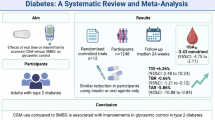
Abstract
Background
The use of minimally and non-invasive monitoring systems (including continuous glucose monitoring) has increased rapidly over recent years. Up to now, it remains unclear how accurate devices can detect hypoglycaemic episodes. In this systematic review and meta-analysis, we assessed the diagnostic accuracy of minimally and non-invasive hypoglycaemia detection in comparison to capillary or venous blood glucose in patients with type 1 or type 2 diabetes.
Methods
Clinical Trials.gov, Cochrane Library, Embase, PubMed, ProQuest, Scopus and Web of Science were systematically searched. Two authors independently screened the articles, extracted data using a standardised extraction form and assessed methodological quality using a review-tailored quality assessment tool for diagnostic accuracy studies (QUADAS-2). The diagnostic accuracy of hypoglycaemia detection was analysed via meta-analysis using a bivariate random effects model and meta-regression with regard to pre-specified covariates.
Results
We identified 3416 nonduplicate articles. Finally, 15 studies with a total of 733 patients were included. Different thresholds for hypoglycaemia detection ranging from 40 to 100 mg/dl were used. Pooled analysis revealed a mean sensitivity of 69.3% [95% CI: 56.8 to 79.4] and a mean specificity of 93.3% [95% CI: 88.2 to 96.3]. Meta-regression analyses showed a better hypoglycaemia detection in studies indicating a higher overall accuracy, whereas year of publication did not significantly influence diagnostic accuracy. An additional analysis shows the absence of evidence for a better performance of the most recent generation of devices.
Conclusion
Overall, the present data suggest that minimally and non-invasive monitoring systems are not sufficiently accurate for detecting hypoglycaemia in routine use.
Systematic review registration
PROSPERO 2018 CRD42018104812
Similar content being viewed by others
Background
Hypoglycaemia is a common side effect of diabetes treatment. On average, a patient with type 1 diabetes has two episodes of symptomatic hypoglycaemia per week and experiences 1.0 to 1.7 episodes of severe hypoglycaemia per year [1, 2]. The consequences of hypoglycaemia do not just include the immediate symptoms and mortality [3], hypoglycaemic events also have an enormous impact on the long-term outcome (increased cardiovascular risk, impaired cognitive function) [4, 5]. Therefore, current guidelines recommend that patients with type 1 diabetes self-monitor their blood glucose (SMBG) 4–10 times a day [6]. However, the adherence to SMBG via glucometer was reported to be as low as 44% for adults with type 1 diabetes and 24% for adults with type 2 diabetes [7, 8]. Minimally (MID) and non-invasive devices (NID) aim to facilitate diabetes control and improve patients’ adherence. With hypoglycaemia being one of the most threatening complications of diabetes mellitus, it is critical that these devices are capable of accurately detecting hypoglycaemic episodes, especially in those patients who are unaware of their hypoglycaemic episodes. Comparison of different devices and between different studies is challenging as there is no consensus on how to optimally assess the general accuracy over the whole glycaemic range and the binary accuracy of hypoglycaemia detection of MID and NID [9]. Consequently, studies report diagnostic accuracy in many different ways (e.g. sensitivity/specificity, MARD (mean absolute relative difference)), which are often not directly comparable to each other and/or of uncertain clinical relevance.
While many manufacturers of MID and NID advertise the safety and convenience with which those devices warn of hypoglycaemic episodes, there is no clear evidence how accurately they can actually detect hypoglycaemia. Therefore, in this systematic review, we aim to assess the diagnostic accuracy of hypoglycaemia detection of MID and NID.
Methods
The study protocol for this review was registered on PROSPERO on 27/07/2018 (CRD42018104812).
Data sources and searches
A literature search was conducted in June 2018 using the following databases: Clinical Trials.gov, Cochrane Library, Embase, PubMed, ProQuest, Scopus and Web of Science. Search phrases used for the search are given in supplement 1. These were reviewed with a healthcare librarian (NR) specialised in planning systematic reviews. We did not apply any language restriction. The references of included articles were scanned and the “related articles” feature in PubMed was used. We contacted manufacturers of MID and NID to seek unpublished data. To screen for newly published articles, we performed two updated searches (29th of March 2019 and 19th of December 2019). To search for articles investigating diagnostic accuracy of recently released devices, we additionally performed a pragmatic search on 26th of October 2020 in PubMed.
Study selection
We included any prospective, clinical diagnostic test accuracy study including children or adults with type 1 diabetes or type 2 diabetes, where MID or NID was compared to venous, capillary or arterial blood as a reference standard. Studies with only a sub-group eligible for inclusion were also included. The target condition was hypoglycaemia, determined by biochemical criteria with a glucose concentration of at least ≤100 mg/dl. Studies investigating different thresholds at the same time were also included. Studies eligible for inclusion should provide sufficient information on sensitivity and specificity of hypoglycaemia detection. Excluded were retrospective simulated data analyses of pre-existing data sets, in vitro studies, in vivo studies in species other than human and studies in participants with other types of diabetes (e.g. gestational diabetes or cystic fibrosis-related diabetes).
