Abstract
Background
The multitargeting tyrosine kinase inhibitor (TKI) sunitinib is currently the first-line drug therapy for metastasizing renal cell carcinoma (RCC). TKIs have profound effects on tumor angiogenesis, leading to modifications of the tumor microenvironment. The goal of this study was to determine whether these treatment-induced changes can be detected with [18F]FAZA.
Methods
The present study utilized positron emission tomography (PET) to analyze tumor oxygenation status during and after sunitinib therapy in the murine Caki-1 RCC tumor model. Dynamic and static scans were performed, as well as ex vivo biodistributions at 3 h post injection (p.i.). Immunohistochemical analysis of tumor tissue was carried out for the quantification of pimonidazole binding and the hypoxia-associated factors CD-31, Ki-67, and Von Willebrand factor (VWF). In addition, in vitro cellular uptake studies were done to analyze the direct effects of sunitinib on the Caki-1 cells.
Results
During therapy with sunitinib (40 mg/kg/day), uptake of [18F]FAZA into Caki-1 mice decreased by 46±5% (n = 4; 5 days) at 3 h post injection (p.i.) during the first study and 22±5% (n = 8; 9 days) during the long-term study, indicating a decrease in the tumor's hypoxia level. However, when drug therapy was stopped, this effect was reversed completely, and the tumor [18F]FAZA uptake increased to 126±6% (n = 6) of the control tumor uptake, indicative of an even higher level of tumor hypoxia compared to the therapy starting point. Sunitinib had no direct effect on [18F]FAZA uptake into Caki-1 cells in vitro.
Conclusion
[18F]FAZA PET could be used to monitor drug response during sunitinib therapy in RCC and may guide combination therapies based on the tumor's hypoxia status.
Similar content being viewed by others
Background
Renal cell carcinoma (RCC) is the third most diagnosed urological malignancy, with 75% of cases being the clear cell RCC histological subtype [1]. RCC arises in the proximal tubule of the nephron within the kidney; its symptoms present late, often preventing diagnosis early enough to lead to a favorable prognosis [2]. The American Cancer Society estimated 65,150 new cases and 13,680 deaths in the USA in 2013, a significant increase compared to the 2005 prediction statistics of 36,160 new cases and 12,660 deaths [3],[4]. Between 2005 and 2009, the overall incidence of kidney cancer in the USA increased 3.1% per year [3], which is comparable to the observed 2% increase over the past two decades worldwide and within Europe [5]. Barring early intervention, many patients will experience metastatic disease and require systemic therapy. Metastatic RCC (mRCC) rarely shows a complete and durable response to immuno- or radiation therapy [6]. Significant improvements in patient management have been achieved, however, with the development of targeted small molecules including tyrosine kinase inhibitors (TKIs) such as sunitinib, sorafenib, pazopanib, and axitinib [7]-[9]. Two types of TKIs are known to date. Termed type I and type II, they differ in their mode of action at the ATP binding site of the tyrosine kinase [10]. Sunitinib represents a TKI of type I, which targets mainly the vascular endothelial growth factor receptor 2 (VEGFR 2) and the platelet-derived growth factor receptors (PDGFR) α and β, the stem cell growth factor c-KIT (CD117), the RET proto-oncogene, and the Fms-like tyrosine kinase 3 (FLT-3). In addition, sunitinib has also been shown to inhibit 72 other kinases [3). As dynamic PET experiments revealed, lower levels of radiotracer were detected and these decreased over the 3-h time frame. In treated animals, [18F]FAZA uptake into the Caki-1 tumors reached its maximum at 30 min p.i. (SUVmean of 0.38 ± 0.02 (n = 4/2)) and decreased to a final SUVmean,3h of 0.23 ± 0.02 (n = 4/2; p < 0.05). In control tumors, a SUVmean of 0.44 ± 0.05 was measured after 60 min and 0.42 ± 0.05 (n = 4 tumors/two mice) after 3 h p.i. This observation showed clearance of [18F]FAZA and therefore less trap** of the radiotracer, indicative for a lower level of hypoxia in the treated versus the non-treated tumors. Biodistribution analysis of tumor tissue after the PET experiments confirmed these findings: 2.20 ± 0.30% ID/g in the controls versus 1.38 ± 0.13% ID/g (n = 8/4; p < 0.05) in the sunitinib-treated tumors.
