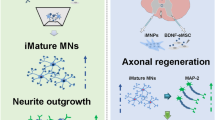
Abstract
Background
Spinal cord injury (SCI) is an intractable neurological disease in which functions cannot be permanently restored due to nerve damage. Stem cell therapy is a promising strategy for neuroregeneration after SCI. However, experimental evidence of its therapeutic effect in SCI is lacking. This study aimed to investigate the efficacy of transplanted cells using stepwise combined cell therapy with human mesenchymal stem cells (hMSC) and induced pluripotent stem cell (iPSC)-derived motor neuron progenitor cells (iMNP) in a rat model of SCI.
Methods
A contusive SCI model was developed in Sprague-Dawley rats using multicenter animal spinal cord injury study (MASCIS) impactor. Three protocols were designed and conducted as follows: (Subtopic 1) chronic SCI + iMNP, (Subtopic 2) acute SCI + multiple hMSC injections, and (Main topic) chronic SCI + stepwise combined cell therapy using multiple preemptive hMSC and iMNP. Neurite outgrowth was induced by coculturing hMSC and iPSC-derived motor neuron (iMN) on both two-dimensional (2D) and three-dimensional (3D) spheroid platforms during mature iMN differentiation in vitro.
Results
Stepwise combined cell therapy promoted mature motor neuron differentiation and axonal regeneration at the lesional site. In addition, stepwise combined cell therapy improved behavioral recovery and was more effective than single cell therapy alone. In vitro results showed that hMSC and iMN act synergistically and play a critical role in the induction of neurite outgrowth during iMN differentiation and maturation.
Conclusions
Our findings show that stepwise combined cell therapy can induce alterations in the microenvironment for effective cell therapy in SCI. The in vitro results suggest that co-culturing hMSC and iMN can synergistically promote induction of MN neurite outgrowth.
Graphical Abstract

Similar content being viewed by others
Background
Contusive spinal cord injury (SCI) can induce permanent impairment of the sensory and motor nervous systems, leading to restricted muscle movement. Moreover, patients with SCI have a lower quality of life in comparison to their uninjured peers due to low life expectancy and lower employment rates [1, 2]. The pathophysiologic mechanisms of SCI may be primary or secondary phase injury. Primary SCI is caused by direct traumatic injury to the spinal cord (SC). On the other hand, secondary SCI can be categorized into acute, subacute, and chronic phases according to the time and pathological mechanisms after the primary injury [3]. Secondary injury mechanisms include neuronal and glial cell death, primarily due to apoptosis and autophagy in the injured regions. In addition, secondary injuries activate astrocytes over time, resulting in reactive gliosis and subsequent glial scar formation, which acts as a physical and chemical barrier inhibiting axonal regeneration [4, 5]. Therefore, the goal of SCI treatment in clinical practice is to minimize secondary injuries after the primary injury. Therapeutic attempts to overcome SCI are generally based on two pathophysiological mechanisms: neuroprotective and neuroregenerative approaches [3, 5, 6].
Stem cell therapy has demonstrated both neuroprotective and neuroregenerative effects in SCI by minimizing the pathophysiological mechanisms that occur during primary and secondary injuries [7,8,9]. The neuroprotective mechanisms of the transplanted cells include secondary injury mitigation by protecting the injured region of the SC. The basic mechanism has been reported to be preservation of the adjacent tissues at the injured site by stem cells in comparison to the control group [5, 10]. Remyelination after SCI is a therapeutic target in regenerative trials. Myelination plays a critical role in effective action potential propagation along with survival of axons and corresponding neurons in the SC tissue. Enhanced approaches for cell transplantation and endogenous repair processes are being actively investigated to improve remyelination in SCI [3]. Numerous cell types have been transplanted and studied for their neuroprotective and neuro-regenerative effects in SCI. Several cell types, including Schwann cells, neural stem and progenitor cells (NSPCs), mesenchymal stem cells (MSCs), olfactory ensheathing cells (OECs), and oligodendrocyte precursor cells, are being studied in the context of promoting neuroprotection in SCI [10,11,12]. The therapeutic effects of different types of differentiated cells derived from multipotent or pluripotent cell types are also being investigated in SCI [5, 13]. Till date, various cell types have been successfully differentiated from induced pluripotent stem cells (iPSCs) and transplanted into animal models of SCI, suggesting their potential for neuroprotection and neuroregeneration [5, 14]. Moreover, several studies have reported the efficacy of differentiated cell types derived from iPSCs in preclinical and clinical SCI trials [15,43]. Based on previous studies, we confirmed the possibility of stem cell therapy using a rat model of contusive SCI.
