Abstract
Background
Acute kidney injury (AKI) is one of the preventable complications of percutaneous coronary intervention (PCI). This study aimed to develop machine learning (ML) models to predict AKI after PCI in patients with acute coronary syndrome (ACS).
Methods
This study was conducted at Tehran Heart Center from 2015 to 2020. Several variables were used to design five ML models: Naïve Bayes (NB), Logistic Regression (LR), CatBoost (CB), Multi-layer Perception (MLP), and Random Forest (RF). Feature importance was evaluated with the RF model, CB model, and LR coefficients while SHAP beeswarm plots based on the CB model were also used for deriving the importance of variables in the population using pre-procedural variables and all variables. Sensitivity, specificity, and the area under the receiver operating characteristics curve (ROC-AUC) were used as the evaluation measures.
Results
A total of 4592 patients were included, and 646 (14.1%) experienced AKI. The train data consisted of 3672 and the test data included 920 cases. The patient population had a mean age of 65.6 ± 11.2 years and 73.1% male predominance. Notably, left ventricular ejection fraction (LVEF) and fasting plasma glucose (FPG) had the highest feature importance when training the RF model on only pre-procedural features. SHAP plots for all features demonstrated LVEF and age as the top features. With pre-procedural variables only, CB had the highest AUC for the prediction of AKI (AUC 0.755, 95% CI 0.713 to 0.797), while RF had the highest sensitivity (75.9%) and MLP had the highest specificity (64.35%). However, when considering pre-procedural, procedural, and post-procedural features, RF outperformed other models (AUC: 0.775). In this analysis, CB achieved the highest sensitivity (82.95%) and NB had the highest specificity (82.93%).
Conclusion
Our analyses showed that ML models can predict AKI with acceptable performance. This has potential clinical utility for assessing the individualized risk of AKI in ACS patients undergoing PCI. Additionally, the identified features in the models may aid in mitigating these risk factors.
Graphical Abstract

Similar content being viewed by others
Introduction
Coronary artery disease, particularly acute coronary syndrome (ACS), is responsible for approximately one-third of all deaths in adults over 35. Nowadays percutaneous coronary intervention (PCI) is the most widely used treatment for ACS. Acute kidney injury (AKI) is a serious non-cardiovascular complication in patients with ACS, and nearly 12.8% of the patients develop AKI as a major post-PCI complication with a 20.2% attributed mortality rate during or after hospitalization [1, 2]. A growing body of evidence indicates that AKI is significantly associated with an increased risk of long-term morbidities such as repeated coronary revascularization, myocardial infarction, and stroke [3, 4].
To prevent contrast induced-AKI (CI-AKI), physicians can implement preventive measures such as regulating contrast volume and osmolarity, pre-procedural statin intake, and pre- and post-procedural hydration [1, 5]. Identifying PCI-related patient risks allows physicians to tailor strategies based on each individual’s risk profile, leading to fewer complications and improved clinical outcomes after a PCI procedure [1, 6]. Prediction models, such as the NCDR-AKI risk model, have been developed to assess the risk of CI-AKI prior to performing PCI with a c-statistics of 0.71 [7]. Traditional statistical models may not include all possible interactions when there are numerous candidate variables, resulting in a decrease in the model’s accuracy when these interactions are ignored [1, 8]. Machine Learning (ML)-based models do not depend on assumptions about the variables involved or their relationship with the outcome. Instead, they capture complex relationships in a data-driven manner, including nonlinearity and interactions that may be difficult to identify otherwise. These models have been used for the prediction of outcomes in cardiovascular medicine [9,10,11].
This study aims to evaluate novel ML-based models to more accurately predict the risk of PCI-induced AKI in ACS patients and subsequently reduce the risk of long-term complications. The efficacy of ML-based models will be compared with traditional stepwise selection models, and the study will investigate whether machine learning-based models can sufficiently reduce the variables needed for disease prognosis prediction.