[18F]FAZA images in Caki-1 tumor mice in the absence (left) and presence (right) of sunitinib. The insets show the respective transaxial slides of both mice. Time-activity curves for the radioactivity uptake into Caki-1 tumor in the absence and presence of sunitinib in vivo (bottom left). Image data are shown as maximum intensity projection (MIP) at 3 h post injection and TAC data as SUV and mean ± SEM from four tumors grown in two mice. Kinetic constants k1 to k4 were calculated based on a two-compartment model (bottom right). Changes were significant for k2 and k4 (*p < 0.05).
Uptake and retention of [18F]FAZA in Caki-1 tumors was further analyzed by kinetic modeling using a two-compartment model [41]. All four kinetic parameters k1 to k4 were calculated for control as well as for the sunitinib-treated tumors (Figure 3, bottom right). In the presence of sunitinib, all constants were found to be increased, significantly even for k2 and k4 with a p < 0.05. Once diffused into the cell, [18F]FAZA either diffuses back out of the cell or is reduced for further intracellular binding. The first process is governed by the rate constant k2 and is elevated under sunitinib therapy, as is the latter, which is governed by k3. The ratio k3/(k2 + k3), however, remains unchanged, indicating that once it has crossed the cell membrane, a similar fraction of [18F]FAZA will be further reduced in the presence or absence of sunitinib. Once reduced, re-oxygenation of [18F]FAZA is governed by the rate constant k4. It is amplified in the presence of sunitinib, resulting in the observed reduction in uptake under treatment (Figure 3). Analysis of the fractional blood volume (fbv) revealed similar values in the absence and presence of sunitinib: 0.160 ± 0.032 and 0.148 ± 0.036 (n = 4), respectively, indicating similar overall perfusion of the tumor volume (yet not necessarily the microenvironment, which can display hypoxia).
Following PET imaging and biodistribution experiments, tumor tissue slices were analyzed immunohistochemically. Figure 4 summarizes the results for pimonidazole, CD-31, VWF, and Ki67 staining. Treatment of Caki-1 tumor-bearing mice with sunitinib led to reduced staining of Ki67, CD-31, and an increase in VWF. Although no significant difference in the quantification of the pimonidazole staining was detectable, a qualitative trend of decreased staining density was observed in the long-term study (see Figure 5). A potential reason for this observation could be that animals were sacrificed at 1 h p.i. of pimonidazole for the short-term experiments. Since pimonidazole, like FAZA, is a 2-nitromidazole, no effects of sunitinib were detected after 1 h p.i. in accordance with the PET results (Figure 3). A 1.83 ± 0.10% CD-31 binding was determined in the tumor tissue from the control mice, whereas the samples from sunitinib-treated animals showed reduced binding of 0.58 ± 0.05% CD-31 (n = 8/4 - tumors from four mice; p < 0.001). Additionally, in the controls, 0.53 ± 0.05% VWF positive cells were found versus 0.31 ± 0.08% in sunitinib-treated samples (n = 8/4; p < 0.001). Ki67 immunohistochemistry showed a decreased binding from 75 ± 2% Ki67 positive cells in the controls to 54 ± 6% Ki67 positive cells in the treated samples (n = 8/4; p < 0.001).
Immunohistochemical staining with pimonidazole HCl, CD-31, Ki-67, and VWF in Caki-1 tumor tissue slices. Slices are shown from control animals and mice treated with sunitinib (40 mg/kg/day) for 5 days (top). Quantified staining data for the percent positive stained tissue. Data as mean ± SEM with **p < 0.01; ***p < 0.001 and n.s. for not significant.
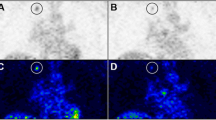
Static [18F]FAZA images in BALB/c nude mice bearing two Caki-1 tumors on each upper flank. Image data are presented as maximum intensity projection (MIP) at 3 h post injection. Top left: control mouse versus sunitinib-treated mouse (9 days 40 mg/kg). Top right: control mouse versus a sunitinib-treated mouse which had received 13 days of drug therapy followed by 12 days after therapy was finished. Bottom: semi-quantified PET data as SUV in comparison to percent pimonidazole binding in tumor slices. Data as mean ± SEM from n analyzed tumors grown in x mice. *p < 0.05.
