Abstract
Background
Menstrual blood-derived cells show regenerative potential as a mesenchymal stem cell and may therefore be a novel stem cell source of treatment for refractory infertility with injured endometrium. However, there have been few pre-clinical studies using cells from infertile patients, which need to be addressed before establishing an autologous transplantation. Herein, we aimed to investigate the therapeutic capacity of menstrual blood-derived cells from infertile patients on endometrial infertility.
Methods
We collected menstrual blood-derived cells from volunteers and infertile patients and confirmed their mesenchymal stem cell phenotype by flow cytometry and induction of tri-lineage differentiation. We compared the proliferative and paracrine capacities of these cells. Furthermore, we also investigated the regenerative potential and safety concerns of the intrauterine transplantation of infertile patient-derived cells using a mouse model with mechanically injured endometrium.
Results
Menstrual blood-derived cells from both infertile patients and volunteers showed phenotypic characteristics of mesenchymal stem cells. In vitro proliferative and paracrine capacities for wound healing and angiogenesis were equal for both samples. Furthermore, the transplantation of infertile patient-derived cells into uterine horns of the mouse model ameliorated endometrial thickness, prevented fibrosis, and improved fertility outcomes without any apparent complications.
Conclusions
In our pre-clinical study, intrauterine transplantation of menstrual blood-derived cells may be a novel and attractive stem cell source for the curative and prophylactic therapy for injured endometrium. Further studies will be warranted for future clinical application.
Similar content being viewed by others
Background
Infertility is defined as failed conception after 12 months of regular unprotected sexual intercourse, and it is estimated to affect 8–12% of couples of reproductive age [1]. The number of worldwide infertile patients has increased to more than 186 million people, the majority being residents of developed countries [2]. Among various etiologies of infertility, endometrium function is an essential factor for successful embryonic implantation and subsequent maintaining of pregnancy [3]. Fundamentally, endometrium is structurally comprised of two distinct layers, outer functional layer and underlying basal layer proximal to the myometrium. It is dynamic and cyclic significant cell turnover during reproductive age [4]. However, once normal endometrium is damaged by invasive interventions as curettage, hysteroscopic surgery, or uterine artery embolization for gynecological diseases several times, endometrium becomes thin and fibrotic, leading to the disturbance of normal conception, called Asherman’s syndrome. Although several treatments for this disease, including hysteroscopic endometrial adhesiolysis and intrauterine injection of granulocyte-colony-stimulating factor or platelet-rich plasma, have been in use, their therapeutic efficacy is quite limited and relapse often occurs after the treatment [5, 6]. Therefore, a novel therapeutic approach needs to be established for repair of endometrial receptivity when endometrium is injured.
According to recent studies, stem cell therapy using mesenchymal stem cells (MSCs) may be the breakthrough needed for the treatment of Asherman’s syndrome [6,7,8]. MSCs are heterogeneous subsets of stromal/mesenchymal regenerative cells, usually derived from bone marrow, adipose tissue, dental pulp, umbilical cord, and amniotic membrane [9]. Since they possess a powerful self-renewal potency and a high ability of immunomodulation, wound healing, and neovascularization, they have been widely studied as a novel stem cell resource for treatments to various diseases related to immune rejection, autoimmunity, degeneration, and severe inflammation [10,11,12]. Due to their regenerative ability, previous pre-clinical studies reported that intrauterine transplantation of MSCs from bone marrow, adipose tissue, and umbilical cord ameliorates thin endometrium and improves fertility [8, 13,14,15]. However, since these MSC resources have concerns such as invasiveness of collection, low availability, minimal cost-effectiveness, and ethical issues [5, 7], novel MSC resources are warranted.
In recent years, it has been shown that endometrium contains various types of stem cells including MSCs, and these cells play an important role in dynamic endometrial regeneration during the regular menstruation cycle [4, 16, 17]. Endometrial MSCs are located in the perivascular region in the functional layer. Hence, numerous endometrial MSCs shed from the endometrium into menstrual blood during menstruation and are called menstrual blood-derived stem cells (MenSCs), first reported in 2007 [18, 19]. Therefore, menstrual blood is attractive as a novel noninvasive, periodic, and cost-effective MSC resource with few ethical concerns [20]. Until now, several pre-clinical studies showed the regenerative efficacy of MenSCs on infertility caused by injured endometrium; [21,Statistical analysis Continuous data were presented as mean ± standard error of the mean (SEM) or standard deviation (SD) and analyzed using Student’s t test or one-way ANOVA test. Categorical data were presented as percentages and analyzed using Chi-square test. Prism 9.4.0 software (GraphPad, Inc.) was used for statistical analyses. P < 0.05 was considered statistically significant for all analyses.
