Abstract
In tropical countries, a mysterious tubulo-interstitial chronic renal disease (CKD), unrelated to diabetes, hypertension, and immunological causes, manifested four decades ago. Approximately 25,000 primarily middle-aged male farmers succumb annually to this crystal-tubular nephropathy (CTN). Without any known causative factors, it was identified as CKD of unknown aetiology (CKDu). Because multiple factors contribute to causing it later, was changed to CKD of multi-factorial (CKDmfo). Despite no evidence, it was hypothesised to cause by agrochemicals or heavy metals in food or drinking contaminated water. However, current data suggest that the CKD-CTN is due to natural geogenic water contamination. Consumption of concentrated stagnant groundwater from deep-dug wells and tube wells containing hard water and fluoride, overdecades is necessary for its clinical manifestations. In all affected countries have prolonged annual dry seasons that led to the evopo-concentration of ions and minerals in groundwater, making hard water even more unpalatable, thus, peasants consume lesser amounts of water. They develop chronic dehydration from daily exposure to hot climatic conditions aggravated by regular alcohol intake. These conditions provide a highly conducive environment—a perfect storm for calcium phosphate (CaPO4) crystal formation in renal tissues. Our recent histological and preliminary electron microscopic data reveal deposition of CaPO4 crystals and nano-tubes in kidneys. While CaPO4 nano-minerals are unstable, the presence of fluoride ions stabilises and allows their growth. This new concept paves the path for highly cost-effective, straightforward local solutions to protect farm workers and eliminate the disease, without embarking on expensive medications, interventions, or building hospitals. Chronic dehydration-associated CKD–CTN is preventable by increased consumption of potable water. Increasing clean water consumption reduces CKD–CTN incidence, and associated morbidities and premature deaths. However, the damage becomes irreversible when the disease advances beyond CKD stage IIIB. The incidence of this deadly renal failure can be prevented by its education, lifestyle changes, and increased water consumption, not by treating the renal disease or expanding dialysis centres/hospitals, or transplantation services. Eradication of CKD-CTN cost significantly less than the current approach of treating affected persons and unnecessarily expanding health infrastructure. Since the manifestation of CKD-CTN is due to consuming naturally contaminated drinking water (with calcium containing hard water and fluoride), it is not difficult to remove these to prevent CKD-CTN: thus, international assistance is unwarranted for its eradication. The straightforward approaches described here will prevent CKD–CTN and save thousands of lives in affected farming communities.
Keypoints
-
During the past four decades, an unusual tubulointerstitial chronic renal disease (CKD) unrelated to diabetes, hypertension, or immunological causes, manifested in over twenty tropical countries located north of the equator.
-
Previously, this crystal-tubular nephropathy (CKD–CTN) was assumed to arise from agrochemical or heavy metal contamination.
-
Natural geogenic conditions/events contaminate groundwater. Drinking such concentrated, stagnant groundwater in the presence of chronic dehydration over a prolonged period leads to the development of CKD–CTN.
-
Nano-minerals stabilised with fluoride ions grow perniciously in kidney tissues in the presence of dehydration. It provides a conducive environment for CaPO4 crystal formation in renal tubules and interstitial tissues.
-
In conjunction with lifestyle changes and providing affordable clean drinking water will prevent the disease and eventually eradicate CKD–CTN. Those with CKD stages below stages IIIB can reverse by drinking plenty of clean water. These approaches would save thousands of lives in affected communities and healthcare costs.
Similar content being viewed by others
Introduction
Over forty million people in tropical countries who consume groundwater naturally contaminated with geogenic components are at risk of develo** an unusual form of chronic kidney disease (CKD). Most affected are adult males in farming communities in affected tropical countries. They create unique chronic tubulointerstitial chronic kidney disease (CKD–CTN) unrelated to commonly known factors, such as diabetes, hypertension, toxins, or immunological disorder. Each year approximately 25,000, primarily middle-aged males, die from it [1].
However, it neither exclusively affects agricultural communities nor is it related to agrochemicals. Those who were never involved with agriculture and engaged in other professions also developed this fatal CKD; what matters are their location and drinking water source—the quality of water, and behaviour [2]. There is no affirmative and reliable scientific evidence that fertilisers, arsenic, pesticides, arsenic, heavy metals, algae toxins, genetic susceptibility, ayurvedic or traditional medications, other known chemical nephrotoxins, or direct human interventions cause CKD–CTN [3].
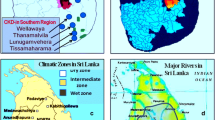
There had been over thirty conjectures proposed as the cause for CKD–CTN; none were examined meticulously or proven to be the cause. Since the exact cause was unknown, it was initially identified as CKD of unknown etiology (CKDu) [4, 5]. Subsequent data firmly pointed towards the need for multiple factors to precipitate CKDu; hence, it was re-named CKD of multi-factorial etiology (CKDmfo) [1, 6, 7]. This fatal renal failure primarily affects countries that are geographically restricted—located north and closer to the equator (Fig. 1).
The similarities of diseases and characteristic differences between the central (meso)American and Sri Lankan nephropathy have been described [8, 9]. Most recent data confirmed that prolonged exposure to multiple factors (usually more than 10 years), including environmental conditions, chronic dehydration, and favorable internal milieu is necessary to develop CKD–CTN [10]. These various factors lead to the precipitation of CaPO4 crystals and nano-tubes in renal tubules and interstitial spaces in renal tissue, causing chronic renal failure [10].
Ongoing research suggests that it is a geo-environmental-induced water-borne disease. CKD–CTN is due to inflammatory CaPO4 nano-particles in renal tubular tissues [10, 11]. For mineral precipitation, it is necessary to have prolonged dehydration and low urine output. Urine pH of around 6.8, hypercalcemia, hypercalciuria, hypomagnesuria, and low urinary Mg2+-to-Ca2+ ratio, these conditions are favourable for CaPO4 precipitation in the kidneys [12].
Crystal induces chronic inflammation and oxidative stress, inflammatory cell infiltration and renal tubular cell damage, apoptosis, and tissue fibrosis; causing renal shrinkage, leading to chronic renal failure (CRF). With the advancing knowledge and considering the above, we coined the terminology CKD–crystal tubular-nephropathy (CKD–CTN) to describe this disease. This acronym parallels CKD–mineral and bone disorders (CKD–MBD) [13] and is logical [14]. Whereas, when there is no scientific evidence or correlation between agrochemicals (or heavy metals) and CKD–CTN, attempted usage of agriculture-based acronyms, such as CINAC, ACN, KDUCAL, NUCAL, etc., is flawed, inappropriate, and misleading [3, 15,16,17]. Such diversion does not help peasants but further stigmatises farming communities. Even abbreviations CKDu and CKDmfo are superfluous as these do not describe an etiology.