Secondary SCI can be categorized into acute, subacute (or intermediate), and chronic phases depending on the time elapsed after injury and the pathological mechanism. Acute SCI phase is expressed in the last 48 h after the initial physical insult to the SC. The main phenomena observed in the acute SCI phase are vascular disruption, hemorrhage, and subsequent ischemia of the injured SC. After disruption of the microenvironment in the injured SC, pathological changes such as ion dysregulation, excitotoxicity, excessive production of free radicals, and inflammatory responses cause further damage to the neurons and glial cells at the lesional site [3, 44, 45]. The sub-acute (intermediate) phase is considered to last up two weeks in the injured SC. Characteristic features of the subacute phase are phagocytic response and reactive proliferation of astrocytes at the lesional site. Increase in the number of reactive astrocytes at the lesional site results in the formation of a glial scar. Glial scarring is a major cause of limited neuroregeneration and axonal regeneration in SCI [3, 46, 47]. A characteristic feature of chronic SCI phase is the maturation of the lesion, including scar formation and development of a syrinx at the lesional site. Endogenous regenerative capacity is affected by the release of growth-inhibitory molecules and glial scarring around the epicenter of the lesion [3, 44].
Neuroprotective and neuroregenerative approaches are being used to treat SCI [3]. Transplantation of various types of stem cells reportedly has great potential to preserve damaged tissues and promote the recovery of nerve function [46, 48]. Previous studies have reported that bone marrow-derived MSC (BM-MSC) transplantation in acute and chronic SCI rat models increased clinical improvement, neuroprotection, and neuroregeneration via differentiated astrocytes and oligodendrocytes at the lesional sites [7,8,9, 27]. The findings of the aforementioned studies on transplantation of BM-MSCs in SCI suggests the potential for cell therapy in acute and chronic SCI models. It has been shown that the densities of astrocytes and oligodendrocytes return to near-normal levels in the residual white matter within several weeks after SCI. However, chronic SCI causes loss of gray matter neurons due to damage at the lesional site and permanent damage to motor function. In chronic SCI, a disorder characterized by progressive loss of motor neurons, a feasible treatment approach is to replace lost or degenerated motor neurons [20].
Recently, high-purity iMNPs and iMNs were successfully established, and their transplantation in SCI was studied [19, 30]. Another study reported that transplantation of human embryonic stem cell (hESC)-derived motor neurons progenitor (MP) enhanced astrogliosis at the lesional site four-months after SCI. In addition, it was confirmed that increased astrogliosis at the lesional site favored the survival and differentiation of hESC-derived neurons and correlated with improved motor function recovery [21]. In another study, when a high-purity population of human motor neuron progenitors (hMNP) derived from hESC was transplanted into an SCI rat model, it was reported that hMNP at the lesional site suppressed the intracellular signaling pathway associated with SCI pathogenesis, which correlated with greater endogenous neural survival and neurite branching [22]. However, studies on their therapeutic effects in SCI are lacking. We aimed to confirm the increased differentiation of gray matter neurons and recovery of motor function at the lesional site by transplantation of iPSC-derived motor neuron progenitor cells (iMNP) in a chronic SCI model. In the context of subtopic 1 of the study, we confirmed the efficacy of iMNP in neuroregeneration in a chronic SCI model. We generated iPSC-derived iMNPs and iMNs in vitro and selected iMNPs with high proliferation for successful transplantation into the SC of chronic SCI model (Fig. 2a and b). The iMNPs transplanted at the lesional site showed possible in vivo MN differentiation and maturation. Moreover, the cells also showed behavioral recovery through BBB locomotor scale scores (Fig. 2c-g). Our findings suggest successful engraftment and MN differentiation of implanted iMNP in chronic SCI. However, the subsequent formation of glial scars and their microenvironment could not be effectively decreased at the injured site. In addition, the transplanted iMNP did not decrease the expression of reactive gliosis in the round cystic area in chronic SCI (Additional file 1: Fig.S1a-g). However, subtopic 1 of the study had a limitation in that few animals were used due to the pilot study concept.