Monitoring therapy effects and withdrawal of sunitinib with [18F]FAZA
Following the investigation of short-term effects of sunitinib on tumor oxygenation, a second experimental series was performed in order to analyze the longitudinal effects of anti-angiogenic therapy on Caki-1 tumor oxygenation. Figure 5 summarizes the PET results with [18F]FAZA for the effect of sunitinib during the therapy and after therapy withdrawal. Treated Caki-1 tumors clearly showed higher radioactivity uptake levels than the control tumors, confirming that in the presence of sunitinib, the amount of tumor hypoxia is decreased in the tumor microenvironment. After 9 days of sunitinib therapy (Figure 5, left), the SUVmean,3h of control tumors was found to be 0.24 ± 0.02, while the SUVmean,3h of sunitinib-treated tumors was 0.19 ± 0.01 (n = 8; p < 0.05). Similar results were obtained when analyzing SUVmax values, which are more relevant for clinical use: 0.38 ± 0.03 (control) versus 0.29 ± 0.02 (9 days of sunitinib; n = 8, p < 0.05). Pimonidazole staining from tissue slices confirmed a reduction in hypoxia of the treated tumors. Twelve days after sunitinib therapy was withdrawn, [18F]FAZA uptake into the treated tumors increased again, leading now to even higher uptake levels than the control tumors (Figure 5, right): SUVmean, 3 h of 0.18 ± 0.01 (control) versus 0.23 ± 0.01 (sunitinib-treated; n = 6, p < 0.05). Analysis of SUVmax revealed the following values: 0.30 ± 0.02 (control) versus 0.36 ± 0.02 (after therapy withdrawal; n = 6, p < 0.05). This was indicative of higher levels of hypoxia and corresponded to a flare effect after withdrawal of sunitinib. The pimonidazole staining confirmed this phenomenon with similar levels of binding in both the control and the treated groups.
In vitro cell uptake
In order to assess the overall functional effects of a sunitinib treatment in vivo, uptake of [18F]FAZA into the Caki-1 cells was analyzed directly in the absence and presence of sunitinib in vitro. Hypoxia selective uptake and retention (= trap**) of [18F]FAZA occurs only under hypoxic conditions. After a 4-h incubation time, [18F]FAZA uptake was significantly higher in the Caki-1 cells (Figure 6): 2.42 ± 0.09% (hypoxia) versus 0.24 ± 0.02% radioactivity/mg protein (n = 9; p < 0.001; normoxia). For comparison and proof of the experimental setup, MCF-7 cells, known to have a higher uptake of the hypoxia PET tracer under hypoxic conditions, were also analyzed: 2.63 ± 0.44% (hypoxia) versus 0.37 ± 0.06% radioactivity/mg protein (n = 9; p < 0.001; normoxia). Cellular trap** of [18F]FAZA into the Caki-1 and MCF-7 cells was increased approximately eightfold to tenfold under hypoxic conditions, confirming increased cellular retention of [18F]FAZA under hypoxic conditions. The presence of sunitinib (3 µM) did not change the uptake properties of [18F]FAZA into the cell lines (Figure 6), indicating no direct effect occurring from this compound on the cellular uptake of a hypoxic radiotracer.
Uptake of [18F]FAZA into Caki-1 (renal cell carcinoma) and MCF-7 cells (human breast cancer cell line). Cell uptake was normalized to percent radioactivity per milligram protein, and data are shown as mean ± SEM from n analyzed cell culture dishes from x number of experiments. Cell uptake was measured under normoxic and hypoxic conditions and in the presence and absence of 3 µM sunitinib.
Discussion
Four main findings of the current study may be summarized as follows: (i) xenograft Caki-1 RCC tumors show uptake of [18F]FAZA, revealing a base level of hypoxia; (ii) sunitinib therapy reduces [18F]FAZA tumor uptake, indicating reduced tumor hypoxia; (iii) TKI withdrawal flare phenomenon was observed after sunitinib was discontinued, resulting in a once more increased tumor hypoxia; and (iv) sunitinib did not influence the direct cellular uptake and retention of [18F]FAZA in the Caki-1 and MCF-7 cells under normoxic or hypoxic conditions in vitro.
Currently, treatment of metastatic RCC with the TKI sunitinib is recommended as one of the first therapy options [7],[15],[16]. Sunitinib causes reduced tumor size or growth rate. In the current study, smaller Caki-1 tumor sizes were observed in the treated mice. Meta-analysis of metastatic RCC studies has shown that higher exposure to sunitinib is correlated with improved treatment outcomes. Patients with the highest exposure to sunitinib had longer times to progression, and the probability of decreases in tumor size or of halting tumor growth was higher in patients with the highest exposure to sunitinib [16],[43]. However, after therapy, Caki-1 tumors reached similar sizes again, indicating that the tumors regained their molecular characteristics which are essential for tumor development and growth. Changes occurring during therapy with sunitinib seem to be reversed after treatment disruption, leading to further tumor progression. The goal in patients would be to have a maximum exposure time to sunitinib which is associated with longer time to tumor progression, longer overall survival, and greater decrease in tumor size [43].