The cause of CKD–CTN
The role of Ca2+, PO4 3- and F− in nano-crystal formation in the kidney
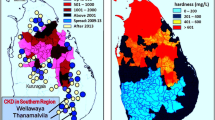
In the affected communities, consumed groundwater has high dissolved solids and electrical conductivity, and Ca2+, phosphate (PO43), and fluoride (F−). For example, the median range of these in groundwater, in affected regions are TDS 500 mg/L, EC 1,800 μS/cm, calcium 125 mg/L, Mg2+ 50 mg/L, alkalinity 250 mg/L, SO42- 200 mg/L, F− 0.8 mg/L, and PO43- 0.025 mg/L [18]. Most of these water sources are unhealthy to consume over time, and mentioned components are above the EPA and WHO-mandated safety standards [18]. However, no reliable published research studies reported higher (or even detectable) levels of agrochemicals, heavy metals, or other known nephrotoxins [3, 19,20,21,22,23]. Besides, because of the unpalatability of water, people in these hot tropical climatic regions consume less water, and the habit of daily alcohol consumption worsens dehydration [11, 24].
Under the conditions mentioned above, renal tissues chronically exposed to high concentrations of Ca2+, (PO4)3-, and F− provide a conducive milieu for calcium phosphate (CaPO4) and other nano-mineral crystal formation. Although CaPO4-hydroxyapatite formation in kidneys is not unusual: these structures are unstable [Ca5(PO4)3(OH) = Ca2+ + 3(PO4)3− + OH−] under the normal range of urine pH. In these regions, groundwater contains higher F− concentrations [20, 25] but is sporadic [8, 9]. At the proper concentrations, F− substitute hydroxyl or PO4 [3] groups in CaPO4 hydroxyapatite through ionic and hydrogen bonds form more stable fluorapatite crystals [fluoroapatite: 5Ca2+ + 3(PO4)3− + Ca5(PO4)3-F] [10].
This phenomenon is analogous to incorporating F− into CaPO4 apatite in the enamel component in teeth and skeletal tissues [8, 9]. It strengthens existing hydroxyapatite scaffolds despite requiring a minute F concentration (e.g., between one and three mg/L). It produces stable fluoroapatite crystals resistant to decay [8, 9], and known to grow gradually. Consequent cell-mediated fluorapatite, carbonatoapatite and incorporation of matrix proteins make these nano-crystals stiffer [26]. In teeth, incorporating F− leads to brownish discolouration of teeth and bones to become brittle [8, 9, 27].
Nano-crystal and nano-tube formation in renal tissues
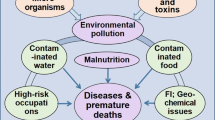
Ultra-structural studies in human kidney tissues with end-stage CKD consistently reported 50 to 1500 nm multi-lamellar mineral particles [28]. Presence of CaPO4 crystals was also reported in renal biopsy samples in those with several chronic renal diseases [14, 29,30,31]. While the generic crystal formation in renal tissues is not unique, the condition leading to crystal formation and classic pattern of CRF in CKD–CTN are unique [10]. Mentioned nano-mineral particles of polycrystalline CaPO4 are produced in both extracellular matrices and tubular cells [10, 30, 31]. Figure 2 shows a conceptual diagram illustrating Geo–Bio interactions, pathways, and conditions necessary to precipitate CaPO4 nano-crystal in renal tissues in renal tubules, causing CKD–CTN. These are not present in other types of nano crystallisation of minerals in the kidney.
Illustrates the geological and biological (Geo–Bio) pathways of forming nano-tubes and nano-crystals in CKD–CTN. A conceptual diagram is illustrating ways for the formation of calcium phosphate (CaPO4) nano-crystal-based hydroxyapatite in renal tubules causing CKD–CTN: a interaction of rocks containing minerals rich in F−, Ca2+, Mg2+, PO43− with groundwater; long duration of water–rock interaction is exacerbate by seismic effects; c consumption of water with high concentrations of ions; d biochemical reactions and super-saturation, precipitating CaPO4 nano-crystals and nano-tubes in kidney tubules; e common factors enhancing the nano-mineral precipitation; f Low-grade persisting chronic inflammation and tissue fibrosis; and g Onset of chronic renal failure (adapted from Wimalawansa and Dissanayake, 2022; Frontiers of Water and human health) [10]
Hydroxy- and fluorapatite nano-particle growth occurs slowly in vivo. Generally, it takes years before producing symptoms [32,33,34]. These are inflammatogenic and, thus, attract fibroblasts and leukocytes, low-grade inflammation, tissue fibrosis, and eventually renal failure. In vivo, nano-crystal complexes attract matrix proteins [35] incorporated into hydroxy-fluorapatite, further protecting nano-crystal dissolution and from enzymatic degradation [36, 37]. These allow a steady growth of fluoroapatite crystals, eventually blocking and perforating renal tubules, causing intense local inflammation, cellular apoptosis, and fibrosis [10].
Diagnosis of CKD–CTN
The diagnosis of tubule-interstitial nephritis/nephropathies (TIN) is generally considered when there are no common causes present in the history and presented with unexplained renal failure dysfunction [38,39,40]. Routinely used urine and serum biochemical makers, such as urine and renal profiles capture all types of CKDs, but none are specific to CKD–CTN. Similarly, none of the routinely used in vivo imaging and scanning methods, distinguish between CRF.
A history of exposure, living location and clusters of persons with CRF, and the environmental circumstance should lead to the clinical suspicion of CKD–CTN [41]. History of not having diabetes and hypertension, lack of history of exposure to known nephrotoxins (most are acute onset), snake bites and immunological disorders virtually exclude most common types of CKDs. The primary causes of TIN are illustrated in Table 1.
Proteinuria is an early manifestation of glomerular diseases, such as diabetes and hypertension, but protein in urine appears late in renal tubular disorders [8, 41,42,43]. Consequently, using proteinuria of 30 mg/L as a cutoff point, which is standard for glomerular renal disease [44, 45], is inappropriate for CKD–CTN [8, 46]. Using such would significantly underestimate the diagnosis and prevalence of CKD–CTN in the community [26], as proteinuria is a later finding of CKD-CTN. This significantly delays the diagnosis of CKD-CTN, which is detrimental for the prognosis. Standard diagnostic method even with renal biopsies with routine histology would not differentiate CKD–CTN from other CKDs; as crystals are not visualised under standard microscopy and magnifications.