Data extraction
Two reviewers (NL and AK) independently assessed the eligibility of identified articles in a two-step approach ((1) abstract and title screening, (2) full-text screening). Endnote X5 and X8 (Clarivate Analytics, PA, USA) and Excel 2016 (Microsoft, Redmond, WA, USA) were used to catalogue the results. Disagreements among reviewers were resolved through consensus. The study selection process was reported in a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. Two reviewers (NL and AK) independently extracted data using a standardised data extraction form (supplement 3). Outstanding data were sought by a pre-specified procedure (two e-mails separated by a time interval of 2 weeks to the corresponding author).
Quality assessment
Two reviewers (NL and AK) independently assessed the quality of included studies using a review-tailored Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool [10]. Disagreements among reviewers were resolved through consensus. The outcome of the methodological quality assessment was presented in two tables, showing the individual study with their risk of bias in each of the four domains and a summary graph of all of studies. The tables were created using the Review Manager 5 Software [11]. The risk of bias was explored in sensitivity analyses by excluding studies with overall high risk of bias. The overall risk of bias was rated as high when two domains of the QUADAS-2 tool were at high risk of bias.
Data synthesis and analysis
For each study, contingency tables of hypoglycaemia detection comparing index test to reference standard were constructed and sensitivity and specificity for each study were calculated. If authors had performed diagnostic accuracy analysis for multiple thresholds, the main analysis was performed using the threshold value most commonly employed among the included studies. As data for multiple thresholds were available, we additionally analysed diagnostic accuracy with regard to the level of glucose (basis of this analysis was the hypoglycaemia definition of the American Diabetes Association, which defines a blood glucose value equal to or below 70 mg/dl (3.9 mmol/l) as hypoglycaemic) [4]. If data for more than one reference standard were available, the superior reference standard (venous blood and not capillary blood) was used for the main analysis. If data for more than one insertion site was given, the data on the officially approved insertion site was used for the main analysis.
To calculate pooled estimates for meta-analysis, the bivariate random effects model of Reitsma et al. [12] implemented in the madad package [13] for R software for statistical computing [14] was used. Paired forest plots and hierarchical summary receiver operating characteristic (SROC) curves were drawn using metaplot version 0.4 [15]. The magnitude of heterogeneity was visually examined in SROC curves and forest plots as recommended by the Cochrane Collaboration [16]. In addition, the effects of pre-specified covariates were explored via meta-regression and sub-group analyses. Sensitivity and specificity were calculated individually for pre-specified sub-groups and a likelihood-ratio test was used to assess the difference of sub-groups. The primary analysis included all eligible studies. To prove the robustness of findings, we excluded studies with high overall risk of bias according to the QUADAS-2 tool in the sensitivity analysis. Tests for funnel plot asymmetry have low power to detect publication bias in diagnostic accuracy studies when there is considerable heterogeneity and were therefore not performed [16,17,18].
Results
Literature search led to inclusion of 14 studies
The search was performed in December 2019 and identified 3416 nonduplicate results. Of those, 502 articles were identified as eligible by abstract and title screening and 14 articles containing 15 studies were included after the full-text screening. Figure 1 shows the PRISMA flow diagram. Supplement 2 gives an overview of reasons of the exclusion of studies which partly fulfilled inclusion criteria.
Details of included studies
Fourteen articles including 15 studies with a total of 733 patients were included into the final analysis. Characteristics of those studies are shown in Table 1. Eight studies were performed in North America, six in Europe and one in Asia. Most of the trials investigated the diagnostic accuracy of MID (MID: 13 studies, NID: 2 studies). Seven studies used capillary blood as the only reference standard test, six compared MID or NID to capillary and venous blood and two studies had venous blood as the only reference standard test. Eight studies addressed diagnostic accuracy in individuals with type 1 diabetes only, and in two of the included studies, just a sub-group of participants had diabetes. Different thresholds ranging from 40 to 100 mg/dl for hypoglycaemia detection were used. The most common threshold was 70 mg/dl (10 studies), which corresponds to the hypoglycaemia definition of the American Diabetes Association [4]. Three studies investigated diagnostic accuracy at different thresholds simultaneously. The mean age of participants ranged from 9.6 to 61.6 years and three studies included children. Laffel et al. encompass two independent trials. Therefore, the two trials were included separately: Laffel 2016, study 1, corresponds to the trial investigating diagnostic accuracy of the CGM (continuous glucose monitoring) G4 Platinum with its regular algorithm, whereas Laffel 2016, study 2, corresponds to the trial investigating diagnostic accuracy of G4 Platinum with a modified (Software 505) algorithm [21].
Methodological quality of included studies was often insufficient
The methodological quality of the included studies was assessed in four key domains ((1) patient selection, (2) index test, (3) reference standard and (4) flow and timing) using the established Quality Assessment of Diagnostic Accuracy Studies (QUADAS) 2 tool [10]. Figure 2 summarises the overall risk of bias and applicability concerns. In general, across all of the studies, the methodological quality was often classified as either insufficient or unclear. (1) With regard to patient selection, the risk of bias was generally unclear or high as most of the studies included non-random series of participants or excluded participants inappropriately. (2) Regarding the risk of bias for the index test, only two studies were rated as low risk of bias. Looking at the other studies, insufficient information or biased interpretation of the reference standard led to classification as unclear or high risk of bias. (3) The risk of bias for the reference standard test was rated as high in eight studies because of the use of an inferior reference standard (capillary instead of venous blood) or interpretation of the reference standard with knowledge on the index test result. (4) In general, the risk of bias with regard to the flow of timing was high because of an inappropriate interval between the index test and reference standard.