Therapy of metastatic RCC with TKIs can be monitored with non-invasive imaging methods to analyze therapy success as well as predict patient outcomes [22],[23]. Several preclinical and clinical studies have been carried out to analyze therapeutic effects of a TKI such as sunitinib or sorafenib utilizing PET [31]-[38]. Besides monitoring tumor metabolism in RCC with [18F]FDG, [18F]FMISO was used to analyze effects on tumor oxygenation status and [18F]FLT for determination of proliferation. During the present study, [18F]FAZA was used. Although [18F]FAZA in comparison to [18F]FMISO may exhibit a better clearance pattern from non-targeting muscle tissue based on its lower lipophylicity, there is still a debate about the radiotracer of choice for imaging tumor hypoxia [26]-[28]. In Caki-1 tumors, [18F]FAZA uptake amounted to 1.5% to 2.2% ID/g after 3 h p.i. As analyzed from the dynamic PET studies, initial uptake reached a maximum level after approximately 40 to 60 min which did not further increase. Image contrast with [18F]FAZA is achieved based on the non-target tissue clearance over time. In Caki-1 tumors, a tumor-to-muscle ratio of approximately 1.5 was reached after 3 h p.i.. The current findings are in line with observations in other murine and human xenograft models of different origin, where 1.3% to 3% ID/g or even lower levels were measured at the same time p.i. [26],[27],[44]. Similar to the Caki-1 model, low uptake of [18F]FMISO was found in A498, another human RCC xenografted tumor model [33].
PET is an ideal non-invasive imaging tool to use for monitoring response to therapy. However, there are still ongoing discussions of how patients would benefit from monitoring tumor hypoxia status and how hypoxia-directed therapy approaches could be used clinically [30],[45]. During the present study, sunitinib therapy led to a reduction of tumor hypoxia levels by 22% to 46% as determined with [18F]FAZA. Although sunitinib mainly targets tumor vasculature and function, it can be concluded that it also leads to a reduction of tumor hypoxia. As Verwer et al. [41] have proposed, a reversible two-tissue compartment model best describes uptake through passive diffusion and initial retention based on a chemical reduction of a nitroimidazole such as [18F]FAZA. Both steps are reversible: higher oxygen levels can re-oxygenate the reduced radiotracer, and [18F]FAZA may leave cells again via diffusion into the plasma. Kinetic analysis of the present dynamic data revealed that k1 to k4 were all elevated in the presence of sunitinib, k2 and k4 even significantly. Increased k1 shows that sunitinib-treated tumors allow even faster cellular uptake of [18F]FAZA. Delivery of the radiotracer through the tumor vasculature is rather improved and not impaired. On the other hand, elevated k2 indicates a higher rate of back diffusion into the plasma for treated mice. One can conclude that in this case, more re-oxygenated [18F]FAZA is present in the intracellular compartment. The intracellular presence or absence of [18F]FAZA is determined in this work to be due chiefly to the difference in k4, i.e., the re-oxygenation of the reduced [18F]FAZA, which is significantly elevated under sunitinib therapy. The perfused percentage of each tumor, as determined by the fbv, was found to be equal (within error margins) in each case, meaning that delivery of the radiotracer through the blood vessels is not impaired under sunitinib therapy. In summary, sunitinib treatment leads to decreased uptake in tumor cells due to increased re-oxygenation and back diffusion of [18F]FAZA caused by overall lower hypoxia levels.
Congruent with the current findings, sunitinib also reduces [18F]FMISO uptake by 22% to 35% in metastatic RCC patients [32]. The EGFR-blocker gefitinib showed even higher inhibition of [18F]FAZA uptake (60% to 70%) in xenograft A431 human squamous cell carcinoma [37]. Similar observations with [18F]FAZA and [18F]FMISO were described in A431 tumor-bearing mice under therapy with CI-1033, a pan-Erb-B inhibitor [46]. Studies with [18F]FDG analyzing glucose metabolism revealed 17% to 75% reduction in uptake during a sunitinib therapy [34]-[36], although with high variability for basic [18F]FDG levels in RCC patients [47],[48], reducing its utility in the clinics. In addition, the proliferation marker [18F]FLT also showed a 16% reduced uptake in RCC patients in the presence of sunitinib [38]. Taken together, this supports the conclusion that systemic therapy with the TKI sunitinib achieves therapeutic efficacy by decreasing tumor hypoxia, metabolism, and proliferation. Monitoring of tumor oxygen levels is feasible in lesions with a well-defined population of hypoxic cells. This allows detection of reduced hypoxia during sunitinib therapy, while non-hypoxic metastases would not be expected to change [32].
