 2-h video segments and calculated as the average number of pecks per bird per hour (the number of pecks per cage per hour = total number of pecks/2 h/the number of chickens in each cage).
Paired behavioral test
At week 5 and 16, paired behavioral tests were conducted between the treatments following the procedure published previously [37]. Briefly, chickens with similar BW among the treatments were randomly assigned to pairs for aggression tests in novel cages (n = 7) and the body weight of chickens at each test time was shown in Table 1. All tests were conducted during the same period in the morning between 8:00 to 24:00 in the test room and each pair was continuously recorded for 30 min. Afterward, the frequency of aggressive behavior was counted and calculated as the average number of aggressive pecks per bird per pair for statistical analysis.
Cecal microbial profiles
16S rRNA amplicon sequencing
Total genomic DNA was extracted from the cecal samples using the QIAamp Fast DNA Stool Mini kit (Catalog #: 51504, Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. The 16S rRNA gene sequence was sequenced and analyzed to characterize the effects of CMT on the gut microbial community diversity and structures of the recipient chickens [38]. Briefly, the V4 region of the 16S rRNA genes was amplified from the genomic DNA using 515F (5'-GTGCCAGCMGCCGCGGTAA-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3'). Each 25 µL PCR reaction consisted of 9.5 µL of PCR-grade water, 12.5 µL of QuantaBio’s AccuStart II PCR ToughMix (2 × concentration, 1 × final), 1 µL Golay barcode tagged Forward Primer (5 µmol/L, 200 pmol/L final), 1 µL Reverse Primer (5 µmol/L, 200 pmol/L final), and 1 µL of template DNA. The conditions for PCR were as follows: 94 °C for 3 min to denature the DNA, 35 cycles at 94 °C for 45 s, 50 °C for 60 s, and 72 °C for 90 s; then 10 min at 72 °C to ensure complete amplification. The amplicons were quantified using the PicoGreen assay (Invitrogen, Carlsbad, CA, USA). Afterward, the volume were pooled into a single tube and cleaned up using the AMPure XP Beads (Beckman Coulter, Brea, CA, USA), then quantified using a fluorometer (Qubit, Invitrogen, Carlsbad, CA, USA). After quantification, the molarity of the pool was determined and diluted down to 2 nmol/L, denatured, then diluted to a final concentration of 6.75 pmol/L with a 10% PhiX spike [39]. The amplicons were sequenced on an Illumina Miseq platform using 2 × 150 bp paired-end reads at the Argonne National Laboratory (Chicago, IL, USA).
Sequence analysis
The amplicons were trimmed at the 13th base of each sequence for both the forward and reversed sequences and denoised with the Divisive Amplicon Denoising Algorithm (DADA 2) pipelines via the Quantitative Insights into Microbial Ecology (QIIME, v2020.2). The DADA generated amplicon sequence variants (ASVs) were classified using the 99% Silva v132 taxonomic database as a reference (https://data.qiime2.org/2020.2/common/silva-132-99-515-806-nb-classifier). Cecal samples were rarefied to 13,580 and 24,907 reads at week 5 and 16, respectively. The α-diversity was evaluated by the Faith’s PD index. To visualize the dissimilarity of microbial communities, the plots of principal coordinate analysis (PCoA) based on unweighted UniFrac were performed using the Phyloseq, DESeq2, and ggplot2 packages in addition to custom scripts in the R studio (version 3.6.2). Treatment effects were evaluated using the Adonis, and the pairwise comparison was assessed using the permutational multivariate analysis of variance (PERMANOVA) test. The false discovery rate (FDR) adjusted P-value (q-value) was used for multiple comparisons with the QIIME 2. The R studio (version 3.6.2) was performed to determine the treatment effect on the differential abundant ASV at the phylum and genus levels using the DESeq2 package (version 1.30.1). Significant differences in log2 fold changes were determined by the Wald test with P-value correction using the Benjamin and Hochberg test. Levels of significance were determined when P < 0.05 and LDA score > 2.