Methods
Study design
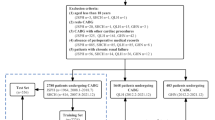
We retrospectively reviewed all patients with ACS [ST-elevation myocardial infarction (STEMI), non-STEMI, and unstable angina (UA)] who underwent PCI at Tehran Heart Center between 2015 and 2020. The ethics committee of Tehran Heart Center approved this study (IR.TUMS.MEDICINE.REC.1402.178). The informed consent was waived due to the retrospective design of this study.
Variable’s definition and outcome
Pre-procedural variables used were: gender, age, left ventricular ejection fraction (LVEF), atrial fibrillation (AF), fasting plasma glucose (FPG), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), drug history (lipid-lowering, anti-diabetes, anti-hypertension, anti-arrhythmia, and anti-thrombotic), hematocrit, body mass index (BMI), estimated glomerular filtration rate (eGFR), creatinine (Cr), type of diabetes management, past medical histories (cardiac, renal, previous PCI, previous CABG), and CAD risk factors.
Procedural variables were: non-ST elevation myocardial infarction (NSTEMI) in coronary angiography (CAG), acute MI in CAG, treated vessel, procedure result, stenosis, stent diameter, stent length, stent inflation pressure, post-procedural complications (arrhythmia, cardiopulmonary resuscitation (CPR), aborted cardiac arrest, and procedure-induced shock).
AKI, the primary outcome in this study, was defined based on the acute kidney injury necrosis (AKIN) as an absolute increase of ≥ 0.3 mg/dL or a relative increase of ≥ 50% in serum creatinine after the procedure [12].
Data cleaning
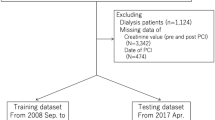
At first, patients with missing data for follow-up were removed and missing data for other features were handled through imputation with median values for numerical features and mode for categorical ones. Notably, features with more than 40% missing data were removed from the models. Then, the patients with end-stage renal disease (ESRD) (eGFR < 15 mL/min) were excluded. Moreover, we excluded individuals with implausible creatinine values (Cr < 0.3 mg/dL or Cr > 4.0 mg/dL). Label encoder (from the scikit-learn library) was used to change categorical variables into numerical variables.
Train/test split and feature selection
We randomly assigned each patient to the train (80%) or test (20%) dataset using stratified splitting. Five-fold cross-validation was used in this study for feature selection and hyperparameter tuning. To find the most important variables among the vast number of procedural and post-procedural features, and to reduce the complexity of our models, we first trained an RF model on these features from our training dataset as our feature selector. We selected the top 15 features based on the feature importance given by this model. This cutoff was defined as we wanted to use features, sum of which contributed to 80% of the total feature importance. The selected features are used as our procedural features to train the main models in this study. Moreover, SHapley Additive ExPlanations (SHAP), as a game-based feature analysis technique [38].
In a study, Lasso and SHAP methods in ML selected that ST-elevation MI, eGFR, age, preprocedural hemoglobin, non-ST-elevation MI/unstable angina, heart failure at admission, and cardiogenic shock as the pertinent predictor for AKI risk after PCI [8]. On the other hand, Ma et al. reported 11 important predictors of CI-nephropathy after PCI, including uric acid, peripheral vascular disease, cystatin C, creatine kinase-MB, hemoglobin, N-terminal pro-brain natriuretic peptide, age, diabetes, systemic immune-inflammatory index, total protein, and low-density lipoprotein, using SHAP method [28]. Also, age, serum creatinine level, and LVEF were among the top 20 ranked important variables concerning CI-AKI risk stratification after acute MI, using the Boruta ML algorithm [1].
Given the potential importance of AKI as an adverse event after PCI, models such as the ones investigated in this study can have clinical applications in the prediction of AKI post-PCI in patients with ACS, after further confirmation in larger studies. With implementing easy-to-use variables both pre-procedural and procedural, these ML-based models provided acceptable predictions. Our models showed similar prediction ability between models with and without procedural variables. It is of importance since intra-procedural features are dependent on the skill of the team performing PCI which makes it subjective and, hence, makes the inherent risk of patients less highlighted [18, 39]. Individualized risk stratification in predicting PCI can lead to better prevention of AKI after PCI. LVEF, age, and FPG were the main predictors of AKI which are easy to measure in patients with ACS admitted to PCI units. Clinicians could take advantage of these models for the prediction of AKI and therefore, provide better care for those at higher risk. These kinds of models could be used regionally or even internationally when assessed in different settings and on different populations.