Immunostaining with pimonidazole did only show a tendency for decreased binding to hypoxic cells in the short-term study. This may be explained by the short incubation time of only 1 h compared to 3 h in the long-term study. As the dynamic PET study reveals, effects of sunitinib on [18F]FAZA are likewise not detectable at 1 h p.i. In theory, pimonidazole should behave similarly to [18F]FAZA since both are 2-nitroimidazoles. Pimonidazole binding was reduced after 3 h p.i. by 30% which is similar to the 21% reduction of [18F]FAZA. Considering the small numbers of animals, n, and thus relatively large SEM, this difference was insignificant. Immunohistochemistry with CD-31 and Ki67 resulted in a reduction in endothelial cells (and therefore mean vessel density and cell proliferation), pointing to a decrease in oxygen consumption and demand. This may have contributed to improved oxygenation of the sunitinib-treated Caki-1 tumors which correlates well with the fact that [18F]FAZA delivery to the tumor cells was not impaired under sunitinib. From a clinical perspective, improved oxygenation has been shown to improve response to conventional chemotherapy, and radiotherapy, as well as reduce metastatic potential [49].
While sunitinib therapy leads to a pronounced reduction in tumor hypoxia, termination of therapy resulted in an increased hypoxia with even higher levels of [18F]FAZA uptake compared to the control tumors. Following the release of TK inhibition, the tumor cell activity rebounds and experiences accelerated proliferation. This withdrawal flare phenomenon has not been thoroughly investigated subsequent to discontinuation of anti-angiogenic therapy. Similar rebound effects were observed in human renal cell cancer, where the SUV of [18F]FLT increased by 15% after sunitinib withdrawal [38]. Currently, patients receive sunitinib in an on-and-off regimen [50]. Following treatment discontinuation, patients sometimes experience recurrent pain in metastatic sites, as a result of flare re-growth. It has been proposed that during this period of withdrawal, tumor cells are stuck in the `S' phase and therefore are more vulnerable to combination cytotoxic chemotherapy [38]. This may be a useful strategy for therapy monitoring of RCC with PET, e.g., determining the hypoxia status.
Since direct effects of sunitinib on [18F]FAZA uptake into the Caki-1 cells can be excluded from the in vitro studies, the observed reduction in tumor hypoxia in vivo must be related to other effects of sunitinib. Sunitinib was originally designed to specifically inhibit VEGFR, PDGFR, and C-kit; however, it has been found to inhibit other types of kinases as well, including non-receptor tyrosine kinases, receptor tyrosine kinases, tyrosine kinase-like kinases, cyclin-dependant kinases, and mitogen-activated protein kinase [ DC, JM, LW, MW, and RM contributed to the concept and study design. DC, IM, and MW collected the data. DC and MW performed the data analysis. DC, JM, LW, MW, and RM were involved in the interpretation of the data. HSJ performed the kinetic analysis. All authors were involved in the writing process and all approved the manuscript before submission.Authors' contributions
References
Linehan WM, Walter MM, Zbar B: The genetic base of cancer of the kidney. J Urol 2003, 170: 163–172.
Tan PH, Cheng L, Rioux-Leclercq N, Merino MJ, Netto G, Reuter VE, Shen SS, Grignon DJ, Montironi R, Egevad L, Srigley JR, Delahunt B, Moch H: Renal tumors: diagnostic and prognostic biomarkers. Am J Surg Pathol 2013, 37: 1518–1531. 10.1097/PAS.0b013e318299f12e
Cancer Facts and Figures. American Cancer Society, Atlanta ACS; 2013.
Cohen HT, McGovern FJ: Renal-cell carcinoma. N Engl J Med 2005, 353: 2477–2490. 10.1056/NEJMra043172
Ljungberg B, Bensalah K, Bex A, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders PFA, Staehler M, Volpe A: Guidelines on Renal Cell Carcinoma. European Association of Urology (EAU), Arnhem; 2013.
Basu B, Eisen T: Perspectives in drug development for metastatic renal cell cancer. Target Oncol 2010, 5: 139–156. 10.1007/s11523-010-0149-2
Dutcher JP: Recent developments in the treatment of renal cell carcinoma. Ther Adv Urol 2013, 5: 338–353. 10.1177/1756287213505672
Schrader AJ, Hofmann R: Metastatic renal cell carcinoma: recent advances and current therapeutic options. Anticancer Drugs 2008, 19: 235–245. 10.1097/CAD.0b013e3282f444de
Gross-Goupil M, Massard C, Ravaud A: Targeted therapies in metastatic renal cell carcinoma: overview of the past year. Curr Urol Rep 2012, 13: 16–23. 10.1007/s11934-011-0232-y
Zhang J, Yang PL, Gray NS: Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer 2009, 9: 28–39. 10.1038/nrc2559
Mendel DB, Laird AD, **n X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM: In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2003, 9: 327–337.