Brain serotonergic activities
The examined genes involved in the serotonergic pathways were listed in Table 2. Total RNA of the hypothalamic samples was extracted using the RNeasy Mini Lipid Kit (Catalog #: 74804, Qiagen, Valencia, CA, USA) following the instructions provided by the company. The purity and concentration of total RNA were checked using a NanoDrop 2000 (Thermo Scientific, Wilmington, DE, USA). The reverse transcription was conducted by the Reverse Transcription Reagent Pack (Catalog #: N8080234, Applied Biosystems, Foster City, CA, USA). A mixture of reverse transcription reagents consisted of 2 μL RNase inhibitor, 2.5 μL multi-scribe reverse transcriptase, 5 μL random hexamers, 10 μL of TaqMan reverse transcription buffer, 20 μL deoxy nucleotides, and 22 μL of magnesium chloride. A total mixture of each sample consisted of 61.5 μL with the adjusted volume of RNA sample adjusted volume of RNA sample and RNase-free water for a final 100 μL. The RNA samples were reverse transcribed to cDNA using a Techne TC-3000G PCR Thermal Cycler (Bibby Scientific Limited, Stone, UK). To explore the effects of CMT on the activities of serotonin pathway, mRNA abundance of related genes (Table 2) in the left side of hypothalamic tissues were detected by RT-qPCR using the protocol described previously [40]. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekee** gene. The cycling conditions were 50 °C for 2 min and 95 °C for 10 min of the holding stage, followed by 40 cycles of 95 °C for 15 s, then 60 °C for 1 min. Results were quantitated using the standard curve method. The standards were measured in triplicates with a standard deviation of less than 2.0 and a coefficient of variation less than 2.0%.
Brain monoamine profiles
To determine the effects of CMT on central serotonergic activities of recipient chickens, duplicate samples were run by HPLC (UltiMate™ 3000 RSLCnano System, Thermo Fisher Scientific Inc., Waltham, MA, USA) as previously described [41]. Briefly, the right side of the hypothalamus was weighed and homogenized in 0.2 mol/L perchloric acid at a 10:1 ratio and then vortexed for 1 min. Afterward, the supernatant was centrifuged at 14,000 r/min for 10 min at 4 °C. The supernatants were drawn into a microcentrifuge tube and diluted with MD-TM mobile phase (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 1:1. The flow rate of the mobile phase was 0.8 mL/min. Duplicate samples were run at 10 μL per injection. The concentrations of 5-hydroxyindoleacetic acid (5-HIAA), serotonin (5-HT), tryptophan (TRP), norepinephrine (NE), epinephrine (EP), and dopamine (DA) were calculated as nanograms per gram of wet tissue (ng/g) through a reference curve generated from the corresponding standards.
Peripheral serotonin and tryptophan
The concentrations of 5-HT and TRP (My BioSource, San Diego, CA, USA) in the plasma were measured using the respective ELISA kits following the relative company’s instructions. Briefly, 50 μL of samples were incubated with 100 μL of horseradish peroxidase (HRP) conjugate reagent at 37 °C for 60 min. Afterward, the plates were washed and then 50 μL of chromogen solutions A and B were added for ELISA detection at 37 °C for 15 min. Thereafter, stop solution (0.25 mol/L H2SO4) were added and the absorbance was monitored at 450 nm within 5 min.
Statistical analysis
The fixed effects were treatment and age. Physiological and behavioral data were considered as response variables. Data were analyzed using R studio (version 3.6.2) except for home-cage behavioral data, which were analyzed using PROC MIXED repeated measures procedures of SAS 9.4 (SAS Institute Inc., Cary, NC, USA). The frequency of aggressive pecking in home cages was normalized using Box-Cox transformation before analysis. The behavioral data of the paired test were analyzed using the Kruskal–Wallis test and followed by Dunn’s test for multiple comparisons. Other normalized physiological data was carried out by one-way ANOVA and the Tukey–Kramer test was used to partition any significant differences among the least-square means due to the treatment effect [42]. For each treatment, Spearman’s rank test was used to find the correlation between the significantly differential abundant ASVs and differential neurotransmitter concentrations. Significance was set at P ≤ 0.05 and a trend was defined as 0.05 < P ≤ 0.1.
Results
Differences in aggressive behavior of recipient chickens
Compared to 72-CMT chickens, 63-CMT chickens displayed less aggressive pecking in home cages (P = 0.039, Fig. 2A) and paired tests at week 5 (P = 0.040, Fig. 2B). At week 16, 72-CMT chickens tended to exhibit more aggressive pecking than both 63-CMT and CTRL chickens during paired tests (P = 0.093) but not in home cages (P = 0.455).
Frequency of aggressive pecking of recipient chickens at week 5 and week 16. A Home-cage behavior B Paired test. Values are means ± SEM, n = 7. *Indicates significant differences (P ≤ 0.05), and #shows trend differences (0.05 < P ≤ 0.1). 63-CMT, received cecal content solution from 63 donors; 72-CMT, received cecal content solution from 72 donors; CTRL, received saline, control
Changes of the cecal microbial profiles in recipient chickens
Cecal microbiota transplantation induced different effects on the cecal microbial structure and diversity of the recipient chickens (Table 3 and Fig. 3). At week 5, 63-CMT chickens had the lowest phylogenetic diversity (Faith’s PD index) among the treatments (P = 0.033, Fig. 3A), and tended to be lower than 72-CMT chickens at week 16 (P = 0.090). Also, treatment effects on Unweighted UniFrac were found in the recipient chickens at week 5 (P = 0.005) but not at week 16 (P > 0.05, Fig. 3B). The post-hoc pairwise test showed that there were significant differences in the microbial community structures between 63-CMT chickens and 72-CMT chickens (P = 0.006, Table 3). Community composition at phylum and family levels are shown in Fig. 3C.