Results
Successful identification of mesenchymal stem cells from menstrual blood
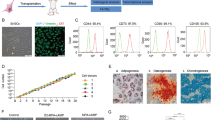
We gathered total 5 menstrual samples from volunteers and were able to isolate menstrual blood-derived cells in all samples. In addition, we collected menstrual blood samples from a total of 16 infertile patients and successfully isolated menstrual blood-derived cells from 10 samples, even from an Asherman’s syndrome patient (Table 1 and Additional file 4: Table S3). We failed to isolate menstrual blood-derived cells from the other 6 samples, which were mainly collected by cotton and consisted of cells that resembled vaginal epithelial cells when seeded on dishes. Following cell culture, spindle-shaped fibroblast-like cells were found from both volunteers and infertile patients (Fig. 1A). Then, we randomly selected 3 samples from volunteers and infertile patients to determine the cellular characteristics as MSCs. In tri-lineage differentiation, menstrual blood-derived cells from both volunteers and infertile patients expressed FABP-4 for adipogenesis, osteocalcin for osteogenesis, and aggrecan for chondrogenesis (Fig. 1B). Additionally, upon flow cytometric analysis for evaluating phenotypic markers of MSCs, these samples expressed CD73, CD90, and CD105 as MSC markers without the expression of CD14, CD19, CD34, CD45, and HLA-DR as hematopoietic cell markers (Fig. 1C). Expression of these MSC markers was similar between menstrual blood-derived cells from volunteers and infertile patients (Additional file 5: Table S4). Further, we also confirmed the cells with characteristics as MSCs from an Asherman’s syndrome (patient #1) (data not shown). These results show that we could identify menstrual blood-derived cells fulfilling the ISCT criteria from volunteers and infertile patients.
Cellular characterization of menstrual blood-derived cells as mesenchymal stem cells. A Phase-contrast photomicrographs of spindle-shaped fibroblast-like menstrual blood-derived cells from volunteers and infertile patients with 100% confluency on a petri dish. Black bar is 500 μm. B Immunocytochemistry of menstrual blood-derived cells after differentiation. Menstrual blood-derived cells from both volunteers and infertile patients were positive for FABP-4 (green), osteocalcin (red), and aggrecan (green) for adipogenesis, osteogenesis, and chondrogenesis, respectively. Cell nuclei were stained using DAPI in blue. White bar is 100 μm. C Flow cytometric analysis for phenotypic markers of menstrual blood-derived cells. Menstrual blood-derived cells at passage 3–4 from both volunteers and infertile patients were positive for CD73, 90, and 105 as makers for mesenchymal stem cells and negative for CD14, CD19, CD34, CD45, and HLA-DR as makers for hematopoietic stem cells (n = 3 in each group)
MenSCs from infertile patients were biologically equivalent to those from volunteers
To investigate therapeutic potential equivalence between volunteer-derived and infertile patient-derived cells, we first examined the proliferative potential of MenSCs. MenSCs from both volunteers and infertile patients were propagated to 100% confluency until day 7 after plating on a 6-well plate (Fig. 2A). Growth of primary menstrual blood-derived cells from infertile patients was similar to that of volunteers (Fig. 2B). Further, to investigate the effect of the freeze–thaw procedure on cell growth, we compared cell proliferation between primary and thawed frozen menstrual blood-derived cells from infertile patients (Additional file 6: Figure S2A, B). The thawed cells were also propagated to 100% confluency at day 7, and there were no significant differences until day 7, compared to the primary cells, suggesting that proliferative potential is preserved even after the freeze–thaw procedure.