Adams VR, Leggas M: Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther 2007, 29: 1338–1353. 10.1016/j.clinthera.2007.07.022
Petrelli A, Giordano S: From single- to mutli-target drugs in cancer therapy: when aspecificity becomes an advantage. Curr Med Chem 2008, 15: 422–432. 10.2174/092986708783503212
Wood L: Sunitinib malate for the treatment of renal cell carcinoma. Expert Opin Pharmacother 2012, 13: 1323–1336. 10.1517/14656566.2012.689130
Escudier B, Albiges L, Sonpavde G: Optimal management of metastatic renal cell carcinoma: current status. Drugs 2013, 73: 427–438. 10.1007/s40265-013-0043-1
Castellano D, Ravaud A, Schmidinger M, De Velasco G, Vazquez F: Therapy management with sunitinib in patients with metastatic renal cell carcinoma: key concepts and the impact of clinical biomarkers. Cancer Treat Rev 2013, 39: 230–240. 10.1016/j.ctrv.2012.04.009
Nilsson MB, Zage PE, Zeng L, Xu L, Cascone T, Wu HK, Saigal B, Zweidler-McKay PA, Heymach JV: Multiple receptor tyrosine kinases regulate HIF-1alpha and HIF-2alpha in normoxia and hypoxia in neuroblastoma: implications for antiangiogenic mechanisms of multikinase inhibitors. Oncogene 2010, 29: 2938–2949. 10.1038/onc.2010.60
Marín-Hernández A, Gallardo-Pérez JC, Ralph SJ, Rodríguez-Enríquez S, Moreno-Sánchez R: HIF-1alpha modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev Med Chem 2009, 9:1084–1101. 10.2174/138955709788922610
Cassavaugh J, Lounsbury KM: Hypoxia-mediated biological control. J Cell Biochem 2011, 112: 735–744. 10.1002/jcb.22956
Chapman JD, Schneider RF, Urbain JL, Hanks GE: Single-photon emission computed tomography and positron-emission tomography assays for tissue oxygenation. Semin Radiat Oncol 2001, 11: 47–57. 10.1053/srao.2001.18103
Krohn KA, Link JM, Mason RP: Molecular imaging of hypoxia. J Nucl Med 2008, 49(Suppl 2):129S-148S. 10.2967/jnumed.107.045914
Sacco E, Pinto F, Totaro A, D'Addessi A, Racioppi M, Gulino G, Volpe A, Marangi F, D'Agostino D, Bassi P: Imaging of renal cell carcinoma: state of the art and recent advances. Urol Int 2011, 86: 125–139. 10.1159/000322724
Krajewski KM, Giardino AA, Zukotynski K, Van den Abbeele AD, Pedrosa I: Imaging in renal cell carcinoma. Hematol Oncol Clin North Am 2011, 25: 687–715. 10.1016/j.hoc.2011.04.005
Carlin S, Humm JL: PET of hypoxia: current and future perspectives. J Nucl Med 2012, 53: 1171–1174. 10.2967/jnumed.111.099770
Wuest M, Wuest F: Positron emission tomography radiotracers for imaging hypoxia. J Labelled Comp Radiopharm 2013, 56: 244–250. 10.1002/jlcr.2997
Sorger D, Patt M, Kumar P, Wiebe LI, Barthel H, Seese A, Dannenberg C, Tannapfel A, Kluge R, Sabri O: [18F]Fluoroazomycinarabinofuranoside (18FAZA) and [18F]Fluoromisonidazole (18FMISO): a comparative study of their selective uptake in hypoxic cells and PET imaging in experimental rat tumors. Nucl Med Biol 2003, 30: 317–326. 10.1016/S0969-8051(02)00442-0
Piert M, Machulla HJ, Picchio M, Reischl G, Ziegler S, Kumar P, Wester HJ, Beck R, McEwan AJ, Wiebe LI, Schwaiger M: Hypoxia-specific tumor imaging with 18F-fluoroazomycin arabinoside. J Nucl Med 2005, 46: 106–113.
Reischl G, Dorow DS, Cullinane C, Katsifis A, Roselt P, Binns D, Hicks RJ: Imaging of tumor hypoxia with [124I]IAZA in comparison with [18F]FMISO and [18F]FAZA–first small animal PET results. J Pharm Pharm Sci 2007, 10: 203–211.
Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ: Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J Nucl Med 1999, 40: 177–183.
Dunphy MP, Lewis JS: Radiopharmaceuticals in preclinical and clinical development for monitoring of therapy with PET. J Nucl Med 2009, Suppl 1: 106S-121S. 10.2967/jnumed.108.057281
Valable S, Petit E, Roussel S, Marteau L, Toutain J, Divoux D, Sobrio F, Delamare J, Barré L, Bernaudin M: Complementary information from magnetic resonance imaging and (18)F-fluoromisonidazole positron emission tomography in the assessment of the response to an antiangiogenic treatment in a rat brain tumor model. Nucl Med Biol 2011, 38: 781–793.
Hugonnet F, Fournier L, Medioni J, Smadja C, Hindié E, Huchet V, Itti E, Cuenod CA, Chatellier G, Oudard S, Faraggi M: Metastatic renal cell carcinoma: relationship between initial metastasis hypoxia, change after 1 month's sunitinib, and therapeutic response: an 18F-fluoromisonidazole PET/CT study. J Nucl Med 2011, 52: 1048–1055. 10.2967/jnumed.110.084517
Murakami M, Zhao S, Zhao Y, Chowdhury NF, Yu W, Nishijima K, Takiguchi M, Tamaki N, Kuge Y: Evaluation of changes in the tumor microenvironment after sorafenib therapy by sequential histology and 18F-fluoromisonidazole hypoxia imaging in renal cell carcinoma. Int J Oncol 2012, 41: 1593–1600.
Revheim ME, Winge-Main AK, Hagen G, Fjeld JG, Fosså SD, Lilleby W: Combined positron emission tomography/computed tomography in sunitinib therapy assessment of patients with metastatic renal cell carcinoma. Clin Oncol (R Coll Radiol) 2011, 23: 339–343. 10.1016/j.clon.2010.11.006
Vercellino L, Bousquet G, Baillet G, Barré E, Mathieu O, Just PA, Desgrandchamps F, Misset JL, Hindié E, Moretti JL: 18F-FDG PET/CT imaging for an early assessment of response to sunitinib in metastatic renal carcinoma: preliminary study. Cancer Biother Radiopharm 2009, 24: 137–144. 10.1089/cbr.2008.0527
Lyrdal D, Boijsen M, Suurküla M, Lundstam S, Stierner U: Evaluation of sorafenib treatment in metastatic renal cell carcinoma with 2-fluoro-2-deoxyglucose positron emission tomography and computed tomography. Nucl Med Commun 2009, 30: 519–524. 10.1097/MNM.0b013e32832cc220
Solomon B, Binns D, Roselt P, Weibe LI, McArthur GA, Cullinane C, Hicks RJ: Modulation of intratumoral hypoxia by the epidermal growth factor receptor inhibitor gefitinib detected using small animal PET imaging. Mol Cancer Ther 2005, 4: 1417–1422. 10.1158/1535-7163.MCT-05-0066
Liu G, Jeraj R, Vanderhoek M, Perlman S, Kolesar J, Harrison M, Simoncic U, Eickhoff J, Carmichael L, Chao B, Marnocha R, Ivy P, Wilding G: Pharmacodynamic study using FLT PET/CT in patients with renal cell cancer and other solid malignancies treated with sunitinib malate. Clin Cancer Res 2011, 15(17):7634–7644. 10.1158/1078-0432.CCR-11-1677
Bruce JY, Kolesar JM, Hammers H, Stein MN, Carmichael L, Eickhoff J, Johnston SA, Binger KA, Heideman JL, Perlman SB, Jeraj R, Liu G: A phase I pharmacodynamic trial of sequential sunitinib with bevacizumab in patients with renal cell carcinoma and other advanced solid malignancies. Cancer Chemother Pharmacol. 2014.