Effects of cecal microbiota transplantation on cecal microbial profiles of recipient chickens at week 5 and week 16 (n = 7). A Faith’s PD index, values are median ± SEM, *Indicates significant differences (P ≤ 0.05), and # shows trend differences (0.05 < P ≤ 0.1). B Principal coordinate analysis (PCoA) of Unweighted UniFrac of recipient chickens at week 5 and week 16. Each dot represents one bird (n = 7), PCo1 and PCo2 represent the percentage of variance explained by each coordinate. C Cecal microbial composition profiles of the recipient chickens at phylum and family (relative abundance > 1%) levels. 63-CMT, received cecal content solution from 63 donors; 72-CMT, received cecal content solution from 72 donors; CTRL, received saline, control
The differentially abundant ASVs among the treatments were further examined (Fig. 4). At week 5, the number of differentially abundant ASVs was 38, 68, and 52, respectively, between the 63-CMT chickens vs. 72-CMT chickens, the 72-CMT chickens vs. CTRL chickens, and the 63-CMT chickens vs. CTRL chickens (Fig. 4A). Compared with the CTRL chickens, 9 ASVs belonging to GCA-900066225, Lachnoclostridium, Christensenellaceae R7 group, Flavonifractor, Ruminococcaceae UCG-014, Faecalibacterium, Alistipes, Fournierella, and Ruminococcaceae UCG-005 were enriched in the 63-CMT recipient chickens; while the 72-CMT recipient chickens had 12 enriched ASVs belonging to Alistipes, Candidatus Arthromitus, Dielma, Merdibacter, Faecalibacterium, Flavonifractor, GCA-900066225, Anaerofilum, Ruminococcus 2, Ruminococcaceae UCG-005, Ruminococcaceae NK4A214 group, and Lachnoclostridium. Compared with the 63-CMT recipient chickens, 10 ASVs belonging to Akkermansia, Dielma, Merdibacter, Anaeroplasma, Ruminococcaceae UCG-008, Faecalibacterium, GCA-900066225, Ruminococcaceae UCG-014, CAG-56, and Blautia were more abundant than those in the 72-CMT recipient chickens.
DESeq2 analysis of differentially abundant ASVs between 63-CMT group and CTRL group, 63-CMT group and 72-CMT group, 72-CMT group and CTRL group at A week 5 and B week 16. Estimations of log2 fold change values for each ASV were computed and each point represents an ASV that was significantly different (P ≤ 0.05). 63-CMT, received cecal content solution from 63 donors; 72-CMT, received cecal content solution from 72 donors; CTRL, received saline, control
At week 16, there were 30, 14, and 25 differentially abundant ASVs between the 63-CMT chickens vs. 72-CMT chickens, the 72-CMT chickens vs. CTRL chickens, and the 63-CMT chickens vs. CTRL chickens, respectively (Fig. 4B). Those ASVs belong to the phyla Bacteroidetes, Desulfobacterota, Firmicutes, and Proteobacteria. Compared with the CTRL chickens, 5 ASVs were more abundant (Alistipes, Clostridia_UCG-014, Incertae_Sedis, Lachnoclostridium, and Lachnospiraceae) in the 63-CMT chickens, while the 72-CMT chickens had 2 enriched ASVs belonging to genera Bacteroides and Clostridia_UCG-014. Compared with the 63-CMT recipient chickens, 3 ASVs (Bacillus, Escherichia-Shigella, Bacteroides) were more abundant in the 72-CMT chickens.
Changes of brain monoamines in recipient chickens
Cecal microbiota transplantation-caused changes in the biomarkers of the central serotonergic system in recipient chickens are illustrated in Fig. 5. At week 5, the 63-CMT chickens had higher concentrations of hypothalamic 5-HT (P = 0.037) and 5-HIAA (P = 0.007, Fig. 5A), while no differences were found for serotonin turnover (P > 0.05, Fig. 5B). Although there were no treatment effects on the levels of 5-HT and 5-HIAA at week 16 (Fig. 5C), 5-HT turnover in the 63-CMT chickens was elevated compared to the CTRL chickens (P = 0.011, Fig. 5D). In addition, the 63-CMT chickens tended to have higher concentrations of TRP in the hypothalamus compared with the CTRL chickens at week 5 (P = 0.089) and 72-CMT chickens at week 16 (P = 0.063). Microbiota modulation also affected the activation of the noradrenergic system. At week 5, the 72-CMT chickens had lower NE (P = 0.072, Fig. 6A) and DA concentrations (P = 0.031, Fig. 6C) compared to the CTRL chickens but not the 63-CMT chickens. There were no treatment effects on NE, EP, and DA levels at week 16 (P > 0.05, Fig. 6). No differences were found for gene expressions of TPH2, Htr1a, Htr1b, and 5-HTT, except that MAOA mRNA abundance was increased in the 63-CMT group at week 16 (P = 0.024, Table 4).