However, applicability concerns were generally lower as all of the studies included patients with type 1 or type 2 diabetes, and all of the studies investigated the detection of hypoglycaemia in MID or NID defined by an acknowledged reference standard. With regard to the patient selection, applicability concerns were high in two of the studies as only a sub-group of participants had diabetes and unclear in one study as there was not enough information provided on the participants.
a Risk of bias graph. b Risk of bias summary. Methodological quality was assessed on four key domains (1. patient selection, 2. index test, 3. reference standard and 4. flow and timing). Therein none of the studies was assessed as low risk of bias in all of the four key domains. Applicability concerns were assessed in three key domains (1. patient selection, 2. index test, 3. reference standard) with the QUADAS-2 tool. Applicability concerns were generally lower
MID and NID had a pooled mean sensitivity of 69.3% and a mean specificity of 93.3%
Pooling the data resulted in a relatively low mean sensitivity of 69.3% [95% CI: 56.8 to 79.4] and a mean specificity of 93.3% [95% CI: 88.2 to 96.3]. Diagnostic accuracy showed a great variation reflecting that in individual studies, sensitivity varied between 33.3 and 91% and specificity between 66 and 98.9%. Figure 3 displays the paired forest plot of sensitivity and specificity and the resulting summary receiver operating characteristic (SROC) curve.
a Forest plot of sensitivity and specificity with 95% confidence interval in brackets of minimally invasive devices (MID) and non-invasive devices (NID) for detection of hypoglycaemia in each study. Pooling all of the studies resulted in a mean sensitivity of 69.3% [95% CI: 56.8 to 79.4] and a mean specificity of 93.3% [95% CI: 88.2 to 96.3], n number of participants, threshold in mg/dl. b Summary receiver operating characteristic (SROC) curve for overall diagnostic accuracy to detect hypoglycaemia of MID and NID. SROC, solid curve; individual studies, circles; summary estimate, triangle; 95% confidence region, contour ellipsoid
Impaired diagnostic accuracy in the hypoglycaemic range of latest generation devices
The field of continuous glucose monitoring (CGM) is rapidly evolving, and devices with advanced techniques and algorithms are introduced regularly. Several studies investigating the latest generation devices were found in our systematic literature screening. However, none of those provided the data necessary to determine diagnostic accuracy in the hypoglycaemic range in terms of sensitivity and specificity [33,34,35,36]. This impeded formal inclusion of those studies into the meta-analysis. To avoid losing the valuable information contained in these studies, we moved on to perform an explorative sub-analysis on any data on diagnostic accuracy in the hypoglycaemic range provided in those studies: Wadwa et al. in their study report a missed detection rate of 26% and a false alert rate of 30% for hypoglycaemia < 60 mg/dl for the Dexcom G6 [37]. In the adult study population of Alva et al., FreeStyle Libre 2 missed 24% of the hypoglycaemic events (< 60 mg/dl) and 28% of alarms were false [38]. Szadkowska et al. investigated the diagnostic accuracy of Free Style Libre with new glucose algorithm measurement in children. They state that accuracy was best in stable glycaemic conditions and deteriorated significantly when glucose was falling abruptly. Furthermore, they report a significant tendency of Free Style Libre 2 FSL to overestimate blood glucose. Therefore, they recommend to double-check CGM values with SMBG measurement in hypoglycaemia [39]. Table 2 provides an overview on data on diagnostic accuracy in the whole glycaemic range and in hypoglycaemia of more recent devices.
Meta-regression analysis shows that heterogeneity is explained by 4 covariates
Next, we investigated if the studied index test technique (MID vs. NID) influenced sensitivity and specificity. MID were more often studied than NID: 13 studies investigated the diagnostic accuracy of MID while NID were only assessed in two of the included studies. For MID, sensitivity and specificity varied greatly and pooling the studies resulted in a mean sensitivity of 71.1% [95% CI: 57.6 to 81.7] (range 33.3 to 93) and a mean specificity of 94.2% [95% CI: 89.3 to 96.9] (range 66 to 99.1). Two studies assessed the performance of NID (sensitivity: Hathout et al. [31], 48% [95% CI: 33 to 62] and Johansen et al. [32], 67% [95% CI: 35 to 88]; specificity: Hathout et al. [31], 93% [95% CI: 89 to 95] and Johansen et al. [32], 69% [95% CI: 55 to 80]). Those are the only two studies included investigating the diagnostic accuracy of NID. Moreover, these NIDs are not commercially available anymore. Thus, the validity of a comparison of MID vs. NID is limited. Figure 3b summarises the study results colour coded by technique.
The included studies compared NID or MID to different reference standards. Seven studies used capillary blood as the only reference standard, six compared MID or NID to capillary and venous blood and two studies had venous blood as the only reference standard. Studies using venous blood as the reference standard indicated a higher sensitivity than studies using capillary blood as the reference standard (venous 81.6% [95% CI: 68.7 to 89.9] vs. capillary 52.9% [95% CI: 41.3 to 64.3], p-value: < 0.001***). Yet, a significant difference in specificity could not be observed (venous 94.5% [95% CI: 84.1 to 98.3] vs. capillary 92.1% [95% CI: 86.6 to 95.5], p-value: 0.55). The likelihood-ratio test confirmed the result (χ2: 9.81, p-value: 0.007**). The corresponding SROC is displayed in Fig. 4a. As venous reference standard test, YSI (Yellow springs instrument, YSI Inc, OH, USA) was used in all studies except one (Bay et al. [24] used Hitachi, Roche, Basel, Switzerland), whereas a number of different devices were used as capillary reference standard (e.g. Accu-Chek (Roche), StatStrip Xpress (Nova Biomedical), OneTouch Ultra 2 meter (Onetouch)).