The characteristic interstitial infiltrate comprises lymphocytes, macrophages, eosinophils, and plasma cells, which can rapidly transform into interstitial fibrosis leading to chronic kidney disease (CKD). The critical biochemical differential diagnosis aid is tubular-specific markers in urine, which is straightforward and not expensive [9, 41, 47]. Examples of tubular specific makers (when the primary damage is to renal tubules) include Cystatin C, Kidney injury molecule-1 (KIM-1), Monocyte chemotactic protein-1 (MCP-1) N-acetyl-β-D-glucosaminidase (NAG) Nephrin, Netrin-1, Neutrophil gelatinase-associated lipocalin (NGAL) Smooth Muscle alpha-Actin (α-SMA), Urinary Vitamin D binding protein (uVDBP), and Vascular endothelial growth factor (VEGF) [41, 48, 49].
Mechanisms of formation of nano-tubes in renal tubules
In 2019 the authors postulated that repeated chronic dehydration generated peaks of Ca2+ within renal interstitial tissues and in tubules and excess PO4−3, intensifying the formation of nano-crystals and nano-tube formation and growth. This established the inflammatory process attracts fibroblasts and tissue fibrosis, causing CKD–CTN and premature deaths [10]. This process requires over a decade of consuming contaminated water containing higher mineral content.
The formation of intra-tubular Ca2− and other minerals increases the risks for clinically significant mineral crystallisation in kidneys. A charitable foundation headed by the lead author, Water Board, and others have provided potable water to CKD–CTN-affected villages through reverse osmosis units. These efforts have led to over a 50% reduction in two to three years: the improvement was continued [50].
With higher intakes of mentioned minerals, PO4-3 and F-, at the body temperature the solubility of the “Ca2+ PO43−” product exceeds a threshold thus, leads to the formation of CaPO4 nano-particles. In addition, super-saturation of CaOx, urates, or other minerals also occurs, albeit at a lesser quantities than CaPO4 -hydroxy apatite in renal tissues. When nano-crystals exceed 1 mm, such accumulations can be visualised with in vivo imaging methods, such as high-resolution computed tomography [14, 29, 51]: Nano-particles, even when combined, are too small to be visible in other routine radiological investigations. It takes over a decade to develop symptomatic renal disease and full-blown renal failure [22, 52]. However, renal biopsy with high-resolution computed tomography or electron microscopic studies can confirm CKD-CTN diagnosis, early in the disease.
Magnesium deficiency increases the risks of renal nano-crystal formation
Mg2+ competes with Ca2+ in physiological situations and is a cofactor for enzymatic reactions: it is also essential for releasing hormones from endocrine cells. Under the above-discussed conditions, hypomagnesemia enhances the accumulation and precipitation of Ca2+ within renal cells. Moreover, with saturation in stagnant tissue fluids within the counter-current multiplier mechanisms in kidneys, the lack of antagonist effect of Mg2+ on Ca2+ synergises CaPO4 nano-crystal formation in renal parenchyma.
When rats are fed an Mg-deficient diet, the harmful effects of calcium are accentuated and worsened by low dietary potassium. Likewise, circulatory Mg2+ concentration is reduced when rats are fed high-calcium diets [53]. In contrast, renal accumulation of calcium (CaPO4 and other minerals) is mitigated with Mg2+ supplements. Contrary to suggestions based on incidental and isolated findings [23] and an animal study [54, 55], Mg2+ does not cause CKD–CTN. Instead, magnesium protects renal tissues from excess Ca2+ [56,57,58] and against mineral crystallisation [10]. Also, physiological intra-cellular Mg2+ concentration is essential for proper biological and physiological activities in all cells [59,60,61].
Besides, lower circulatory total magnesium Mg2+ concentrations usually measured in blood neither correlate with the intracellular Mg2+ nor its biological activity [60, 61] and facilitate CaPO4 precipitation. In addition to dietary factors, these data emphasise the importance of the quality of drinking water, multiple ion interactions, and their ratios and competition in vivo physiological situations—multi-factor effects. These either mitigate or aggravate the ionic reactions, nano crystallisation, and clinical outcomes [10, 59, 61].
Dietetic fructose in renal tubular CaPO4 nanotube formation
Rats fed on an Mg-deficient diet develop hypomagnesemia, and clinical outcomes are worsened when fed with fructose (to a lesser extent with glucose) than those fed unrefined, complex carbohydrates. In the fructose-fed group, renal calcium content was eight times greater than in the control group [62]. High fructose diet also worsens existing hypercalcemia, hypercalciuria, and hypomagnesuria, enhancing CaPO4 crystallisation in kidneys and renal failure. Excess fructose in the presence of low Mg2+ increases risks for nephrocalcinosis and renal failure [59, 62,63,64].
Meanwhile, higher F concentrations increase Ca2+ precipitation in soft tissues, arteries, and kidneys. Those who live in regions with a higher prevalence of CKD–CTN are economically poor, and their diets are predominantly carbohydrates. In addition, these diets are deficient in micronutrients and Mg2, whereas fructose content is high and represents a significant component of their carbohydrate intake. In the presence of low cell/tissue Mg2+ concentration, higher fructokinase activity further increases the risks of renal calcification [11].
Synergistic ion interactions exacerbate the nano-crystal formation
Physiological concentrations of Mg2+ counteract the adverse effects of increased intracellular Ca2+ concentration and modulation of Ca2+ channels. Hypomagnesemia increases N-methyl-D-aspartate receptors and nuclear factor-kappaB activity, stimulating the renin–angiotensin system and reducing renal blood flow [64]. Aggregated apatite nano-tubes attach to the luminal cell surfaces [30], causing blockage and/or rupturing renal tubules and reducing renal functions.
Insufficiency of crystallisation modulators, such as Tamm–Horsfall protein, osteopontin, sodium phosphate co-transporter, or sodium–hydrogen exchanger regulatory factor-1, also increases the risks of tubular and interstitial nephrocalcinosis [65]. Whereas the crystal-induced interstitial tissue inflammation and oxidative stress further reduce intra-renal blood flow via activation of the renin–angiotensin system, causing further impairment of renal functions.
Other contributory factors
Renal failure increases PO4 retention, which leads to increased Klotho levels. Meanwhile, advanced renal failure disrupts fibroblast growth factor-23 (FGF23), signalling that increases the accumulation of PO4 [66]. Therefore, modulation of Klotho activity should be investigated as a target for intervention in those with moderate CKD–CTN. For example, using Klotho or its synthetic agonist reduces PO4 toxicity and severity in CKD–CTN and accelerated ageing [67].
Hypomagnesemia worsens the retention of Klotho-induced PO4 tubular load and renal functions. Therefore, prophylactically correcting Mg2+ deficiency could be a cost-effective approach for those living in endemic areas to reduce PO4 load and an economical way to prevent and reverse CKD–CTN in its early stages. Figure 3 illustrates the fundamental mechanisms and pathways, forming CaPO4 crystals in renal tissue.