Effects of cecal microbiota transplantation on the parameters of the serotonergic activities in the hypothalamus of recipient chickens at A-B week 5 and C-D week 16. Values are least square means ± SEM, n = 7. *Indicates significant differences (P ≤ 0.05), and #shows trend differences (0.05 < P ≤ 0.1). 5-HIAA, 5-Hydroxuindoleacetic acid; 5-HT, serotonin; 63-CMT, received cecal content solution from line 63 donors; 72-CMT, received cecal content solution from line 72 donors; CTRL, received saline, control; TRP, tryptophan
Effects of cecal microbiota transplantation on levels of A norepinephrine, B epinephrine, and C dopamine in the hypothalamus of recipient chickens at week 5 and week 16. Values are least square means ± SEM, n = 7. *Indicates significant differences (P ≤ 0.05), and #shows trend differences (0.05 < P ≤ 0.1). 63-CMT, received cecal content solution from line 63 donors; 72-CMT, received cecal content solution from line 72 donors; CTRL, received saline, control
Alterations in plasma tryptophan and serotonin
At week 5, the 63-CMT chickens tended to have higher 5-HT concentrations in the plasma as compared to the CTRL chickens (P = 0.086, Fig. 7A), while no treatment effects on TRP levels (P > 0.05, Fig. 7B). At week 16, the 72-CMT recipient chickens had higher levels of plasma 5-HT (P = 0.033) and TRP (P = 0.037) as compared to the 63-CMT chickens.
Effects of cecal microbiota transplantation on the plasma A 5-HT and B TRP concentration of recipient chickens at week 5 and week 16. Values are least square means ± SEM, n = 7. *Indicates significant differences (P ≤ 0.05), and #shows trend differences (0.05 < P ≤ 0.1). 5-HT, serotonin; 63-CMT, received cecal content solution from line 63 donors; 72-CMT, received cecal content solution from line 72 donors; CTRL, received saline, control; TRP, tryptophan
Correlations between neurotransmitter levels and differentially abundant ASVs
At week 5, two ASVs belonging to Lachnospiraceae (r = 0.87 and 0.67, P = 0.012 and 0.100, Fig. 8A) and one Fournierella ASV (r = 0.98, P < 0.001) were positively correlated with the brain TRP in the 63-CMT recipient chickens. In addition, there was a positive correlation between one Ruminococcaceae UCG-005 ASV (r = 0.79, P = 0.036) and the brain 5-HT levels in the 63-CMT chickens. No correlations were found in the 63-CMT chickens at week 16 (Fig. 8B). In the 72-CMT, one Mollicutes RF39 ASV (r = −0.79, P = 0.035) and one GCA-900066225 ASV (r = −0.78, P = 0.039) were negatively associated with the brain 5-HIAA, and one CAG-56 ASV was negatively correlated with the brain TRP (r = −0.85, P = 0.015) at week 5, while at week 16, one Bacteroides ASV (r = −0.74, P = 0.048) was negatively correlated with the plasma 5-HT.
Spearman correlations of neurotransmitter (and its metabolites) concentration with bacterial taxa (ASVs) in 72-CMT and 63-CMT chickens at A week 5 and B week 16. **Represents P < 0.001, *indicates significant differences (P ≤ 0.05), and #shows trend differences (0.05 < P ≤ 0.1). 5-HIAA, 5-Hydroxuindoleacetic acid; 5-HT, serotonin; 63-CMT, received cecal content solution from line 63 donors; 72-CMT, received cecal content solution from line 72 donors; CTRL, received saline, control; DA, dopamine; EP, epinephrine; MAOA, monoamine oxidase; NE, norepinephrine; TRP, tryptophan
Discussion
Growing evidence has highlighted the involvement of gut microbiota in the regulation of host physiological and behavioral homeostasis and the potential implications of gut microbiota for treating patients with psychiatric and mental disorders [43]. Here, we found transplantation of cecal content from nonaggressive and aggressive donors exerted different developmental patterns of the cecal microbial community, and the activities of central serotonergic and catecholaminergic systems, contributing to divergent behavior phenotypes in recipients, Dekalb XL.