Transplantation in chronic SCI results in low rate of engraftment and functional restoration due to the subsequent formation of glial scars [8, 29]. Therefore, the timing of transplantation must be taken into consideration and alterations in the microenvironment of chronic SCI tissues should be induced to enhance the effectiveness of transplantation [29, 49]. MSC transplantation can prevent the secretion of various inflammatory cytokines, apoptosis, and inflammation to exert neuroprotection during acute SCI [7, 50,51,52]. Based on the results of previous studies and our initial findings, we attempted to increase the transplantation effect at the lesional site through multiple hMSC injections for acute SCI (Subtopic 2), and found that it increased the cell transplantation efficacy in comparison to single hMSC injection by increasing neuronal cells via NGF and axonal regeneration at the lesional site (Fig. 3f and g). Interestingly, multiple hMSC injections promoted clinical recovery by increasing neuronal cell differentiation, whereas a single hMSC injection promoted clinical recovery by increasing astrocyte and oligodendrocyte differentiation in the injured SC (Additional file 2: Fig.S2a-b). C3 is a specific marker of A1 neuroinflammation-reactive astrocytes in SCI, and evaluation of the phenotype of reactive astrocytes in SCI have been reported by several studies [23, 32, 33, 53]. One research reported that an IV injection of MSC-derived exosomes reduced the number of C3- or GFAP-positive astrocytes at the lesional site in acute SCI [53]. Our findings suggest that multiple and single injections of hMSCs can effectively promote functional behavioral recovery by decreasing C3 expression at the lesional site in acute SCI (Additional file 3: Fig.S3a-c).
Stem cell-derived MN and MNP offer promising strategies for cellular replacement in SCI. However, in our pilot experiment, the data suggested that iMNP cell transplantation confirmed the limitations of cellular replacement strategies in chronic stage astrocyte and Neurocan formation scar cavity. Thus, we used a stepwise cell therapy strategy for SCI to increase the efficacy of the transplanted cells at the lesional site. This main topic of the study included confirmation of the neuroprotective and neuroregenerative effects of multiple preemptive hMSC injections and increased MN differentiation at the lesional site through transplanted iMNP. Interestingly, we found that stepwise cell therapy promoted MN maturation and axonal regeneration at the lesional site. We also confirmed that cell transplantation with iMNP alone increased MN differentiation at the lesional site (Additional file 4: Fig.S4a-d). Another study reported that MNP cell transplantation resulted in MN lineage differentiation in the ventral horns at the lesional site. However, the failure of MNPs to mature in all other regions of the SC likely reflected the gliogenic nature of the SCI environment [22]. Our findings suggest that the increased neuroprotective effects of multiple preemptive hMSC injections in the acute SCI phase can enhance MN differentiation and maturation at the lesional site. More importantly, it was demonstrated in vitro that hMSC and iMN co-culture significantly increased neurite outgrowth during the MN maturation stage (Fig. 6b-g). Another study reported that differentiated Schwann cells (SC), human bone marrow-MSCs, and umbilical-cord-blood MSCs significantly promoted neurite outgrowth and elongation in comparison to untreated MSCs [54]. We found that 2D and 3D co-cultured hMSC and iMN induced neurite outgrowth and elongation compared to hMSC and iMN separately (Fig. 6f-g). However, hMSC alone did not significantly promote neurite outgrowth and elongation as compared to iMN. Another study suggested that hMSC promotes neurite outgrowth via a paracrine effect through growth factors including BDNF and NGF [55,56,57].