a Summary ROC for MID and NID to detect hypoglycaemia (SROC curve, solid curve; study data, circles; summary estimate, triangle; 95% confidence region, contour ellipsoid; venous blood as the reference standard, grey; capillary blood as the reference standard, black). Pooled sensitivity was significantly higher in trials using venous blood as the reference standard, whereas the influence on pooled specificity was not significant. b Summary receiver operating characteristic (SROC) curve for overall diagnostic accuracy to detect hypoglycaemia of MID and NID. SROC, solid curve; study data, circles; summary estimate, triangle; 95% confidence region, contour ellipsoid; high number of participants (> 50), black; low number of participants (≤50), grey. The pooled sensitivity was significantly higher in studies investigating a larger study cohort
Most studies included a limited number of participants (2 studies investigated only 12 participants [27, 28] and one study only 14 participants [20]). Yet, the cohort of the largest study included 176 participants [21]. We investigated whether there is an association of study size with observed diagnostic accuracy. Indeed, the pooled sensitivity was higher in trials with a larger study cohort or multi-centre trials (larger study cohort (> 50 participants) or multi-centre: 81.1% [95% CI: 67.8 to 89.8] vs. low number of participants or single-centre: 52.2% [95% CI: 40.6 to 63.5], p-value: < 0.001***, χ2: 12.79, p-value: 0.00167**). There was no significant effect on pooled specificity. Corresponding SROC is given in Fig. 4b. There was also a high variability in the number of paired measurements. One study relied on only 99 paired measurements [32], while the highest number of paired measurements was 16,653 [19]. Analogous to the association of study size and sensitivity, the pooled sensitivity was higher in trials with a larger number of measurements (large number of measurements (≥1000): 74.8% [95% CI: 58.5 to 86.2] vs. small number of measurements: 58.8% [95% CI: 47.2 to 69.5], p-value: 0.207).
In many studies, in addition to sensitivity and specificity of hypoglycaemia detection, further parameters of accuracy were reported. Ten studies described accuracy in terms of mean absolute relative difference (MARD), which is a parameter that shows overall device accuracy over the whole glycaemic range. In trials showing a better overall accuracy expressed in a lower MARD, the mean sensitivity was higher (low MARD (≤ 10%): 92% [95% CI: 89.9 to 93.6] vs. high MARD: 61.7% [95% CI: 48.2 to 73.6], p-value: < 0.001***, χ2: 12.38, p-value: 0.00205**). An insignificant difference of mean specificity was also observed (low MARD: 97.5% [95% CI: 86.3 to 99.6] vs. high MARD: 93.2% [95% CI: 86.4 to 96.8], p-value: 0.216). Corresponding SROC is given in supplement 4. In this context, also other parameters of accuracy, like the correlation coefficient and percentage of measurements in zones A and B of the Clarke Error Grid Analysis, showed a similar relationship with pooled sensitivity and specificity.
Covariates relating to the study setting were analysed. Here (1) artificial adjustment of blood glucose, (2) funding by manufacturers and (3) age of the study showed an influence on diagnostic accuracy. (1) Artificial adjustment of blood glucose via insulin administration (“insulin challenge”) was associated with a highly significant increase of pooled sensitivity (insulin administration: 85.6% [95% CI: 72 to 93.2] vs. no insulin administration: 55.6% [95% CI: 45.5 to 65.2], p-value: < 0.001***). An association with specificity was not found (insulin administration: 95% [95% CI: 80.7 to 98.9] vs. no insulin administration: 93.5 % [95% CI: 89.3 to 96.1], p-value: 0.693). (2) Furthermore, in studies funded by manufacturers, there was a significant difference of pooled sensitivity (manufacturer-funding: 82% [95% CI: 65.9 to 91.5] vs. no-manufacturer-funding: 59.2% [95% CI: 44 to 72.9], p-value: 0.031*, χ2: 6.717, p-value: 0.0348*), while no influence on specificity was seen (manufacturer-funding: 92.5 [95% CI: 75 to 98.1] vs. no-manufacturer-funding: 93.8% [95% CI 88.5 to 96.8], p-value 0.793). (3) The age of the study showed a relationship with measured diagnostic accuracy. Newer studies revealed a non-significantly higher sensitivity (new studies: 75.8% [95% CI: 59.4 to 87] vs. old studies: 57.5% [95% CI: 45.9 to 68.3], p-value: 0.086) and a slightly higher specificity (new studies: 94.9% [95% CI: 88.2 to 97.9] vs. old studies: 90% [95% CI: 82.4 to 94.6], p-value: 0.258). The location of the study (hospital vs. other (home/outdoor)) did not have a significant influence on pooled sensitivity or specificity.
Interestingly, no association of participant characteristics (including mean age, gender, proportion of participants with type 1 diabetes and BMI) with pooled sensitivity and specificity was observed. Two studies also included participants that did not have diabetes (Lee et al. [27], 42% of participants had diabetes; Rabiee et al. [30], 64% of participants had diabetes). The sensitivity was notably lower in studies including patients without diabetes (pooled sensitivity: 42.2% [95% CI: 21.9 to 65.59] vs. 71.4% [95% CI: 58.3 to 81.6]), whereas there was no difference in specificity (pooled specificity: 91.5% [95% CI: 73.9 to 97.6] vs. 93.6% [95% CI: 87.8 to 96.7]).