Early-life gut microbiota development has long-term effects on health and disease in humans [44]. Similar outcomes have been revealed in chickens [45, 79] and the mesocorticolimbic dopamine system [80]. Patients with Parkinson's disease have lower levels of DA in the nigrostriatal pathways with a great number of risky decisions [81]. Taken together, the reduced DA in the 72-CMT chickens further support our hypothesis that gut microbiota may work together with the catecholaminergic system to modulate aggression.
Conclusion
The current results indicate that CMT at an early age affects the development of aggressive behavior in recipient chickens via regulating the activities of brain serotonergic and catecholaminergic systems. The outcomes provide new insights into understanding the mechanism of aggression in poultry through the microbiota-gut-brain axis. These results suggest a potential strategy of early intervention with the gut microbiota to reduce aggressive behavior and improve welfare for poultry production.
Availability of data and materials
The datasets used and analyzed in the current study are available from corresponding authors on reasonable request.
All data generated and analyzed during this study are included in this published article.
Abbreviations
- 5-HIAA:
-
5-Hydroxuindoleacetic acid
- 5-HT:
-
Serotonin
- 5-HTergic:
-
Serotonergic
- 5-HTT:
-
Serotonin transporter
- 63-CMT:
-
Chickens with cecal solution of line 63
- 72-CMT:
-
Chickens with cecal solution of line 72
- ASVs:
-
Amplicon sequence variants
- BW:
-
Body weight
- CMT:
-
Cecal microbiota transplantation
- CNS:
-
Central nervous system
- CTRL:
-
Chickens with saline, control
- DA:
-
Dopamine
- ELISA:
-
Enzyme-linked immunosorbent assay
- EP:
-
Epinephrine
- FMT:
-
Fecal microbiota transplantation
- GF:
-
Germ-free
- HPLC:
-
High-performance liquid chromatography
- Htr1a:
-
5-HT 1a receptor
- Htr1b:
-
5-HT 1b receptor
- MAO:
-
Monoamine oxidase
- NE:
-
Norepinephrine
- SCFAs:
-
Short-chain fatty acids
- SPF:
-
Specific pathogen-free
- TPH:
-
Tryptophan hydroxylase
- TRP:
-
Tryptophan
References
Buss DM, Duntley JD. The evolution of aggression. In: Schaller M, Simpson JA, Kenrick DT, editors. Evolution and social psychology. New York: Psychology Press; 2006. p. 263–85.
Craig JV. Behavioral and genetic adaptation of laying hens to high-density environments. Bioscience. 1982;32:33–7.
D’Eath RB, Keeling LJ. Social discrimination and aggression by laying hens in large groups: from peck orders to social tolerance. Appl Anim Behav Sci. 2003;84:197–212.
Liu J, Lewis G, Evans L. Understanding aggressive behaviour across the lifespan. J Psychiatr Ment Health Nurs. 2013;20:156–68.
Raine A. Biosocial studies of antisocial and violent behavior in children and adults: a review. J Abnorm Child Psychol. 2002;30:311–26.
Hubbard AJ, Foster MJ, Daigle CL. Impact of social mixing on beef and dairy cattle—a sco** review. Appl Anim Behav Sci. 2021;241:105389.
Cheng HW, Muir WM. Mechanisms of aggression and production in chickens: genetic variations in the functions of serotonin, catecholamine, and corticosterone. World’s Poult Sci J. 2007;63:233–54.
Cannon WB. The influence of emotional states on the functions of the alimentary canal. Am J M Sc. 1909;137:480–7.
Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. J Physiol. 2004;558:263–75.
Hand TW, Vujkovic-Cvi** I, Ridaura VK, Belkaid Y. Linking the microbiota, chronic disease, and the immune system. Trends Endocrinol Metab. 2016;27:831–43.
Zheng P, Wu J, Zhang H, Perry SW, Yin B, Tan X, et al. The gut microbiome modulates gut–brain axis glycerophospholipid metabolism in a region-specific manner in a nonhuman primate model of depression. Mol Psychiatry. 2021;26:2380–92.
Nikolova VL, Hall MR, Hall LJ, Cleare AJ, Stone JM, Young AH. Perturbations in gut microbiota composition in psychiatric disorders: a review and meta-analysis. JAMA Psychiat. 2021;78:1343–54.
Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, et al. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal. 2017;138:231–9.
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry. 2016;21:786–96.
Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–63.
Zhu F, Guo R, Wang W, Ju Y, Wang Q, Ma Q, et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol Psychiatry. 2020;25:2905–18.
Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. 2019;9:1–13.