In summary, this study confirmed that clinical behavioral outcomes were restored through induction of mature motor neuron differentiation and axonal regeneration at the lesional site using stepwise combined cell transplantation of hMSCs and iMNPs in a contusive SCI model, suggesting the therapeutic efficacy of stepwise combined cell transplantation strategy in a severed contusion SCI rat model. The stepwise combined cell transplantation strategy has the advantage of not only suggesting ideal stem cell selection for each stage of SCI, but also confirming the function of the transplanted cells. However, a limitation of this study is the lack of an explanation for the mechanisms underlying the synergistic effect of stepwise combined cell transplantation in a contusion SCI model. In future studies, it will be necessary to confirm the synergistic effects of stepwise combined cell transplantation mechanisms using time-dependent RNA sequencing (RNA-seq) or single-cell analysis at the lesional site. In addition, selecting a sample size for animal experiments requires calculating a sample size sufficient for statistical analysis using a few free software packages (G power, power sample size). There is a need to overcome the limitations of stem cell therapy for SCI using a stepwise combined cell transplantation strategy with a 3D iPSC-derived motor neuron source.
Conclusion
Our study demonstrated that stepwise cell therapy increased MN differentiation and axonal regeneration compared to single-cell therapy in severed SCI model. Stepwise cell therapy increased behavioral recovery and the rate of BBB locomotor scale grade 3 (BBB score, 10–15). Moreover, it also induced alterations in the microenvironment for effective cell therapy in severed SCI model. These in vitro results suggest that co-cultured hMSC and iMN synergistically promoted induction of MN neurite outgrowth. Taken together, we report a proof-of-concept study showing that stepwise combined transplantation can increase cell engraftment and SC recovery based on cell type and transplantation timing in SCI.
Data availability
All datasets generated during the study are included within the article.
Abbreviations
- AVMA:
-
American veterinary medical association
- ARRIVE:
-
Animal research: reporting of in vivo experiments
- BBB:
-
Basso–Beattie–Bresnahan
- BSA:
-
Bovine serum albumin
- BDNF:
-
Brain-derived neurotrophic factor
- CCK-8:
-
Cell counting kit-8
- C3:
-
Complement component 3
- ChAT:
-
Choline acetyltransferase
- DMEM:
-
Dulbecco’s modified eagle’s medium
- DMH1:
-
Dorsomorphin homologue 1
- DAPI:
-
4′,6-diamidino-2-phenylindole
- ESC:
-
Embryonic stem cell
- GFAP:
-
Glial fibrillary acidic protein
- hMSC:
-
human mesenchymal stem cells
- iPSC:
-
Induced pluripotent stem cell
- iNEP:
-
Induced pluripotent stem cell derived Neuro epithelial progenitor
- iMNP:
-
Induced pluripotent stem cell derived motor neuron progenitor cells
- iMN:
-
Induced pluripotent stem cell derived motor neuron
- iMature MNs:
-
Induced pluripotent stem cell derived mature motor neurons
- IL:
-
Intra lesional
- IV:
-
Intra venous
- MEA:
-
Multi-electrode array
- MNs:
-
Motor neuron cells
- MASCIS:
-
Multicenter animal spinal cord injury study
- NSPCs:
-
Neural stem and progenitor cells
- NGF:
-
Nerve growth factor
- OECs:
-
Olfactory ensheathing cells
- OCT:
-
Optimal cutting temperature
- PBS:
-
Phosphate-buffered saline
- PBMCs:
-
Peripheral blood mononuclear cells
- PFA:
-
Paraformaldehyde
- PMSF:
-
Phenyl methyl sulfonyl fluoride
- Pur:
-
Pumorphamine
- RT:
-
Room temperature
- RA:
-
Retinoic acid
- SCI:
-
Spinal cord injury
- SC:
-
Spinal cord
- SD:
-
Sprague–Dawley
- TEM:
-
Transmission electron microscopy
- TBS:
-
Tris-buffered saline
- TBST:
-
tris-buffered saline with 0.05% Tween-20
- VEGF:
-
Vascular endothelial growth factor
References
Ramotowski C, Qu X, Villa-Diaz LG. Progress in the Use of Induced Pluripotent Stem cell-derived neural cells for traumatic spinal cord injuries in animal populations: Meta-Analysis and Review. Stem Cells Transl Med. 2019;8:681–93.
Iyer NR, Wilems TS, Sakiyama-Elbert SE. Stem cells for spinal cord injury: strategies to inform differentiation and transplantation. Biotechnol Bioeng. 2017;114:245–59.