Multiple pathways and interactions leading to nano-mineral forming in renal tubular and cortical tissues. Chronic dehydration-induced high mineral concentration within the renal fluid and tissues is conducive to forming mineral crystals. Multiple factors aggravate renal ischemia, anaemia, and inflammatory cell infiltration. These leads to chronic inflammation and oxidative stress. Collectively, these lead to disruption of tubular cell functions and fibrosis, causing CKD–CTN [TDS: total dissolved solids; Ca2+ and PO43-, Mg2+, etc.); CaPO4: calcium phosphate; CaOx: calcium oxalate; Mg2+: magnesium; F−: fluoride] (modified from Wimalawansa and Dissanayake) [10]
It is not climate change but the associated disasters that arose from the willful destruction of the environment through uncontrolled development and agriculture and irresponsible behaviour of people that endanger nature, causing many preventable human diseases. Since CKD–CTN originates from an environmental cause—natural Geo–Bio interactions (Fig. 2)—it is feasible to reduce the risk of CKD-CTN by addressing the root causes: without embarking on expensive pharmaceutical agents. As we and others have previously reported [1, 34, 68].
The most critical intervention is supplying potable water to all affected regions [8, 52]. This can be achieved via mentioned cost–benefit analysis and practical steps to help eradicate CKD–CTN [52, 69] and adopting previously described economic longer term chronic disease management programs [69, 70]. The following section describes ways to control and eradicate CKD–CTN.
Eradication of CKD–CTN is straightforward
Interventions for CKD–CTN in affected regions need to focus on providing potable water at an affordable price and educating them on ways to avoid chronic dehydration. Providing centrally purified pipe-borne water to the affected areas is expensive and estimated to take more than three decades. Even then, the methodologies used by Water Boards unlikely to remove sufficient hardness (CaPO4) and fluoride from water. In the interim, the most cost-effective and practical way to provide a scalable potable water supply is through ionexchange systems, reverse osmosis or water descaling/softening to remove hardness, which can also eliminate excess F- scattered across affected regions [46, 71, 72]. A program to educate the public, starting from schools and extending to the community, on avoiding harmful behaviour-like daily alcohol intake is critical to preventing the younger generation from getting affected.
In addition, when a breadwinner or any family member acquires CKD–CTN, their expenses escalate, and their income dwindles. This initiates a vicious cycle within the family that affects children’s education, and ability to do manual activities to generate revenue. Consequently, it escalates the poverty, escalating malnutrition: a universal phenomenon observed in all affected regions [73, 74]. Therefore, programs to alleviate CKD–CTN must encompass poverty alleviation. The authors recommend implementing straightforward, cost-effective muti-prong programs to prevent the fatal CKD–CTN [8, 10, 52, 74], as we previously described [52]. A broader holistic and affirmative approach based on the concepts described [46, 52], encompassing education, awareness, prevention of environmental pollution, lessening malnutrition, correcting unhealthy behaviours and habits that acquired during the past four decades, and providing clean water would be sufficient to reduce the incidence and eradicating this deadly disease rapidly from affected communities [75,76,77].
The way forward and future research
Based on the aetiology as described here, future studies should focus on minimising the risks of mineral crystal formation in kidneys in affected regions. Such processes would lay a firm path and optimal ways to minimise human costs through public education, alleviate micronutrient malnutrition, and provide affordable clean drinking water to affected people. Such actions are a thousand-fold more economical than the current approach of waiting for people to develop and then treat the disease. It reduces the incidence of CKD–CTN in affected countries, as we previously described with feasibility and cost–benefit analyses[52].
Additive and synergistic effects and ion interactions, intertwined with multiple factors, trigger the onset of renal failure (Fig. 3). Therefore, in vivo models of CKD–CTN detailed biochemical studies that simulate the environmental and Gio–Bio conditions, using “bioavailable” components (not the “total” measured amounts), are necessary [7]. Noteworthy that in vitro chemical interactions are not necessarily extrapolatable to in vivo complex biochemical interactions and pathophysiology.
Additional in vitro biochemical and in vivo electron microscopic studies using renal tissues and bioavailable Ca2+, PO−4, and F−, simulating the CKD–CTN situations, are needed to assess potential antagonistic, additive, or synergistic interactions in the formation of nano-minerals to understand the exact mechanisms. Such would also reveal additional ways to eliminate this deadly condition.
Some in vitro data and in vivo animal studies cannot extrapolate to humans
Compared to in vitro observations and reactions, dozens of compounds interact simultaneously in vivo to buffer, neutralise, and scavenge to function as agonists or antagonists. Numerous published examples have reported the inappropriateness of direct extrapolation of in vitro studies in isolation or in vivo small animal studies [1, 55], to in vivo human situations [70]; such interpretations could be misleading. Moreover, generalising such data is unreliable and insufficient for understanding complex multi-functional interactions in vivo to deduce clinically meaningful conclusions in humans.
Therefore, in vitro, laboratory examination of compounds in isolation is reasonable initially to generate hypotheses but should not be extrapolated to in vivo complex situations, such as CKD–CTN. This is analogous to interpreting data from individually isolated ayurvedic medications: such experiments would not generate meaningful and extrapolatable inferences. An example of CKD–CTN is mis-interpreting marginally higher concentrations of Mg2+ reported in sporadic drinking water samples [1, 20, 78] and data from non-reproducible laboratory rat studies [55]. Broader interpretation and generalisation of data from such experiments are not only unscientific and ambiguous but also unphysiological to make a swee** conclusion that Mg2+ or F− interactions alone cause CKD–CTN [8,9,10]. We provided proper experimental formats, capital and annual cost estimations, and written protocols to prevent CKD-CTN to successive three presidents in Sri Lanka, department of health and university staff. However, hardly any of these were implemented to date.
Types of in vivo experiments needed now
Despite the established biochemical paths and physiological functions, there are different views of the role of Mg2+ on kidneys and the causation of CKD–CTN. Few consider higher Mg2+ in drinking water with or without F- to cause CKD [54]. In contrast, most evidence supports that Mg2+ has a protective effect on kidneys [79,80,81]. To understand the accurate picture, researchers need to take into account ionic interactions, their ratios, and combinations (based on Gibbs free energy calculations) coupled with bioavailable (effective) components in (e.g., F-) in vivo models.
Data obtained using bioavailable components and the ratios of ions (e.g., Ca2+ to Mg2+) would allow a better understanding of in vivo biological interactions to improve clinical outcomes. These may explain the outcome discrepancies, such as renal damage with low F- intake and low incidence of CKD–CTN despite high F- intake [8, 9]. For example, personal observations and published data exemplify that water F- contents as low as 0.5 mg/L (i.e., acceptable limits) cause dental fluorosis in children in some villages in dry zones. Ironically, chronic water ingestion with F- concentration above 3 mg/L in other regions and America does not cause dental or skeletal flurosis [8, 9].