Berman ME, Tracy JI, Coccaro EF. The serotonin hypothesis of aggression revisited. Clin Psychol Rev. 1997;17:651–5.
da Cunha-Bang S, Knudsen GM. The modulatory role of serotonin on human impulsive aggression. Biol Psychiatry. 2021;90:447–57.
Pourhamzeh M, Moravej FG, Arabi M, Shahriari E, Mehrabi S, Ward R, et al. The roles of serotonin in neuropsychiatric disorders. Cell Mol Neurobiol. 2021;42:1671–92.
Dennis RL, Chen ZQ, Cheng HW. Serotonergic mediation of aggression in high and low aggressive chicken strains. Poult Sci. 2008;87:612–20.
Yıldırım A, Kraimi N, Constantin P, Mercerand F, Leterrier C. Effects of tryptophan and probiotic supplementation on growth and behavior in quail. Poult Sci. 2020;99:5206–13.
Shea MM, Mench JA, Thomas OP. The effect of dietary tryptophan on aggressive behavior in develo** and mature broiler breeder males. Poult Sci. 1990;69:1664–9.
Wolkers CP, Serra M, Hoshiba MA, Urbinati EC. Dietary L-tryptophan alters aggression in juvenile matrinxa Brycon amazonicus. Fish Phys Biochem. 2012;38:819–27.
Bacon LD, Hunt HD, Cheng HH. Genetic resistance to Marek’s disease. Curr Top Microbiol Immunol. 2001;255:121–41.
Stone HA. Use of highly inbred chickens in research. Washington: Agricultural Research Service, US Department of Agriculture; 1975.
Bacon LD, Palmquist D. Chicken lines differ in production of interferon-like activity by peripheral white blood cells stimulated with phytohemagglutinin. Poult Sci. 2002;81:1629–36.
Yonash NI, Bacon LD, Smith EJ. Concentration of immunoglobulin G in plasma varies among 6C. 7 recombinant congenic strains of chickens. Poult Sci. 2002;81:1104–8.
Hu J, Johnson TA, Zhang H, Cheng HW. The microbiota–gut–brain axis: gut microbiota modulates conspecific aggression in diversely selected laying hens. Microorganisms. 2022;10:1081.
Dennis R, Zhang HM, Bacon LD, Estevez I, Cheng HW. Behavioral and physiological features of chickens diversely selected for resistance to avian disease. 1. selected inbred lines differ in behavioral and physical responses to social stress. Poult Sci. 2004;83:1489–96.
Dennis RL, Cheng HW. Differential serotonergic mediation of aggression in roosters bred for resistance and susceptibility to Marek’s disease. Br Poult Sci. 2014;55:13–20.
Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantaset G, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. PNAS. 2011;108:6252–7.
Kuenzel WJ, Masson M. A stereotaxic atlas of the brain of the chick (Gallus domesticus). Baltimore: Poultry Science Faculty Publications and Presentations; 1988.
Puelles L, Martinez-De-La-Torre M, Paxinos G, Watson C, Martinez S. The chick brain in stereotaxic coordinates: an atlas correlating avian and mammalian neuroanatomy. 1st Ed. San Diego: Academic Press; 2007.
Dekalb White management guideline. 2019. https://www.dekalb-poultry.com/documents/1827/Dekalb_White_CS_management_guide__North_American_Version_L2221-1.pdf.
Daigle CL, Rodenburg TB, Bolhuis JE, Swanson JC, Siegford JM. Use of dynamic and rewarding environmental enrichment to alleviate feather pecking in non-cage laying hens. Appl Anim Behav Sci. 2014;161:75–85.
Hu JY, Chen H, Cheng HW. Effect of direct-fed microbials, Bacillus subtilis, on production performance, serotonin concentrations and behavioral parameters in a selected dominant strain of White Leghorn hens. Int J Poult Sci. 2018;17:106–15.
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(supplement_1):4516–22.
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6:1621–4.
Dennis RL, Cheng HW. The dopaminergic system and aggression in laying hens. Poult Sci. 2011;90:2440–8.
Yan FF, Wang WC, Cheng HW. Bacillus subtilis–based probiotic promotes bone growth by inhibition of inflammation in broilers subjected to cyclic heating episodes. Poult Sci. 2020;99:5252–60.
Steel RGD, Torrie JH, Dicky DA. Principles and procedures of statistics, A biometrical approach. 3rd Ed. New York: McGraw Hill, Inc. Book Co.; 1997. p. 352–8.
Settanni CR, Ianiro G, Bibbò S, Cammarota G, Gasbarrini A. Gut microbiota alteration and modulation in psychiatric disorders: Current evidence on fecal microbiota transplantation. Prog Neuropsychopharmacol. 2021;109:110258.