Kim YH, Ha KY, Kim SI. Spinal cord Injury and related clinical trials. Clin Orthop Surg. 2017;9:1–9.
Tzekou A, Fehlings MG. Treatment of spinal cord injury with intravenous immunoglobulin G: preliminary evidence and future perspectives. J Clin Immunol. 2014;34(Suppl 1):S132–138.
Khazaei M, Siddiqui AM, Fehlings MG. The potential for iPS-Derived stem cells as a therapeutic strategy for spinal cord Injury: opportunities and challenges. J Clin Med. 2014;4:37–65.
Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2.
Kang ES, Ha KY, Kim YH. Fate of transplanted bone marrow derived mesenchymal stem cells following spinal cord injury in rats by transplantation routes. J Korean Med Sci. 2012;27:586–93.
Kim JW, Ha KY, Molon JN, Kim YH. Bone marrow-derived mesenchymal stem cell transplantation for chronic spinal cord injury in rats: comparative study between intralesional and intravenous transplantation. Spine (Phila Pa 1976). 2013;38:E1065–1074.
Kim YC, Kim YH, Kim JW, Ha KY. Transplantation of mesenchymal stem cells for Acute spinal cord Injury in rats: comparative study between Intralesional Injection and Scaffold based transplantation. J Korean Med Sci. 2016;31:1373–82.
Assinck P, Duncan GJ, Hilton BJ, Plemel JR, Tetzlaff W. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20:637–47.
Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, Smithson LJ, et al. A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma. 2011;28:1611–82.
Raisman G. Olfactory ensheathing cells - another miracle cure for spinal cord injury? Nat Rev Neurosci. 2001;2:369–75.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Kramer AS, Harvey AR, Plant GW, Hodgetts SI. Systematic review of induced pluripotent stem cell technology as a potential clinical therapy for spinal cord injury. Cell Transpl. 2013;22:571–617.
Saremi J, Mahmoodi N, Rasouli M, Ranjbar FE, Mazaheri EL, Akbari M, Hasanzadeh E, Azami M. Advanced approaches to regenerate spinal cord injury: the development of cell and tissue engineering therapy and combinational treatments. Biomed Pharmacother. 2022;146:112529.
Zhang L, Zhuang X, Chen Y, **a H. Intravenous transplantation of olfactory bulb ensheathing cells for a spinal cord hemisection injury rat model. Cell Transpl. 2019;28:1585–602.
Yamazaki K, Kawabori M, Seki T, Houkin K. Clinical trials of stem cell treatment for spinal cord Injury. Int J Mol Sci 2020, 21.
Sareen D, O’Rourke JG, Meera P, Muhammad AK, Grant S, Simpkinson M, Bell S, Carmona S, Ornelas L, Sahabian A, et al. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med. 2013;5:208ra149.
Du ZW, Chen H, Liu H, Lu J, Qian K, Huang CL, Zhong X, Fan F, Zhang SC. Generation and expansion of highly pure motor neuron progenitors from human pluripotent stem cells. Nat Commun. 2015;6:6626.
Nogradi A, Pajer K, Marton G. The role of embryonic motoneuron transplants to restore the lost motor function of the injured spinal cord. Ann Anat. 2011;193:362–70.
Lukovic D, Valdes-Sanchez L, Sanchez-Vera I, Moreno-Manzano V, Stojkovic M, Bhattacharya SS, Erceg S. Brief report: astrogliosis promotes functional recovery of completely transected spinal cord following transplantation of hESC-derived oligodendrocyte and motoneuron progenitors. Stem Cells. 2014;32:594–9.
Rossi SL, Nistor G, Wyatt T, Yin HZ, Poole AJ, Weiss JH, Gardener MJ, Dijkstra S, Fischer DF, Keirstead HS. Histological and functional benefit following transplantation of motor neuron progenitors to the injured rat spinal cord. PLoS ONE. 2010;5:e11852.
Mukhamedshina YO, Gracheva OA, Mukhutdinova DM, Chelyshev YA, Rizvanov AA. Mesenchymal stem cells and the neuronal microenvironment in the area of spinal cord injury. Neural Regen Res. 2019;14:227–37.