In vitro and in vivo experiments designed with bioavailability components and varied ratios (e.g., Ca2+/Mg2+), in clinically relevant dose exposures, compared to controls, are essential to make sensible conclusions. In humans, it takes over 10 years of exposure to develop CKD–CTN. Therefore, future research should simulate realistic environmental conditions, such as concentrations, temperature, duration of exposure, and appropriate physical activity in age-specific animal models relevant to humans [53]. Such experiments are valuable for understanding the in vivo conditions causing nano-crystal formation and the additional ways to mitigate them [82, 83]. Prospective, multi-discipline, multifaceted, and multi-centre clinical studies using realistic dose/duration exposure relevant to humans are warranted [10]. Figure 4 summarises multiple factors that work together to provide a conducive micro-environment and increase the vulnerability of persons to contract CKD–CTN.
Reasons why CKD–CTN has not been controlled to date
Despite that mentioned evidence, the CKD–CTN-affected communities are neglected. Governments in none of the affected countries have made it a priority to control or eradicate this deadly disease. All these countries are classified as develo** economies and failed to prioritise preventing this CRF. Affected communities are located in rural regions, and peasants live in a subsidiary economy with daily labour. They do not have a voice to get the attention of the government. Despite these, most of the work of provision of clean water conducted today is by individual philanthropists and smaller charitable organisations.
Larger charities have not been involved in hel** the affected villages, as there is not much for them to gain from these rural communities. What has successfully reduced the incidence of CKD–CTN in the affected regions is providing potable water, preventing dehydration, and changing lifestyles. Construction of renal clinical, dialysis units, and even renal hospitals had no impact on reducing the incidence of CKD–CTN, as they all work on the tail-end of this fatal disease. Overall, respective governments have taken only a slight interest in providing clean water, which too was done haphazardly in a few locations. There is a widespread conflict of interest among politicians, bureaucrats, and those who have made this fatal disease a significant business, including intermediaries, governmental departments, and doctors.
Conclusion
Longer term consumption (exposure) of stagnant groundwater concentrated with ions and chronic dehydration creates a favourable internal milieu for CaPO4 and other mineral crystallisation in kidneys. Over-exploitation of groundwater and prolonged droughts lead to increased poor-quality groundwater and lack of potable water, aggravated by unhealthy behavioural issues that collectively cause this crystal-tubular nephropathy (CTN). Because of the described Geo–Bio pathways (Fig. 2) leading to disease, the populations that live in the immediate geo-environment are the most vulnerable to develo** CKD–CTN [10].
Consumption of stagnant groundwater with high ion content over many years with chronic dehydration due to lesser water intake is necessary for crystal development in vivo. The ions and minerals in those stagnant wells get concentrated due to annual prolonged dry periods. This makes such groundwater unpalatable; therefore, residents consume less water. Daily alcohol intake worsens chronic dehydration, creating a perfect storm in the internal milieu in renal tissues—a conducive in vivo environment for crystal formation.
Willful destruction of the environment through greed-driven, lass-scale deforestation, over-extraction of groundwater for agriculture, irresponsible behaviour of people that endanger nature, poorly planned human settlements, and disregarding the environmental consequences have paved the path for the genesis of this deadly kidney disease. These frequent fires and flooding, large-scale waste- air- and water pollution, and prolonged droughts threaten human and animal health, including chronic renal failure—CKD–CTN.
The mechanisms that cause CKD–CTN are illustrated here to facilitate an understanding of the actions needed to overcome CKD–CTN. It also provides cost-effective early intervention strategies to prevent and eradicate CKD–CTN from tropical countries. Besides the nano-crystal/nano-tube concept (Fig. 3), there is overwhelming evidence that a balanced diet enriched with micronutrients, antioxidants (selenium, Zn, etc.), and Mg2+ protect kidneys from all forms of CKDs, especially the crystal formation of CKD–CTN.
Providing potable water, increasing water consumption, and avoiding harmful behaviour are fundamental in affected regions to protect the renal health of farm labourers and others who regularly engage in strenuous physical work in hot and dry environments. CKD–CTN is a chronic, Geo–Bio mediated disease that arises from natural causes (unrelated to agrochemicals or heavy metals), epitomised by multiple environmental and behavioural factors. With the proactive deployment of strategic practices and economic measures, CKD–CTN can be entirely preventable and eradicable.
Historical evidence shows that ancient Kingdoms in Sri Lanka of Anuradhapura, Polonnaruwa, Dambadeniys, Sigiriya, Yapahuwa, etc., time to time had been moved to new locations, mainly within the dry zonal regions that are currently affected by CKD-CTN. Whether these forced relocations were made for better protect from invading armies, severe malarial epidemics, or deaths due to CKD-CTN during those periods, is uncertain: perhaps the combination of all three.
Summary
CKD–CTN is caused by the consumption of concentrated and naturally contaminated groundwater (i.e., underground, geological reasons), where people live for decades: this fulfils the Bradford–Hill criteria of causation. The described novel concepts facilitate a deeper understanding of the aetiology of CKD–CTN and provide cost-effective opportunities to prevent the development of renal failure and premature deaths. A fundamental understanding of the new concept of develo** crystalline nephropathy, causing CKD–CTN, paves the path for cost-effective targeted solutions to protect peasants. In addition to providing potable water for affected communities, multiple interventions are necessary to overcome this silent killer [52]. Further research will confirm these concepts and develop improved, practical solutions.
Such approaches would save thousands of lives of people in CKD–CTN-affected tropical countries who regularly engage in strenuous physical work in hot environments and consume insufficient water. It is crucial to systematically address lifestyles and dietary habits to protect their renal health and improve micro-nutrition to mitigate CKD–CTN in vulnerable populations. The way to eradicate CKD–CTN is by preventing the disease, not by aggressively treating end-stage renal diseases, such as expanding renal clinics, dialysis centres, and renal transplantation services.
Data availability
Not applicable.
References
Wimalawansa SJ. The role of ions, heavy metals, fluoride, and agrochemicals: critical evaluation of potential aetiological factors of chronic kidney disease of multifactorial origin (CKDmfo/CKDu) and recommendations for its eradication. Environ Geochem Health. 2016;38(3):639–78. https://doi.org/10.1007/s10653-015-9768-y.
Ileperuma O, Weeraratne S, Wimalawansa SJ. Acronyms, CINAC, ACN, KDUCAL or NUCAL and so on are inappropriate to use for describing CKDu. J Epidemiol Community Health. 2018;72(10):967–8. https://doi.org/10.1136/jech-2018-210959.