Sarkar A, Yoo JY, Valeria Ozorio Dutra S, Morgan KH, Groer M. The association between early-life gut microbiota and long-term health and diseases. J Clin Med. 2021;10:459.
Shehata AM, Paswan VK, Attia YA, Abdel-Moneim AM, Abougabal MS, Sharaf M, et al. Managing gut microbiota through in ovo nutrition influences early-life programming in broiler chickens. Animals. 2021;11:3491.
Li X, Bi R, **ao K, Roy A, Zhang Z, Chen X, et al. Hen raising helps chicks establish gut microbiota in their early life and improve microbiota stability after H9N2 challenge. Microbiome. 2022;10:1–2.
Zhang F, Yue L, Fang X, Wang G, Li C, Sun X, et al. Altered gut microbiota in Parkinson’s disease patients/healthy spouses and its association with clinical features. Parkinsonism Relat Disord. 2020;81:84–8.
Wan Y, Zuo T, Xu Z, Zhang F, Zhan H, Dorothy CH, et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut. 2021;71:910–8.
Zhou BH, Jia LS, Wei SS, Ding HY, Yang JY, Wang HW. Effects of Eimeria tenella infection on the barrier damage and microbiota diversity of chicken cecum. Poult Sci. 2020;99:1297–305.
Li Q, Hu J, Nie Q, Chang X, Fang Q, **e J, et al. Hypoglycemic mechanism of polysaccharide from Cyclocarya paliurus leaves in type 2 diabetic rats by gut microbiota and host metabolism alteration. Sci China Life Sci. 2021;64:117–32.
Reigstad CS, Salmonson CE, Rainey JF III, Szurszewski JH, Linden DR, Sonnenburg JL, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–403.
Meyer B, Zentek J, Harlander-Matauschek A. Differences in intestinal microbial metabolites in laying hens with high and low levels of repetitive feather-pecking behavior. Physiol Behav. 2013;110:96–101.
Lobzhanidze G, Japaridze N, Lordkipanidze T, Rzayev F, MacFabe D, Zhvania M. Behavioural and brain ultrastructural changes following the systemic administration of propionic acid in adolescent male rats. further development of a rodent model of autism. Int J Dev Neurosci. 2020;80:139–56.
Cheng Y, Liu J, Ling Z. Short-chain fatty acids-producing probiotics: A novel source of psychobiotics. Crit Rev Food Sci Nutr. 2022;62(28):7929–59.
Dicks LM, Hurn D, Hermanus D. Gut bacteria and neuropsychiatric disorders Microorganisms. 2021;9:2583.
Chen H, Meng L, Shen L. Multiple roles of short-chain fatty acids in Alzheimer disease. Nutrition. 2022;93:111499.
Pierz KA, Thase ME. A review of vilazodone, serotonin, and major depressive disorder. Prim Care Companion CNS Disord. 2014;16:23088.
Dennis RL, Fahey AG, Cheng HW. Alterations to embryonic serotonin change aggression and fearfulness. Aggress Behav. 2013;39:91–8.
Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, et al. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;15:1485–91.
Takayama K, Maehara S, Tabuchi N, Okamura N. Anthraquinone-containing compound in rhubarb prevents indole production via functional changes in gut microbiota. J Nat Med. 2021;75:116–28.
Jiang HY, Zhang X, Yu ZH, Zhang Z, Deng M, Zhao JH, et al. Altered gut microbiota profile in patients with generalized anxiety disorder. J Psychiatr Res. 2018;104:130–6.
Sun L, Zhang H, Cao Y, Wang C, Zhao C, Wang H, et al. Fluoxetine ameliorates dysbiosis in a depression model induced by chronic unpredicted mild stress in mice. Int J Medical Sci. 2019;16:1260.
Goehler LE, Park SM, Opitz N, Lyte M, Gaykema RP. Campylobacter jejuni infection increases anxiety-like behavior in the holeboard: possible anatomical substrates for viscerosensory modulation of exploratory behavior. Brain Behav Immun. 2008;22:354–66.
Kriegebaum C, Song NN, Gutknecht L, Huang Y, Schmitt A, Reif A, et al. Brain-specific conditional and time-specific inducible Tph2 knockout mice possess normal serotonergic gene expression in the absence of serotonin during adult life. Neurochem Int. 2010;57:512–7.
Hemmings SM, Xulu K, Sommer J, Hinsberger M, Malan-Muller S, Tromp G, et al. Appetitive and reactive aggression are differentially associated with the STin2 genetic variant in the serotonin transporter gene. Sci Rep. 2018;8:1–9.