Reischl G, Ehrlichmann W, Bieg C, Solbach C, Kumar P, Wiebe LI, Machulla H-J: Preparation of the hypoxia imaging PET tracer [18F]FAZA: reaction parameters and automation. Appl Radiat Isot 2005, 62: 897–901. 10.1016/j.apradiso.2004.12.004
Verwer EE, van Velden FHP, Bahce I, Yaqub M, Schuit RC, Windhorst AD, Raijmakers P, Lammertsma AA, Smit EF, Boellaard R: Pharmacokinetic analysis of [18F]FAZA in non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging 2013, 40: 1523–1531. 10.1007/s00259-013-2462-3
Koch CJ, Stobbe CC, Baer KA: Metabolism induced binding of 14C-misonidazole to hypoxic cells: kinetic dependence on oxygen concentration and misonidazole concentration. Int J Radiat Oncol Biol Phys 1984, 10: 1327–1331. 10.1016/0360-3016(84)90342-0
Houk BE, Bello CL, Poland B, Rosen LS, Demetri GD, Motzer RJ: Relationship between exposure to sunitinib and efficacy and tolerability endpoints in patients with cancer: results of a pharmacokinetic/pharmacodynamic metaanalysis. Cancer Chemother Pharmacol 2010, 66: 357–371. 10.1007/s00280-009-1170-y
Maier FC, Kneilling M, Reischl G, Cay F, Bukala D, Schmid A, Judenhofer MS, Röcken M, Machulla HJ, Pichler BJ: Significant impact of different oxygen breathing conditions on noninvasive in vivo tumor-hypoxia imaging using [18F]-fluoro-azomycinarabino-furanoside ([18F]FAZA). Radiat Oncol 2011, 6: 165–175. 10.1186/1748-717X-6-165
Rischin D, Hicks RJ, Fisher R, Binns D, Corry J, Porceddu S, Peters LJ: Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 2006, 24: 2098–2104. 10.1200/JCO.2005.05.2878
Dorow DS, Cullinane C, Conus N, Roselt P, Binns D, McCarthy TJ, McArthur GA, Hicks RJ: Multi-tracer small animal PET imaging of the tumour response to the novel pan-Erb-B inhibitor CI-1033. Eur J Nucl Med Mol Imaging 2006, 33: 441–452. 10.1007/s00259-005-0039-5
Majhail NS, Urbain JL, Albani JM, Kanvinde MH, Rice TW, Novick AC, Mekhail TM, Olencki TE, Elson P, Bukowski RM: F-18 fluorodeoxyglucose positron emission tomography in the evaluation of distant metastases from renal cell carcinoma. J Clin Oncol 2003, 21: 3995–4000. 10.1200/JCO.2003.04.073
Dilhuydy MS, Durieux A, Pariente A, de Clermont H, Pasticier G, Monteil J, Ravaud A: PET scans for decision-making in metastatic renal cell carcinoma: a single-institution evaluation. Oncology 2006, 70: 339–344. 10.1159/000097946
Arabi M, Piert M: Hypoxia PET/CT imaging: implications for radiation oncology. Q J Nucl Med Mol Imaging 2010, 54: 500–509.
Atkinson BJ, Kalra S, Wang X, Bathala T, Corn P, Jonasch E: Clinical outcomes in patients with metastatic renal cell carcinoma treated with alternative sunitinib schedules. J Urol: Tannir NM. 2013.
Murakami M, Zhao S, Zhao Y, Yu W, Fatema CN, Nishijima KI, Yamasaki M, Takiguchi M, Tamaki N, Kuge Y: Increased intratumoral fluorothymidine uptake levels following multikinase inhibitor sorafenib treatment in a human renal cell carcinoma xenograft model. Oncol Lett 2013, 6: 667–672.
Gaustad JV, Simonsen TG, Leinaas MN, Rofstad EK: Sunitinib treatment does not improve blood supply but induces hypoxia in human melanoma xenografts. BMC Cancer 2012, 12: 388–403. 10.1186/1471-2407-12-388
Gaustad JV, Simonsen TG, Roa AM, Rofstad EK: Tumors exposed to acute cyclic hypoxia show increased vessel density and delayed blood supply. Microvasc Res 2013, 85: 10–15. 10.1016/j.mvr.2012.11.002
Acknowledgements
The authors would like to thank Dr. John Wilson, David Clendening, and Blake Lazurko from the Edmonton PET Center for providing 18F produced on a biomedical cyclotron. In addition, we would like to thank Ali Akbari for the synthesis of [18F]FAZA on an automated synthesis unit, Monica Wang for the help with the in vitro cell experiments, Kevin McCloy from the Technical Management Group at the Cross Cancer Institute (CCI) for maintenance of the small animal PET, and also Gail Hipperson and Dan McGinn from the Vivarium of the CCI for hel** in the animal handling. This work was financially supported by the Edmonton PET Center, the Mr. Lube Foundation, and Alberta Innovates Health Solution (AIHS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chapman, D.W., Jans, HS., Ma, I. et al. Detecting functional changes with [18F]FAZA in a renal cell carcinoma mouse model following sunitinib therapy. EJNMMI Res 4, 27 (2014). https://doi.org/10.1186/s13550-014-0027-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13550-014-0027-5