Several limitations to our research need to be mentioned. Firstly, the single-center nature of our study could affect our findings. Furthermore, it is essential to consider the potential impact of not incorporating confounding variables. It is also important to note that electrocardiogram data and follow-up laboratory data were not available in this databank. Another limitation of our study was missing data that we handled by replacing with median in continuous variables and with mode in categorical ones, which might not have been the optimal way for doing so; however, the prediction of missing data was not possible due to not having a large enough dataset. Moreover, since we tuned the threshold for classifying the groups to optimize sensitivity (recall), we were not able to assess the calibration of our models, and the probabilities in models were only used to identify the optimal threshold. Also, the fact that our data were imbalanced and we tuned our models for better prediction of AKI based on AUC using five-fold cross-validation, led to relatively lower specificities, compared to AUCs and sensitivities. This is a limitation of our study; however, it should be considered that in these types of adverse events, higher sensitivity is much favored over higher specificity since the clinician’s aim is to not miss any potentially high-risk case in terms of AKI. Also, in our study, the threshold was adjusted for higher sensitivity while in other clinical settings, it could be tuned for higher specificity based on clinical settings. Finally, despite using fivefold cross-validation in our training cohort and evaluating the models on an unseen test cohort, the lack of external validation in our study might threaten the generalizability of our findings and models.
Conclusion
In conclusion, the ML models such as RF, LR, CB, MLP, and NB algorithms, showed an acceptable predictive performance for the risk of AKI following PCI, with RF and CB providing the greatest discriminations. Also, the most important features for the AKI prediction were detected, and LVEF demonstrated the largest coefficient in all predicting models. Therefore, it could be suggested that ML models, particularly the RF model, improve the accuracy of AKI prediction in patients undergoing PCI, which has significant implications for clinical decision-making and management to prevent AKI incidence. However, further studies are necessitated to validate the findings of the present study.
Availability of data and materials
The data used in this study will be made available upon reasonable request from the corresponding author.
Abbreviations
- AKI:
-
Acute kidney injury
- PCI:
-
Percutaneous coronary intervention
- ML:
-
Machine learning
- ACS:
-
Acute coronary syndrome
- NB:
-
Naïve Bayes
- LR:
-
Logistic Regression
- RF:
-
Random Forest
- CB:
-
CatBoost
- MLP:
-
Multi-layer perception
- AUC:
-
Area under the receiver operating characteristics curve
- CI-AKI:
-
Contrast induced-AKI
- STEMI:
-
ST-elevation myocardial infarction
- UA:
-
Unstable angina
- LVEF:
-
Left ventricular ejection fraction
- AF:
-
Atrial fibrillation
- FPG:
-
Fasting plasma glucose
- TG:
-
Triglycerides
- TC:
-
Total cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- BMI:
-
Body mass index
- eGFR:
-
Estimated glomerular filtration rate
- Cr:
-
Creatinine
- CABG:
-
Coronary artery bypass grafting
- NSTEMI:
-
Non-ST elevation myocardial infarction
- CAG:
-
Coronary angiography
- CPR:
-
Cardiopulmonary resuscitation
- AKIN:
-
Acute kidney injury necrosis
- ESRD:
-
End-stage renal disease
- PMH:
-
Past medical history
- CI:
-
Confidence interval
- AVM:
-
Support vector machine
- KNN:
-
K-nearest
- GBM:
-
Gradient boosting model
- SHAP:
-
SHapley Additive exPlanations
References
Sun L, Zhu W, Chen X, Jiang J, Ji Y, Liu N, et al. Machine learning to predict contrast-induced acute kidney injury in patients with acute myocardial infarction. Front Med. 2020;7: 592007.
Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interventions. 2014;7(1):1–9.
Chalikias G, Serif L, Kikas P, Thomaidis A, Stakos D, Makrygiannis D, et al. Long-term impact of acute kidney injury on prognosis in patients with acute myocardial infarction. Int J Cardiol. 2019;283:48–54.