The included studies employed different thresholds ranging from 40 to 100 mg/dl for hypoglycaemia detection. The most common threshold was 70 mg/dl (10 studies), which corresponds to the hypoglycaemia definition of the American Diabetes Association [4]. Only pooling data of studies applying the threshold recommended by the American Diabetes Association resulted in a slightly higher pooled sensitivity (71.1% [95% CI: 55.9 to 82.7]) and a slightly higher pooled specificity (95.8% [95% CI: 92.4 to 97.8]). Three studies investigated diagnostic accuracy for different thresholds simultaneously. Inclusion of these data in additional meta-regression analyses showed that, as expected, higher cut-off values were associated with increased sensitivity and decreased specificity. A corresponding forest plot is given in supplement 5.
To investigate whether the findings of this systematic review are robust, sensitivity analyses were undertaken. As occasionally the quality of included studies was unsatisfactory, the influence of studies of poor quality on the results was analysed: Exclusion of studies with high risk of bias according to the QUADAS-2 tool did not have a notable influence on sensitivity.
Rate of device failure is reported as high
Additionally, the performance of different devices was analysed. Ten out of the 15 studies reported on sensor stability. All in all, the device failure rate is reported as high throughout the studies. In the study of Adolfsson et al., 42% of the participants needed a device replacement during the trial of three days duration [28]. However, as this study investigated the diagnostic accuracy of CGMS Gold (Medtronic) in the context of diving, this may underestimate the actual stability in a normal setting. Yet also, Hathout et al. report that 33% of the HypoMon measurements were unusable [31]. Reasons for the high rate of device malfunction are not always discussed, but calibration and transmission failures are reported.
Side effects and adverse events are common
Furthermore, side effects and adverse events of different devices were analysed. Six out of the 15 studies reported on side effects. Two studies reported the occurrence of no side effects or adverse events [24, 29], whereas the rate of reported side effects was high in the other studies. The highest number of side effects was seen by Hathout et. al., where 35% of the participants withdrew because of side effects [31]. The studies from Christiansen et al. and Bode et al. reported both a similar rate of side effects of approximately 10% [19, 26]. Most of the side effects were instances of mild irritation, bleeding or discomfort. However, two more notable side effects were reported by Christiansen et al.: First, two events were described where a small element presumably has been translocated into the participant’s body. Those two events are rated as mild in severity due to small size and biocompatibility. Second, a device could not be removed in local anaesthesia as planned but general anaesthesia was required. This event was adjudicated as serious [19].
Strengths and weaknesses of individual devices
In this presented work, some devices seemed to be more accurate than others. However, in addition to pure accuracy, other factors relating to the use of MID or NID might be important from the patient’s perspective. In this meta-analysis, Eversense (Senseonics, Inc., USA) revealed the highest sensitivity and specificity in detection of hypoglycaemic events. In contrast to other devices, Eversense can be used for relatively long periods (up to 90 days) and the transmitter can be removed and replaced. Calibration is needed twice daily. On the other hand, the sensor cannot be placed by the patients themselves but by a healthcare professional. The placement is more invasive than the procedure for other MID, and the rate of side effects of Eversense was higher and more serious compared to other MID. The second highest accuracy in detection of hypoglycaemic events was seen in Dexcom G4 Platinum (DexCom Inc, USA). However, contemplating the results of this meta-analysis, diagnostic accuracy showed a great variation (sensitivity ranged from 54.7 to 91.2 %). The sensor of this particular device can be worn for up to 7 days, calibration is needed twice daily and the sensor can be placed by the patients themselves. The sensor stability seems to be satisfactory and the rate of side effects seems to be low.
Discussion
In this work, we provide a comprehensive review and meta-analysis on the diagnostic accuracy of MID and NID for hypoglycaemia detection in patients with type 1 diabetes and type 2 diabetes. Fifteen studies with a total of 733 participants evaluating the diagnostic accuracy of hypoglycaemia detection of MID and NID were included. The mean sensitivity was 69.3% and the mean specificity was 93.3%. There was remarkable heterogeneity among the included studies. Meta-regression analyses revealed an association of type of reference standard test (venous vs. capillary blood), number of participants, reported overall performance, artificial manipulation of blood glucose and funding by manufacturers with device performance in hypoglycaemia detection. Pooled sensitivity was significantly higher in studies funded by device manufactures. Different reasons might contribute to this association. The study design might have been more rigorous in trials funded by manufacturers. This concept is supported by the fact that the sample size was generally higher and venous blood was used more often as the reference standard in those studies. On the other hand, in manufacturer-funded studies, trial protocols might have been chosen that tend to overestimate device performance. And indeed, induced hypoglycaemia by insulin administration was more commonly performed in these studies.
Additionally, we found that there is a notable rate of side effects and adverse events (in one case even a serious side effect). Furthermore, the sensor stability was reported as relatively poor throughout the studies.