Khan S, Mafi P, Mafi R, Khan W. A systematic review of mesenchymal stem cells in spinal cord Injury, intervertebral disc repair and spinal Fusion. Curr Stem Cell Res Ther. 2018;13:316–23.
Park N, Rim YA, Jung H, Nam Y, Ju JH. Lupus Heart Disease modeling with combination of Induced Pluripotent Stem Cell-Derived cardiomyocytes and Lupus Patient serum. Int J Stem Cells 2021.
Rim YA, Park N, Nam Y, Ham DS, Kim JW, Ha HY, Jung JW, Jung SM, Baek IC, Kim SY, et al. Recent progress of national banking project on homozygous HLA-typed induced pluripotent stem cells in South Korea. J Tissue Eng Regen Med. 2018;12:e1531–6.
Kim Y, Rim YA, Yi H, Park N, Park SH, Ju JH. The Generation of Human Induced Pluripotent Stem Cells from Blood Cells: An Efficient Protocol Using Serial Plating of Reprogrammed Cells by Centrifugation. Stem Cells Int 2016, 2016:1329459.
Jung H, Rim YA, Park N, Nam Y, Ju JH. Restoration of Osteogenesis by CRISPR/Cas9 genome editing of the mutated COL1A1 gene in Osteogenesis Imperfecta. J Clin Med 2021, 10.
Lee JY, Ha KY, Kim JW, Seo JY, Kim YH. Does extracorporeal shock wave introduce alteration of microenvironment in cell therapy for chronic spinal cord injury? Spine (Phila Pa 1976). 2014;39:E1553–1559.
Lee H, Lee HY, Lee BE, Gerovska D, Park SY, Zaehres H, Arauzo-Bravo MJ, Kim JI, Ha Y, Scholer HR, Kim JB. Sequentially induced motor neurons from human fibroblasts facilitate locomotor recovery in a rodent spinal cord injury model. Elife 2020, 9.
Li H, Wang C, He T, Zhao T, Chen YY, Shen YL, Zhang X, Wang LL. Mitochondrial transfer from bone marrow mesenchymal stem cells to motor neurons in spinal cord Injury rats via Gap Junction. Theranostics. 2019;9:2017–35.
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–7.
Liddelow SA, Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–67.
Guerit S, Fidan E, Macas J, Czupalla CJ, Figueiredo R, Vijikumar A, Yalcin BH, Thom S, Winter P, Gerhardt H, et al. Astrocyte-derived wnt growth factors are required for endothelial blood-brain barrier maintenance. Prog Neurobiol. 2021;199:101937.
Aarabi B, Mirvis S, Shanmuganathan K, Vaccaro AR, Holmes CJ, Akhtar-Danesh N, Fehlings MG, Dvorak MF. Comparative effectiveness of surgical versus nonoperative management of unilateral, nondisplaced, subaxial cervical spine facet fractures without evidence of spinal cord injury: clinical article. J Neurosurg Spine. 2014;20:270–7.
Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord Injury-Repair and Regeneration. Neurosurgery. 2017;80:S9–22.
Hachem LD, Fehlings MG. Pathophysiology of spinal cord Injury. Neurosurg Clin N Am. 2021;32:305–13.
Quadri SA, Farooqui M, Ikram A, Zafar A, Khan MA, Suriya SS, Claus CF, Fiani B, Rahman M, Ramachandran A, et al. Recent update on basic mechanisms of spinal cord injury. Neurosurg Rev. 2020;43:425–41.
Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26:S2–12.
Mortazavi MM, Verma K, Deep A, Esfahani FB, Pritchard PR, Tubbs RS, Theodore N. Chemical priming for spinal cord injury: a review of the literature. Part I-factors involved. Childs Nerv Syst. 2011;27:1297–306.
Kjell J, Olson L. Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech. 2016;9:1125–37.
Lilley E, Andrews MR, Bradbury EJ, Elliott H, Hawkins P, Ichiyama RM, Keeley J, Michael-Titus AT, Moon LDF, Pluchino S, et al. Refining rodent models of spinal cord injury. Exp Neurol. 2020;328:113273.
Metz GA, Curt A, van de Meent H, Klusman I, Schwab ME, Dietz V. Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma. 2000;17:1–17.