Ng AK, Ng PY, Ip A, Lam LT, Ling IW, Wong AS, et al. Impact of contrast-induced acute kidney injury on long-term major adverse cardiovascular events and kidney function after percutaneous coronary intervention: insights from a territory-wide cohort study in Hong Kong. Clin Kidney J. 2022;15(2):338–46.
Maksimczuk J, Galas A, Krzesiński P. What promotes acute kidney injury in patients with myocardial infarction and multivessel coronary artery disease-contrast media, hydration status or something else? Nutrients. 2022;15(1):21.
Huang C, Murugiah K, Mahajan S, Li SX, Dhruva SS, Haimovich JS, et al. Enhancing the prediction of acute kidney injury risk after percutaneous coronary intervention using machine learning techniques: a retrospective cohort study. PLoS Med. 2018;15(11): e1002703.
Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath-PCI Registry. J Am Heart Assoc. 2014;3(6): e001380.
Kuno T, Mikami T, Sahashi Y, Numasawa Y, Suzuki M, Noma S, et al. Machine learning prediction model of acute kidney injury after percutaneous coronary intervention. Sci Rep. 2022;12(1):749.
Negassa A, Ahmed S, Zolty R, Patel SR. Prediction model using machine learning for mortality in patients with heart failure. Am J Cardiol. 2021;153:86–93.
Khalaji A, Behnoush AH, Jameie M, Sharifi A, Sheikhy A, Fallahzadeh A, et al. Machine learning algorithms for predicting mortality after coronary artery bypass grafting. Front Cardiovasc Med. 2022;9: 977747.
de Oliveira Gomes BF, da Silva TMB, Dutra GP, Peres LS, Camisao ND, Junior WSH, et al. Late mortality after myocardial injury in critical care non-cardiac surgery patients using machine learning analysis. Am J Cardiol. 2023;204:70–6.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31.
Lundberg SM, Lee S-I. Consistent feature attribution for tree ensembles. ar**v preprint ar**v:170606060. 2017.
Meacham S, Isaac G, Nauck D, Virginas B, editors. Towards explainable AI: design and development for explanation of machine learning predictions for a patient readmittance medical application. Intelligent Computing. Cham: Springer International Publishing; 2019.
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: machine learning in Python. J Mach Learn Res. 2011;12:2825–30.
Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5(9):1315–6.
Thakker RA, Albaeni A, Alwash H, Gilani S. Prevention and management of AKI in ACS patients undergoing invasive treatments. Curr Cardiol Rep. 2022;24(10):1299–307.
Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–9.
Chen YL, Fu NK, Xu J, Yang SC, Li S, Liu YY, et al. A simple preprocedural score for risk of contrast-induced acute kidney injury after percutaneous coronary intervention. Catheter Cardiovasc Interv. 2014;83(1):E8–16.
Behnoush AH, Khalaji A, Rezaee M, Momtahen S, Mansourian S, Bagheri J, et al. Machine learning-based prediction of 1-year mortality in hypertensive patients undergoing coronary revascularization surgery. Clin Cardiol. 2023;46(3):269–78.
Jiang F, Jiang Y, Zhi H, Dong Y, Li H, Ma S, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230–43.
Mohamadlou H, Lynn-Palevsky A, Barton C, Chettipally U, Shieh L, Calvert J, et al. Prediction of acute kidney injury with a machine learning algorithm using electronic health record data. Can J Kidney Health Dis. 2018;5:2054358118776326.
Li K, Yu N, Li P, Song S, Wu Y, Li Y, et al. Multi-label spacecraft electrical signal classification method based on DBN and random forest. PLoS ONE. 2017;12(5): e0176614.
Amaratunga D, Cabrera J, Lee YS. Enriched random forests. Bioinformatics. 2008;24(18):2010–4.
Speiser JL, Miller ME, Tooze J, Ip E. A comparison of random forest variable selection methods for classification prediction modeling. Expert Syst Appl. 2019;134:93–101.
Chen R-C, Dewi C, Huang S-W, Caraka RE. Selecting critical features for data classification based on machine learning methods. J Big Data. 2020;7(1):52.