While this work, to the best of our knowledge, for the first time reviews systematically the accuracy of MID and NID in detection of hypoglycaemia, a recent non-systematic review also sees limitations in the diagnostic accuracy of MID and NID and raises concerns regarding the frequency of false-positive alarms [44]. Interestingly, Howsmon et al. praise the high sensor accuracy and alarm sensitivity of CGM systems in their non-systematic review [45]. A reason for this discordant conclusion might be the fact that the authors make the assumption that an improved sensor accuracy in the hypoglycaemic range can be translated into providing more accurate hypoglycaemic alarms, which might not always follow. Notably, the authors of the UK recommendation on one particular, currently very popular device (FreeStyle Libre) are aware of these limitations as they recommend to validate hypoglycaemic values measured with FreeStyle Libre via finger-prick blood glucose testing [46].
Even though the present review reveals that an accurate detection of hypoglycaemic events can likely not be achieved with MID and NID, a recent meta-analysis has found that patients using MID spend less time in hypoglycaemia than patients using SMBG [47]. This finding could be due to reduced detection of hypoglycemic events; however, other reasons may lead to a reduction of time spent in hypoglycaemia, for example because users may be able to recognise a trend towards hypoglycaemia and take precautionary steps accordingly.
Interestingly, Koziel et al. found in their non-systematic review that this reduction of time in hypoglycaemia does not correlate with device accuracy in terms of MARD. However, in kee** with our findings, they reported a significant relationship between MARD and the detection of hypoglycaemic events [48].
Implications for clinical practice
The aim of MID and NID is the accurate and user-friendly monitoring of glucose levels. The results of this review indicate that most devices are not yet able to detect hypoglycaemia with sufficient accuracy. In 1 year of using an average MID or NID, according to the results of this meta-analysis, a patient with type 1 diabetes is expected to experience about 17 false-positive alarms and about 32 false-negative measurements. Underlying this estimate is an incidence of two episodes of symptomatic hypoglycaemia per week per patient [1, 2]. The high number of false-positive alarms (especially during the night) may lead to user frustration, alarm fatigue and cessation of device use. Even worse, subsequent alarms may not be taken seriously and true hypoglycaemic events may be missed. The number of false-negative events is equally concerning, as a missed hypoglycaemic episode may be a life-threatening event. This is especially problematic when MID and NID do not confirm hypoglycaemia in the presence of related symptoms, especially during rapid changes in glucose levels [49]. This increases the risk of delayed hypoglycaemia detection. Therefore, based on the available data, MID and NID do not appear to be sufficiently accurate to replace SMBG for the detection of hypoglycaemic episodes on its own. Values measured via MID or NID in or near the hypoglycaemic range should be double-checked with another method (e.g. capillary blood).
Implications for future research
As we also observed a lack of robust high-quality studies, larger and methodologically optimised works are needed to assess the accuracy of hypoglycaemia detection of MID and NID. The risk of bias was specifically high in terms of patient selection. Future studies should take care of including the relevant population (e.g. people unaware of hypoglycaemia should not be excluded). Investigating the comparative diagnostic accuracy among MID and NID is highly challenging [50]. Studies in which all patients are tested with different devices or are randomly assigned to receive one or another device (direct comparative studies/head-to-head) are needed [51]. This systematic review was not designed to provide a complete overview on adverse events and device failure. However, our data are indicative of a high number of adverse events and system failures, and this is likely to be an underestimate as harms may be underreported [52]. Therefore, further studies investigating the actual number and severity of side effects, and analysis of the sensor stability as well as reasons for system failure are mandatory.
Strengths and limitations
This systematic review provides the first comprehensive review of the current evidence on the diagnostic accuracy of MID and NID for the detection of hypoglycaemia. However, some limitations need to be considered: It is generally challenging to investigate the diagnostic accuracy of MID or NID. Therefore, the quality of articles in this field of research often appears imperfect. Frequently, the incomplete reporting in the included studies impeded the assessment of their methodological quality. In particular, there was uncertainty with regard to the index test and the patient selection. This might lead to an overestimation of the accuracy of hypoglycaemia detection of NID and MID by the present systematic review. On the other hand, MID/NID technology is continuously being improved; therefore, our review may demonstrate an underestimation of diagnostic accuracy compared to the most recent devices. However, meta-regression analyses have only revealed an insignificant trend regarding an influence of the year of publication on diagnostic accuracy.
Conclusions
The present data show that MID and NID are not sufficiently accurate for detecting hypoglycaemia in type 1 diabetes and type 2 diabetes in routine use. The indicated diagnostic accuracy was associated with a variety of factors including the type of reference standard test, study size, general device performance, artificial manipulation of blood glucose and study funding source. Additionally, we saw a notable rate of side effects and adverse events and a limited sensor stability.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- CGM:
-
Continuous glucose monitoring
- ISO:
-
International Organisation of Standardisation
- MARD:
-
Mean absolute relative deviation
- MID:
-
Minimally invasive monitoring device
- NID:
-
Non-invasive monitoring device
- NICE:
-
National Institute for Health and Care Excellence
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- QUADAS:
-
Quality Assessment of Diagnostic Accuracy Studies
- SMBG:
-
Self-monitoring of blood glucose
- SROC:
-
Summary receiver operating characteristic
- YSI:
-
Yellow springs instrument
- 95% CI:
-
95% confidence interval
References
BM F. The incidence and impact of hypoglycemia in type 1 and type 2 diabetes. Int Diab Monit. 2009;21:210–8.
McCrimmon RJ, Sherwin RS. Hypoglycemia in type 1 diabetes. Diabetes. 2010;59(10):2333–9. https://doi.org/10.2337/db10-0103.
Lee AK, Juraschek SP, Windham BG, et al. Severe hypoglycemia and risk of falls in type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diab Care. Jul 1 2020.
Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diab Care. 2013;36(5):1384–95. https://doi.org/10.2337/dc12-2480.
Johnson-Rabbett B, Seaquist ER. Hypoglycemia in diabetes: the dark side of diabetes treatment. A patient-centered review. J Diab. Apr 15 2019.
NICE-guideline. Type 1 diabetes in adults: diagnosis and managment. 2015.
Patton SR. Adherence to glycemic monitoring in diabetes. J Diab Sci Technol. 2015;9(3):668–75. https://doi.org/10.1177/1932296814567709.
Mostrom P, Ahlen E, Imberg H, Hansson PO, Lind M. Adherence of self-monitoring of blood glucose in persons with type 1 diabetes in Sweden. BMJ Open Diab Res Care. 2017;5(1):e000342. https://doi.org/10.1136/bmjdrc-2016-000342.
Wentholt IM, Hoekstra JB, DeVries JH. A critical appraisal of the continuous glucose-error grid analysis. Diab Care. 2006;29(8):1805–11. https://doi.org/10.2337/dc06-0079.
Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. Oct 18 2011;155(8):529-536.
The Nordic Cochrane Centre, The Cochrane Collaboration [computer program]. Version Version 5.3. Copenhage; 2014.
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–90. https://doi.org/10.1016/j.jclinepi.2005.02.022.
Doebler P. mada: meta-analysis of diagnostic accuracy. 2017.
R: a language and environment for statistical computing [computer program]. Version. Vienna; 2008.
metaplot [computer program]. Version 0.4; 2019.
Macaskill P GC, Deeks JJ, Harbord RM. Chapter 10: analysing and presenting results. The Cochrane Collaboration. Cochrane handbook for systematic reviews of diagnostic test accuracy version 1.0.0. 2010.
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–93. https://doi.org/10.1016/j.jclinepi.2005.01.016.
van Enst WA, Ochodo E, Scholten RJ, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol. 2014;14:70.
Christiansen MP, Klaff LJ, Brazg R, Chang AR, Levy CJ, Lam D, et al. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol Ther. 2018;20(3):197–206. https://doi.org/10.1089/dia.2017.0142.
Steineck IIK, Mahmoudi Z, Ranjan A, Schmidt S, Jorgensen JB, Norgaard K. Comparison of continuous glucose monitoring accuracy between abdominal and upper arm insertion sites. Diabetes Technol Ther. 2019;21(5):295–302. https://doi.org/10.1089/dia.2019.0014.
Laffel L. Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther. 2016;18(Suppl 2):S223–33. https://doi.org/10.1089/dia.2015.0380.
Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9(2):209–14. https://doi.org/10.1177/1932296814559746.
Nakamura K, Balo A. The accuracy and efficacy of the Dexcom G4 platinum continuous glucose monitoring system. J Diabetes Sci Technol. Mar 23 2015;9(5):1021-1026.
Bay C, Kristensen PL, Pedersen-Bjergaard U, Tarnow L, Thorsteinsson B. Nocturnal continuous glucose monitoring: accuracy and reliability of hypoglycemia detection in patients with type 1 diabetes at high risk of severe hypoglycemia. Diabetes Technol Ther. 2013;15(5):371–7. https://doi.org/10.1089/dia.2013.0004.
Zijlstra E, Heise T, Nosek L, Heinemann L, Heckermann S. Continuous glucose monitoring: quality of hypoglycaemia detection. Diabetes Obes Metab. 2013;15(2):130–5. https://doi.org/10.1111/dom.12001.
Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, et al. Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: the guardian continuous monitoring system. Diabetes Technol Ther. 2004;6(2):105–13. https://doi.org/10.1089/152091504773731285.
Lee JH, Kim K, Jo YH, Rhee JE, Lee JC, Kim KS, et al. Feasibility of continuous glucose monitoring in critically ill emergency department patients. J Emerg Med. 2012;43(2):251–7. https://doi.org/10.1016/j.jemermed.2011.06.037.
Adolfsson P, Ornhagen H, Jendle J. Accuracy and reliability of continuous glucose monitoring in individuals with type 1 diabetes during recreational diving. Diabetes Technol Ther. 2009;11(8):493–7. https://doi.org/10.1089/dia.2009.0017.
Guerci B, Floriot M, Bohme P, Durain D, Benichou M, Jellimann S, et al. Clinical performance of CGMS in type 1 diabetic patients treated by continuous subcutaneous insulin infusion using insulin analogs. Diabetes Care. 2003;26(3):582–9. https://doi.org/10.2337/diacare.26.3.582.
Rabiee A, Andreasik V, Abu-Hamdah R, et al. Numerical and clinical accuracy of a continuous glucose monitoring system during intravenous insulin therapy in the surgical and burn intensive care units. J Diabetes Sci Technol. Jul 1 2009;3(4):951-959.
Hathout E, Patel N, Southern C, Hill J, Anderson R, Sharkey J, et al. Home use of the GlucoWatch G2 biographer in children with diabetes. Pediatrics. 2005;115(3):662–6. https://doi.org/10.1542/peds.2004-0820.
Johansen K, Ellegaard S, Wex S. Detection of nocturnal hypoglycemia in insulin-treated diabetics by a skin temperature--skin conductance meter. Acta Med Scand. 1986;220(3):213–7. https://doi.org/10.1111/j.0954-6820.1986.tb02753.x.