Wijkstrom J, Elinder CG, Hultenby K, Soderberg M, Wernerson A. “Dysmorphic” lysosomes in proximal tubular cells are not specific for CINAC/CKDu and do not provide evidence that CINAC/CKDu is a toxin-induced disease. Kidney Int. 2020;98(3):786–7. https://doi.org/10.1016/j.kint.2020.04.057.
Chandrajith R, Nanayakkara S, Itai K, et al. Chronic kidney diseases of uncertain etiology (CKDue) in Sri Lanka: geographic distribution and environmental implications. Environ Geochem Health. 2011;33(3):267–78. https://doi.org/10.1007/s10653-010-9339-1.
Glaser J, Weiss I, La Isla F. CKDu: strategies for saving lives now. MEDICC Rev. 2014;16(2):81–2.
Wimalawansa SJ, Wimalawansa SA. Chronic kidney disease of multifactorial origin (CKDmfo) in Sri Lanka: escalating incidence and long-term survival estimates. J Nephrol Urol Res. 2015;22(4):1–17.
Dunuweera R, Shimomura RM, Priyankarage M, Jayasingha P, Wimalawansa SJ. Chronic kidney disease of multifunctional origin (CKDmfo) prevailing in Sri Lanka: re-evaluated. World J Pharma Res. 2017;6(16):33–66.
Wimalawansa SJ. Molecular and cellular toxicity of fluoride in mystery, tubulointerstitial chronic kidney disease: a systematic review. Rev Environ Sci Biotechnol. 2019. https://doi.org/10.1007/s11157-019-09521-0.
Wimalawansa SJ. Does fluoride cause the mysterious chronic kidney disease of multifactorial origin? Environ Geochem Health. 2020. https://doi.org/10.1007/s10653-019-00503-3.
Wimalawansa SJ, Dissanayake CB. Factors affecting the environmentally induced, chronic kidney disease of unknown aetiology in dry zonal regions in tropical countries—novel findings. Environments. 2019;7(1):1–26.
Roncal Jimenez CA, Ishimoto T, Lanaspa MA, et al. Fructokinase activity mediates dehydration-induced renal injury. Kidney Int. 2014;86(2):294–302. https://doi.org/10.1038/ki.2013.492.
Koh ET, Min KW. Dietary fructose produces greater nephrocalcinosis in female than in male magnesium-deficient rats. Magnes Res. 1991;4(2):97–103 (In Eng).
Moorthi RN, Moe SM. CKD-mineral and bone disorder: core curriculum 2011. Am J Kidney Dis. 2011;58(6):1022–36. https://doi.org/10.1053/j.ajkd.2011.08.009.
Shiizaki K, Tsubouchi A, Miura Y, et al. Calcium phosphate microcrystals in the renal tubular fluid accelerate chronic kidney disease progression. J Clin Invest. 2021. https://doi.org/10.1172/JCI145693.
Wimalawansa S, Ileperuma O, Weeraratne S. Attempts to change the globally accepted term, CKDu, to KDUCAL, NUCAL, or CINAC are inappropriate. Am J Kidney Dis. 2018;71(6):914. https://doi.org/10.1053/j.ajkd.2018.01.033.
Wimalawansa SJ. There is no evidence that organochlorine pesticides, such as DDT, cause chronic kidney disease of unknown etiology. Sci Total Environ. 2019;649:1636–7. https://doi.org/10.1016/j.scitotenv.2018.09.117.
Wimalawansa SJ. Renal tubular lysosomal vacuoles are a generic toxic manifestation and not particularly associated with agrochemicals and heavy metal toxicity or specific to a disease. Kidney Int. 2020;97(5):1058. https://doi.org/10.1016/j.kint.2020.01.021.
UNICEF, WHO. Progress on drinking water and sanitation: special focus on sanitation. Geneva: UNICEF/WHO; 2008.
Redmon JH, Elledge MF, Womack DS, et al. Additional perspectives on chronic kidney disease of unknown aetiology (CKDu) in Sri Lanka–lessons learned from the WHO CKDu population prevalence study. BMC Nephrol. 2014;15:125. https://doi.org/10.1186/1471-2369-15-125.
Dissanayake CB, Chandrajith R. Groundwater fluoride as a geochemical marker in the etiology of chronic kidney disease of unknown origin in Sri Lanka. Cey J Sci. 2007;46(2):3–12. https://doi.org/10.4038/cjs.v46i2.7425.
Piyathilake ID, Udeshani WA, Hapuarachchi HA, Ranaweera LV, Udayakumara EP, Gunatilake SK, Dissanayake CB. Geochemistry of groundwater in the Uva province, Sri Lanka—implications for chronic kidney disease of uncertain origin. Front Water. 2021;3: 771501. https://doi.org/10.3389/frwa.2021.771501.
Wickramarathna S, Balasooriya S, Diyabalanage S, Chandrajith R. Tracing environmental aetiological factors of chronic kidney diseases in the dry zone of Sri Lanka: a hydrogeochemical and isotope approach. J Trace Elem Med Biol. 2017;44:298–306. https://doi.org/10.1016/j.jtemb.2017.08.013.
Liyanage DND, Diyabalanage S, Dunuweera SP, Rajapakse S, Rajapakse RMG, Chandrajith R. Significance of Mg-hardness and fluoride in drinking water on chronic kidney disease of unknown etiology in Monaragala, Sri Lanka. Environ Res. 2022;203: 111779. https://doi.org/10.1016/j.envres.2021.111779.
Garcia-Trabanino R, Jarquin E, Wesseling C, et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador: a cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res. 2015;142:746–55. https://doi.org/10.1016/j.envres.2015.07.007.
Dharmagunawardhane HAD. CB fluoride problemsc in Sri Lanka. Environ Manag Health. 1993;4(2):9–16.
Aoba T. Recent observations on enamel crystal formation during mammalian amelogenesis. Anat Rec. 1996;245(2):208–18. https://doi.org/10.1002/(SICI)1097-0185(199606)245:2%3c208::AID-AR8%3e3.0.CO;2-S.
Freeman JJ, Wopenka B, Silva MJ, Pasteris JD. Raman spectroscopic detection of changes in bioapatite in mouse femora as a function of age and in vitro fluoride treatment. Calcif Tissue Int. 2001;68(3):156–62. https://doi.org/10.1007/s002230001206.
Wong TY, Wu CY, Martel J, et al. Detection and characterization of mineralo-organic nanoparticles in human kidneys. Sci Rep. 2015;5:15272. https://doi.org/10.1038/srep15272.
Czajka-Jakubowska AE, Liu J, Chang SR, Clarkson BH. The effect of the surface characteristics of various substrates on fluorapatite crystal growth, alignment, and spatial orientation. Med Sci Monit. 2009;15(6):MT84–8.