Niimi N, Shiraishi Y, Sawano M, Ikemura N, Inohara T, Ueda I, et al. Machine learning models for prediction of adverse events after percutaneous coronary intervention. Sci Rep. 2022;12(1):6262.
Ma X, Mo C, Li Y, Chen X, Gui C. Prediction of the development of contrast-induced nephropathy following percutaneous coronary artery intervention by machine learning. Acta Cardiol. 2023;78:1–10.
Khalilia M, Chakraborty S, Popescu M. Predicting disease risks from highly imbalanced data using random forest. BMC Med Inform Decis Mak. 2011;11(1):51.
Wi J, Ko YG, Shin DH, Kim JS, Kim BK, Choi D, et al. Prediction of contrast-induced nephropathy with persistent renal dysfunction and adverse long-term outcomes in patients with acute myocardial infarction using the Mehran Risk Score. Clin Cardiol. 2013;36(1):46–53.
Helber I, Alves CMR, Grespan SM, Veiga ECA, Moraes PIM, Souza JM, et al. The impact of advanced age on major cardiovascular events and mortality in patients with ST-elevation myocardial infarction undergoing a pharmaco-invasive strategy. Clin Interv Aging. 2020;15:715–22.
Hosseini K, Khalaji A, Behnoush AH, Soleimani H, Mehrban S, Amirsardari Z, et al. The association between metabolic syndrome and major adverse cardiac and cerebrovascular events in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Sci Rep. 2024;14(1):697.
Jonas M, Kagan M, Sella G, Haberman D, Chernin G. Cardiovascular outcomes following percutaneous coronary intervention with drug-eluting balloons in chronic kidney disease: a retrospective analysis. BMC Nephrol. 2020;21(1):445.
Wykrzykowska JJ, Garg S, Onuma Y, de Vries T, Goedhart D, Morel MA, et al. Value of age, creatinine, and ejection fraction (ACEF score) in assessing risk in patients undergoing percutaneous coronary interventions in the ‘all-comers’ leaders trial. Circ Cardiovasc Interventions. 2011;4(1):47–56.
Capodanno D, Marcantoni C, Ministeri M, Dipasqua F, Zanoli L, Rastelli S, et al. Incorporating Glomerular filtration rate or creatinine clearance by the modification of diet in renal disease equation or the Cockcroft-Gault equations to improve the Global Accuracy of the Age, Creatinine, Ejection Fraction [ACEF] score in patients undergoing percutaneous coronary intervention. Int J Cardiol. 2013;168(1):396–402.
Araujo GN, Pivatto Junior F, Fuhr B, Cassol EP, Machado GP, Valle FH, et al. Simplifying contrast-induced acute kidney injury prediction after primary percutaneous coronary intervention: the age, creatinine and ejection fraction score. Cardiovasc Interv Ther. 2018;33(3):224–31.
Hertzberg D, Sartipy U, Lund LH, Rydén L, Pickering JW, Holzmann MJ. Heart failure and the risk of acute kidney injury in relation to ejection fraction in patients undergoing coronary artery bypass grafting. Int J Cardiol. 2019;274:66–70.
Schöttker B, Brenner H, Koenig W, Müller H, Rothenbacher D. Prognostic association of HbA1c and fasting plasma glucose with reduced kidney function in subjects with and without diabetes mellitus. Results from a population-based cohort study from Germany. Prev Med. 2013;57(5):596–600.
Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93(12):1515–9.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
AHB, MMS, AK: study conception/data analysis/drafting the manuscript/revision; MA, AY, MR, HS, AS, AA: drafting the manuscript/revision; SY, YJ, FM, MM, MI: critical revision; KH: study conception/drafting the manuscript/critical revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Tehran Heart Center and conformed to the ethical guidelines.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Behnoush, A.H., Shariatnia, M.M., Khalaji, A. et al. Predictive modeling for acute kidney injury after percutaneous coronary intervention in patients with acute coronary syndrome: a machine learning approach. Eur J Med Res 29, 76 (2024). https://doi.org/10.1186/s40001-024-01675-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-024-01675-0