Ha KY, Carragee E, Cheng I, Kwon SE, Kim YH. Pregabalin as a neuroprotector after spinal cord injury in rats: biochemical analysis and effect on glial cells. J Korean Med Sci. 2011;26:404–11.
Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–64.
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–55.
Williams A, Piaton G, Lubetzki C. Astrocytes–friends or foes in multiple sclerosis? Glia. 2007;55:1300–12.
Gao L, Peng Y, Xu W, He P, Li T, Lu X, Chen G. Progress in Stem Cell Therapy for Spinal Cord Injury. Stem Cells Int 2020, 2020:2853650.
Wright KT, El Masri W, Osman A, Chowdhury J, Johnson WE. Concise review: bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells. 2011;29:169–78.
Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal stem cells for spinal cord Injury: current options, limitations, and future of cell therapy. Int J Mol Sci 2019, 20.
Neirinckx V, Cantinieaux D, Coste C, Rogister B, Franzen R, Wislet-Gendebien S. Concise review: spinal cord injuries: how could adult mesenchymal and neural crest stem cells take up the challenge? Stem Cells. 2014;32:829–43.
Nakajima H, Uchida K, Guerrero AR, Watanabe S, Sugita D, Takeura N, Yoshida A, Long G, Wright KT, Johnson WE, Baba H. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J Neurotrauma. 2012;29:1614–25.
Liu W, Wang Y, Gong F, Rong Y, Luo Y, Tang P, Zhou Z, Zhou Z, Xu T, Jiang T, et al. Exosomes Derived from Bone mesenchymal stem cells repair traumatic spinal cord Injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J Neurotrauma. 2019;36:469–84.
Peng J, Wang Y, Zhang L, Zhao B, Zhao Z, Chen J, Guo Q, Liu S, Sui X, Xu W, Lu S. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiate into a Schwann-cell phenotype and promote neurite outgrowth in vitro. Brain Res Bull. 2011;84:235–43.
Mahay D, Terenghi G, Shawcross SG. Schwann cell mediated trophic effects by differentiated mesenchymal stem cells. Exp Cell Res. 2008;314:2692–701.
Cafferty WB, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SW. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J Neurosci. 2001;21:7161–70.
Lin W, Li M, Li Y, Sun X, Li X, Yang F, Huang Y, Wang X. Bone marrow stromal cells promote neurite outgrowth of spinal motor neurons by means of neurotrophic factors in vitro. Neurol Sci. 2014;35:449–57.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, & Future Planning (grant number: NRF-2020R1AC3004123 and NRF-2021R1C1C2004688) and the grant of the Korea Healthcare Technology R&D project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (HI16C2177 and HI20C0495). This research was also supported by grants from the Catholic Institute of Cell Therapy (CRC) in 2022and 2023. The Basic Medical Science Facilitation Program funded by the Catholic Education Foundation through the Catholic Medical Center of the Catholic University of Korea. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI22C1314). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Study design: J.W.K. and J.H.J.; data collection: J.-W.K., Y.A.R., J.K., H.M., H.H and J.H.J., data analysis: J.W.K.,Y.A.R., J.K., and J.H.J.; Drafting manuscript: J.W.K., Y.A.R and J.H.J.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Title of the approved project: Two-step bone marrow derived mesenchymal stem cell and iPSCs derived motor neuron cells cell therapy improves neuro protection and neuro regeneration in contusive spinal cord injury. Name of the institutional approval committee: The Animal Studies Committee of the School of Medicine, the Catholic University of Korea. Approval Number: IACUC approval Number CUMC-2020-0044-04. Date of approval: February 01, 2020.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interest. Affiliation 3 is declare that there is no competing interest. J.K. is employee at YiPSCELL, Inc., and J.H.J. is the employer. J.H.J. is the founder of YiPSCELL. Inc. and also works at the Seoul St. Mary’s hospital, Catholic University of Korea. The two groups do not have competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.






Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kim, JW., Kim, J., Mo, H. et al. Stepwise combined cell transplantation using mesenchymal stem cells and induced pluripotent stem cell-derived motor neuron progenitor cells in spinal cord injury. Stem Cell Res Ther 15, 114 (2024). https://doi.org/10.1186/s13287-024-03714-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-024-03714-3