Zhang M, Zhou W, Liu S, Hao C. Phosphate nephropathy in Gitelman syndrome. Kidney Med. 2021;3(5):864–5. https://doi.org/10.1016/j.xkme.2021.04.021.
Perazella MA, Herlitz LC. The crystalline nephropathies. Kidney Int Rep. 2021;6(12):2942–57. https://doi.org/10.1016/j.ekir.2021.09.003.
Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17-172.
Martel J, Peng HH, Young D, Wu CY, Young JD. Of nanobacteria, nanoparticles, biofilms and their role in health and disease: facts, fancy and future. Nanomedicine (Lond). 2014;9(4):483–99. https://doi.org/10.2217/nnm.13.221.
Abo Markeb A, Alonso A, Dorado AD, Sanchez A, Font X. Phosphate removal and recovery from water using nanocomposite of immobilized magnetite nanoparticles on cationic polymer. Environ Technol. 2016;37(16):2099–112. https://doi.org/10.1080/09593330.2016.1141999.
Lenton S, Nylander T, Teixeira SC, Holt C. A review of the biology of calcium phosphate sequestration with special reference to milk. Dairy Sci Technol. 2015;95:3–14. https://doi.org/10.1007/s13594-014-0177-2.
Robinson C, Connell S, Kirkham J, Brookes SJ, Shore RC, Smith AM. The effect of fluoride on the develo** tooth. Caries Res. 2004;38(3):268–76. https://doi.org/10.1159/000077766.
Iijima M, Moradian-Oldak J. Control of apatite crystal growth in a fluoride containing amelogenin-rich matrix. Biomaterials. 2005;26(13):1595–603. https://doi.org/10.1016/j.biomaterials.2004.05.009.
Praga M, Gonzalez E. Acute interstitial nephritis. Kidney Int. 2010;77(11):956–61. https://doi.org/10.1038/ki.2010.89.
Praga M, Sevillano A, Aunon P, Gonzalez E. Changes in the aetiology, clinical presentation and management of acute interstitial nephritis, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant. 2015;30(9):1472–9. https://doi.org/10.1093/ndt/gfu326.
Tazoe N, Ikezaki N, Ito J, et al. A case of acute interstitial nephritis induced by flurbiprofen. Jpn J Med. 1987;26(2):230–3.
Wyne K, Wimalawansa SJ. Screening and diagnosis of chronic tubular kidney disease of multi-factorial origin. In: Dissanayake R, editor. 5th International conference on sustainable built environment; “environment pollution of prevention of CKD-mfo in Sri Lanka.” Kandy: ICSBE; 2014. p. 11–3.
D’Amico G, Bazzi C. Pathophysiology of proteinuria. Kidney Int. 2003;63(3):809–25. https://doi.org/10.1046/j.1523-1755.2003.00840.x.
Eknoyan G, Hostetter T, Bakris G, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK). Am J Kidney Dis. 2003;42:617–22.
Nanayakkara S, Komiya T, Ratnatunga N, et al. Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ Health Prev Med. 2012;17(3):213–21. https://doi.org/10.1007/s12199-011-0243-9.
Jayatilake N, Mendis S, Maheepala P, Mehta FR. Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a develo** country. BMC Nephrol. 2013;14(1):180. https://doi.org/10.1186/1471-2369-14-180.
Wimalawansa SJ. A cost-effective, interim solution to overcome water-pollution related human diseases. Euro J Biomed Pharma Sci. 2018;5(2):988–98.
Wimalawansa SJ. Agrochemicals and chronic kidney disease of multifactorial origin: environmentally induced occupational exposure disease. Int J Nephrol Kidney Failure. 2015;1(2):1–9. https://doi.org/10.16966/2380-5498.111.
An C, Akankwasa G, Liu J, et al. Urine markers of renal tubular injury in idiopathic membranous nephropathy: a cross sectional study. Clin Chim Acta. 2019;492:7–11. https://doi.org/10.1016/j.cca.2019.01.015.
Garcia-Nieto V, Garcia-Rodriguez VE, Luis-Yanes MI, Monge M, Arango-Sancho P, Garin EH. Renal tubular markers as screening tools for severe vesicoureteral reflux. Eur J Pediatr. 2019;178(4):525–31. https://doi.org/10.1007/s00431-019-03324-9.
Wimalawansa SJ. Escalating chronic kidney diseases in sri lanka: causes, solutions and recommendations. Environ Health Prev Med. 2014;19(6):375–94. https://doi.org/10.1007/s12199-014-0395-5.
Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol. 2009;11(3):134–44.
Wimalawansa SJ. Public health interventions for chronic diseases: cost-benefit modelizations for eradicating chronic kidney disease of multifactorial origin (CKDmfo/ CKDu) from tropical countries. Heliyon. 2019;5(10): e02309. https://doi.org/10.1016/j.heliyon.2019.e02309.
Ericsson Y, Luoma H, Ekberg O. Effects of calcium, fluoride and magnesium supplementations on tissue mineralization in calcium- and magnesium-deficient rats. J Nutr. 1986;116(6):1018–27. https://doi.org/10.1093/jn/116.6.1018. (In Eng).
Wasana HM, Aluthpatabendi D, Kularatne WM, Wijekoon P, Weerasooriya R, Bandara J. Drinking water quality and chronic kidney disease of unknown etiology (CKDu): synergic effects of fluoride, cadmium and hardness of water. Environ Geochem Health. 2016;38(1):157–68. https://doi.org/10.1007/s10653-015-9699-7.
Wasana HM, Perera GD, Gunawardena PS, Fernando PS, Bandara J. WHO water quality standards Vs synergic effect(s) of fluoride, heavy metals and hardness in drinking water on kidney tissues. Sci Rep. 2017;7:42516. https://doi.org/10.1038/srep42516.
Massy ZA, Drueke TB. Magnesium and cardiovascular complications of chronic kidney disease. Nat Rev Nephrol. 2015;11(7):432–42. https://doi.org/10.1038/nrneph.2015.74.
Ter Braake AD, Shanahan CM, de Baaij JHF. Magnesium counteracts vascular calcification: passive interference or active modulation? Arterioscler Thromb Vasc Biol. 2017;37(8):1431–45. https://doi.org/10.1161/ATVBAHA.117.309182.
Sakaguchi Y, Hamano T, Isaka Y. Magnesium and progression of chronic kidney disease: benefits beyond cardiovascular protection? Adv Chronic Kidney Dis. 2018;25(3):274–80. https://doi.org/10.1053/j.ackd.2017.11.001.
Yin S, Zhou Z, Lin T, Wang X. Magnesium depletion score is associated with long-term mortality ichronic kidney diseases: a prospective population-based cohort study. J Nephrol. 2023;36(3):755–65. https://doi.org/10.1007/s40620-022-01489-5.