Guillot FH, Jacobs PG, Wilson LM, et al. Accuracy of the Dexcom G6 glucose sensor during aerobic, resistance, and interval exercise in adults with type 1 diabetes. Biosensors (Basel). Sep 29 2020;10(10).
Tripyla A, Herzig D, Joachim D, Nakas CT, Amiet F, Andreou A, et al. Performance of a factory-calibrated, real-time continuous glucose monitoring system during elective abdominal surgery. Diabetes Obes Metab. 2020;22(9):1678–82. https://doi.org/10.1111/dom.14073.
Denham D. A head-to-head comparison study of the first-day performance of two factory-calibrated CGM systems. J Diabetes Sci Technol. 2020;14(2):493–5. https://doi.org/10.1177/1932296819895505.
Sadhu AR, Serrano IA, Xu J, et al. Continuous glucose monitoring in critically ill patients with COVID-19: results of an emergent pilot study. J Diabetes Sci Technol. Oct 16 2020:1932296820964264.
Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol Ther. Jun 2018;20(6):395–402. https://doi.org/10.1089/dia.2018.0150.
Alva S, Bailey T, Brazg R, et al. Accuracy of a 14-day factory-calibrated continuous glucose monitoring system with advanced algorithm in pediatric and adult population with diabetes. J Diabetes Sci Technol. Sep 19 2020:1932296820958754.
Szadkowska A, Michalak A, Losiewicz A, et al. Impact of factory-calibrated Freestyle Libre System with new glucose algorithm measurement accuracy and clinical performance in children with type 1 diabetes during summer camp. Pediatr Diabetes. Oct 9 2020.
Castorino K, Polsky S, O'Malley G, et al. Performance of the Dexcom G6 continuous glucose monitoring system in pregnant women with diabetes. Diabetes Technol Ther. Apr 23 2020.
Welsh JB, Zhang X, Puhr SA, Johnson TK, Walker TC, Balo AK, et al. Performance of a factory-calibrated, real-time continuous glucose monitoring system in pediatric participants with type 1 diabetes. J Diabetes Sci Technol. 2019;13(2):254–8. https://doi.org/10.1177/1932296818798816.
Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428–33. https://doi.org/10.1089/dia.2018.0143.
Fokkert M, van Dijk PR, Edens MA, et al. Performance of the Eversense versus the Free Style Libre Flash glucose monitor during exercise and normal daily activities in subjects with type 1 diabetes mellitus. BMJ Open Diabetes Res Care. 2020;8(1).
Avari P, Reddy M, Oliver N. Is it possible to constantly and accurately monitor blood sugar levels, in people with type 1 diabetes, with a discrete device (non-invasive or invasive)? Diabet Med. Feb 25 2019.
Howsmon D, Bequette BW. Hypo- and hyperglycemic alarms: devices and algorithms. J Diabetes Sci Technol. Apr 30 2015;9(5):1126-1137.
NICE. FreeStyle Libre for glucose monitoring. 2017.
Ida S, Kaneko R, Murata K. Utility of real-time and retrospective continuous glucose monitoring in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. J Diabetes Res. 2019;2019:4684815.
Koziel CBD, Morel D, Petisce J, Saliu D. Impact of continuous glucose monitors’ accuracy on their clinical utility—a quantitative assessment. Diabetes. 2018;67(Supplement 1).
Hansen EA, Klee P, Dirlewanger M, Bouthors T, Elowe-Gruau E, Stoppa-Vaucher S, et al. Accuracy, satisfaction and usability of a flash glucose monitoring system among children and adolescents with type 1 diabetes attending a summer camp. Pediatr Diabetes. 2018;19(7):1276–84. https://doi.org/10.1111/pedi.12723.
Bailey TS. Clinical implications of accuracy measurements of continuous glucose sensors. Diabetes Technol Ther. 2017;19(S2):S51–4. https://doi.org/10.1089/dia.2017.0050.
Takwoingi Y, Leeflang MM, Deeks JJ. Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Ann Intern Med. Apr 2 2013;158(7):544-554.
Golder S, Loke YK, Wright K, Norman G. Reporting of adverse events in published and unpublished studies of health care interventions: a systematic review. PLoS Med. 2016;13(9):e1002127. https://doi.org/10.1371/journal.pmed.1002127.
Acknowledgements
The authors would like to acknowledge Nia Roberts (NR) for reviewing the search strategy and Annette Pluddemann for her advice on diagnostic accuracy studies.
Funding
No funding was received for this work. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
NL conducted the systematic review, extracted data, researched data and wrote the manuscript. AK helped to conduct the systematic review, extracted data and reviewed and edited the manuscript. TH helped with the interpretation of study findings and reviewed and edited the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1 Supplement 1:
Search Strategies. Supplement 2: Reason for exclusion of articles, which partly met inclusion criteria. Supplement 3: Data extraction sheet. Supplement 4: Summary ROC for MID and NID to detect hypoglycaemia for studies indicating a lower MARD compared to studies indicating a higher MARD. Supplement 5: Forest plot of sensitivity and specificity with 95% confidence interval of MID and NID for detection of hypoglycaemia in studies applying different thresholds simultaneously. All of the supplements are provides as word file (.txt).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lindner, N., Kuwabara, A. & Holt, T. Non-invasive and minimally invasive glucose monitoring devices: a systematic review and meta-analysis on diagnostic accuracy of hypoglycaemia detection. Syst Rev 10, 145 (2021). https://doi.org/10.1186/s13643-021-01644-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-021-01644-2