Kolla NJ, Matthews B, Wilson AA, Houle S, Michael Bagby R, Links P, et al. Lower monoamine oxidase-a total distribution volume in impulsive and violent male offenders with antisocial personality disorder and high psychopathic traits: An [11 C] Harmine positron emission tomography study. Neuropsychopharmacology. 2015;40:2596–603.
Roy A, Linnoila M. Suicidal behavior, impulsiveness and serotonin. Acta Psychiatr Scand. 1988;78:529–35.
van Hierden YM, de Boer SF, Koolhaas JM, Korte SM. The control of feather pecking by serotonin. Behav Neurosci. 2004;118:575–83.
Kops MS, Kjaer JB, Güntürkün O, Westphal KGC, Korte-Bouws GAH, Olivier B, et al. Serotonin release in the caudal nidopallium of adult laying hens genetically selected for high and low feather pecking behavior: an in vivo microdialysis study. Behav Brain Res. 2014;268:81–7.
van der Eijk JAJ, de Vries H, Kjaer JB, Naguib M, Kemp B, Smidt H, et al. Differences in gut microbiota composition of laying hen lines divergently selected on feather pecking. Poult Sci. 2019;98:7009–21.
Nishiwaki H, Hamaguchi T, Ito M, Ishida T, Maeda T, Kashihara K, et al. Short-chain fatty acid-producing gut microbiota is decreased in Parkinson’s disease but not in rapid-eye-movement sleep behavior disorder. MSystems. 2020;5:e00797-e820.
McGaughey KD, Yilmaz-Swenson T, Elsayed NM, Cruz DA, Rodriguiz RM, Kritzer MD, et al. Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety-and depressive-like behavior following social defeat in mice. Sci Rep. 2019;9:3281.
Redweik GA, Daniels K, Severin AJ, Lyte M, Mellata M. Oral treatments with probiotics and live Salmonella vaccine induce unique changes in gut neurochemicals and microbiome in chickens. Front Microbiol. 2020;10:3064.
O’Donnell J, Zeppenfeld D, McConnell E, Pena S, Nedergaard M. Norepinephrine: a neuromodulator that boosts the function of multiple cell types to optimize CNS performance. Neurochem Res. 2012;37:2496–512.
Saboory E, Ghasemi M, Mehranfard N. Norepinephrine, neurodevelopment and behavior. Neurochem Int. 2020;135:104706.
Ohnishi T, Maruyama T, Higashi S, Awazu S. Blood-brain barrier transport of L-iyrosine conjugates: a model study for the brain targeting using large neutral amino acid transport system. J Drug Target. 2000;8:395–401.
Tsuda A, Tanaka M, Ida Y, Shirao I, Gondoh Y, Oguchi M, et al. Expression of aggression attenuates stress-induced increases in rat brain noradrenaline turnover. Brain Res. 1988;474:174–80.
Schlüter T, Winz O, Henkel K, Prinz S, Rademacher L, Schmaljohann J, et al. The impact of dopamine on aggression: an [18F]-FDOPA PET Study in healthy males. J Neurosci. 2013;33:16889–96.
Arias-Carrion O, Stamelou M, Murillo-Rodriguez E, Menendez-Gonzalez M, Poppel E. Dopaminergic reward system: a short integrative review. Int Arch Med. 2010;3:24.
Miczek KA, Fish EW, De Bold JF. Neurosteroids, GABAA receptors, and escalated aggressive behavior. Horm Behav. 2003;44:242–57.
Narvaes R, Martins de Almeida RM. Aggressive behavior and three neurotransmitters: dopamine, GABA, and serotonin—a review of the last 10 years. Psychol Neurosci. 2014;7:601.
Acknowledgements
We sincerely thank Carmen L. Wickware for technical assistance with sample processing. We also thank the farm staff of Purdue Poultry Animal Sciences Research Center, and the technicians of the USDA-ARS, Livestock Behavior Research Unit for their assistance with this project.
Funding
This study was supported by the grant award (No: 2017–67015-26567) of the NIFA-AFRI, USDA.
Author information
Authors and Affiliations
Contributions
YF, JH, TAJ, and HC designed the study. YF and JH conducted animal trials. YF analyzed and interpreted the data. TAJ provided critical guidance in sequence analysis. YF and HC wrote the manuscript. EAM, HZ, TAJ, and HC revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures were approved by the Purdue University Animal Care and Use Committee (PACUC#: 1712001657).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fu, Y., Hu, J., Erasmus, M.A. et al. Cecal microbiota transplantation: unique influence of cecal microbiota from divergently selected inbred donor lines on cecal microbial profile, serotonergic activity, and aggressive behavior of recipient chickens. J Animal Sci Biotechnol 14, 66 (2023). https://doi.org/10.1186/s40104-023-00866-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40104-023-00866-9