Rotondi S, Mazzaferro S. Magnesium: extracellular, intracellular or total magnesium status? Nephrol Dial Transplant. 2023. https://doi.org/10.1093/ndt/gfad059.
Ray E, Mohan K, Ahmad S, Wolf MTF. Physiology of a forgotten electrolyte-magnesium disorders. Adv Kidney Dis Health. 2023;30(2):148–63. https://doi.org/10.1053/j.akdh.2022.12.001.
Koh ET, Reiser S, Fields M. Dietary fructose as compared to glucose and starch increases the calcium content of kidney of magnesium-deficient rats. J Nutr. 1989;119(8):1173–8. https://doi.org/10.1093/jn/119.8.1173. (In Eng).
Koh ET, Min KW. Fructose precipitates calcium phosphate in the kidneys of female rats fed magnesium-deficient diets. Magnes Res. 1991;4(3–4):171–6 (In Eng).
Rayssiguier Y, Gueux E, Nowacki W, Rock E, Mazur A. High fructose consumption combined with low dietary magnesium intake may increase the incidence of the metabolic syndrome by inducing inflammation. Magnes Res. 2006;19(4):237–43 (In Eng).
Khan SR. Nephrocalcinosis in animal models with and without stones. Urol Res. 2010;38(6):429–38. https://doi.org/10.1007/s00240-010-0303-4. (In Eng).
Sakan H, Nakatani K, Asai O, et al. Reduced renal alpha-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS ONE. 2014;9(1): e86301. https://doi.org/10.1371/journal.pone.0086301.
Buchanan S, Combet E, Stenvinkel P, Shiels PG. Klotho, aging, and the failing kidney. Front Endocrinol (Lausanne). 2020;11:560. https://doi.org/10.3389/fendo.2020.00560.
Gobalarajah K, Subramaniam P, Jayawardena UA, Rasiah G, Rajendra S, Prabagar J. Impact of water quality on Chronic Kidney Disease of unknown etiology (CKDu) in Thunukkai division in Mullaitivu district, Sri Lanka. BMC Nephrol. 2020;21(1):507. https://doi.org/10.1186/s12882-020-02157-1.
Wimalawansa SJ. Escalating chronic kidney diseases of multi-factorial origin (CKD-mfo) in Sri Lanka: causes, solutions, and recommendations-update and responses. Environ Health Prev Med. 2015;20(2):152–7. https://doi.org/10.1007/s12199-015-0447-5.
Wimalawansa SJ. Strategic framework for managing non communicable diseases: Preventing chronic kidney disease of multifactorial origin (CKDmfo/CKDu) as an Example. Chronic Dis Int. 2015;2(2):1–9.
Wimalawansa SJ. Purification of contaminated water with reverse osmosis: effective solution of providing clean water for human needs in develo** countries. J Emerg Technol Adv Eng. 2013;3(12):75–89.
Greenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P. Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res. 2009;43(9):2317–48. https://doi.org/10.1016/j.watres.2009.03.010.
Anupama PH, Prasad N, Nzana VB, Tiwari JP, Mathew M, Abraham G. Dietary management in slowing down the progression of CKDu. Indian J Nephrol. 2020;30(4):256–60. https://doi.org/10.4103/ijn.IJN_366_18.
Wimalawansm SJ. Strategic framework for managing non-communicable diseases: Preventing chronic kidney disease of multifactorial origin in Sri Lanka as an example. Chronic Dis Int. 2015;2(2):1–9.
de Francisco ÁL, Rodriguez M. Magnesium - its role in CKD. Nefrologia. 2013;33(3):389–99. https://doi.org/10.3265/Nefrologia.pre2013.Feb.11840.
Wimalawansa SJ. Effect of water hardness on non-communicable diseases including chronic kidney disease of multifactorial origin (CKDmfo/CKDuo). J Environ Health Sci Eng. 2016;2(1):1–11. https://doi.org/10.15436/2378-6841.16.029.
Wimalawansa SA, Wimalawansa SJ. Clean water, healthy environment, and preservation of watersheds: correct, enforceable policies are essential. Jacobs J Hydrol. 2015;1(1):3–15. https://doi.org/10.3390/w50x000x.
Balasooriya S, Munasinghe H, Herath AT, Diyabalanage S, Ileperuma OA, Manthrithilake M, Daniel C, Amann K, Zwiener C, Barth JAC, Chandrajith R. Possible links between groundwater geochemistry and chronic kidney disease of unknown etiology (CKDu): an investigation from the Ginnoruwa region in Sri Lanka. Expoure Health. 2020. https://doi.org/10.1007/s12403-019-00340-w.
Pendon-Ruiz de Mier MV, Rodelo-Haad C, Diaz-Tocados JM, Munoz-Castaneda JR, Rodriguez M. Magnesium: an old player revisited in the context of CKD-MBD. Clin Chim Acta. 2020;501:53–9. https://doi.org/10.1016/j.cca.2019.11.037.
Galassi A, Cozzolino M. Magnesium: a renewed player of vascular ageing in diabetic CKD patients? Clin Kidney J. 2014;7(2):93–6. https://doi.org/10.1093/ckj/sfu011.
Kula AJ, Bansal N. Magnesium and cardiovascular disease in CKD: the mysteries of a humble divalent cation. Kidney Med. 2021;3(2):162–4. https://doi.org/10.1016/j.xkme.2021.02.002.
Yang Y, Deng Y, Wang Y. Major geogenic factors controlling geographical clustering of urolithiasis in China. Sci Total Environ. 2016;571:1164–71. https://doi.org/10.1016/j.scitotenv.2016.07.117.
Edirisinghe E, Manthrithilake H, Pitawala H, Dharmagunawardhane HA, Wijayawardane RL. Geochemical and isotopic evidences from groundwater and surface water for understanding of natural contamination in chronic kidney disease of unknown etiology (CKDu) endemic zones in Sri Lanka. Isot Environ Health Stud. 2018;54(3):244–61. https://doi.org/10.1080/10256016.2017.1377704.
Funding
The authors received no grants funding or writing assistance from any source.
Author information
Authors and Affiliations
Contributions
SJW wrote the paper and contributed related research, while CBD provided intellectual input and editing. Both authors approved it for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors have no conflict of interest. This research received no grant or aid from funding agencies or commercial or not-for-profit sectors. They did not receive any funding or writing assistance.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wimalawansa, S.J., Dissanayake, C.B. Nanocrystal-induced chronic tubular-nephropathy in tropical countries: diagnosis, mitigation, and eradication. Eur J Med Res 28, 221 (2023). https://doi.org/10.1186/s40001-023-01162-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01162-y