Proliferative capacity of MenSCs from volunteers and infertile patients. (A) Phase-contrast photomicrographs of MenSCs at different time points. B Primary cells (1.0 × 10 [5]) from volunteers and infertile patients at passage 4–7 were plated into 6-well plates (n = 3, in each group). Proliferative capacity was not significantly different between MenSCs from volunteers and infertile patients. Statistical significance is shown as *P < 0.05 and **P < 0.01. 'ns' means 'not significant.' Black bars are 500 μm
To screen comprehensively for paracrine factors from menstrual blood-derived cells, CM were collected and assessed by proteomic analysis (n = 1). This analysis revealed more than 200 proteins in the CM. Quantitative scatterplot indicated that the amounts of proteins were similar between the volunteer-derived and infertile patient-derived CM (Additional file 7: Fig. S3A). Furthermore, the number of 'unique proteins' (Additional file 7: Fig. S3B) was similar in the volunteer-derived and infertile patient-derived CM in the categories of 'biological process,' 'cellular component,' and 'molecular function.' In a Venn diagram, 51 proteins common in CM (Additional file 7: Fig. S3C and Additional file 8: Table S5) were categorized into subgroups of 'biological process' such as 'cellular process,' 'biological regulation,' 'developmental process,' 'multicellular organismal process,' 'response to stimulus,' 'localization,' and 'biological adhesion' (Additional file 7: Fig. S3D). In GO term analysis, 9 angiogenesis-related proteins, i.e. thrombospondin 1, fibronectin, lysyl oxidase homolog 2, junction plakoglobin, myosin-9, sushi repeat-containing protein, annexin A2, 14–3-3 protein zeta/delta, and heat shock protein beta-1, were found in both groups; cell proliferation-related 5 proteins, i.e. thrombospondin 1, fibronectin, actin beta, endosialin, and c-type lectin domain family 11 member A, were common; fibroblast proliferation-regulated proteins, i.e. fibronectin, annexin A2, and endosialin, were common; cell adhesion-related 13 proteins, i.e. thrombospondin 1/2, fibronectin, collagen alpha-1(XII) chain, nidogen-1/2, lysyl oxidase homolog 2, junction plakoglobin, amyloid-beta precursor protein, laminin subunit gamma-1/beta-1, galectin-3-binding protein, testican-1, versican core protein, and filaggrin-2, were in both groups. Proteomic analysis showed that infertile patient-derived MenSCs had paracrine factors involved in angiogenesis and wound healing in common with volunteer-derived MenSCs.
We then performed a scratch assay to investigate regenerative ability in injured tissue. The area of the scratch wound in each group was gradually masked by stromal cell migration (Fig. 3A). Wound confluency of infertile patient-derived and volunteer-derived CM groups increased, compared to a negative control (Fig. 3B). Additionally, relative wound density of infertile patient-derived and volunteer-derived CM groups also significantly increased, compared to a negative control (Fig. 3C). To investigate angiogenic potential, we performed a tube-forming assay to investigate whether HUVECs form tubular networks in the presence of the volunteer-derived and infertile patient-derived CM. The capacity of HUVECs to form tubular networks in the volunteer-derived and infertile patient-derived CM significantly increased compared to that in the negative control and equivalent to that in recombinant VEGF-supplemented culture medium, as indicated by tube area measurements (Fig. 3D, E). Further, the capacity of HUVECs to form tubular networks in the infertile-derived CM was equal to the volunteer-derived CM (Fig. 3D, E).
MenSCs show paracrine capacity for tissue repair and angiogenesis. A–C Scratch assay to evaluate the paracrine capacity for wound healing. A The left panels show phase-contrast photomicrographs of scratch wounds at 0, 12, and 24 h after scratching in the following media; M199/0.5% BSA (negative control), infertile patient-derived CM, and volunteer-derived CM. In the right panels, the cell region is yellow, the wound region is pink, and the wound mask region is orange. Black bars are 400 μm. B Wound confluency of infertile patient-derived and volunteer-derived CM groups increased, compared to the negative control (M199/0.5% BSA). C Relative wound density of infertile patient-derived and volunteer-derived CM groups significantly increased, compared to the negative control (M199/0.5% BSA). Each experiment was performed in triplicate. D Tube-forming assay to evaluate the paracrine capacity for angiogenesis. Microscopic appearance of tubular networks of HUVECs stained with Calcein AM in green with following media; M199/0.5% BSA (negative control), M199/0.5% BSA with recombinant VEGF (5 ng/ml) (positive control), infertile patient-derived CM and volunteer-derived CM. White bars are 500 μm. (E) The capacity of HUVECs to form tubular networks in the CM from a volunteer and an infertile patient significantly increased. Each experiment was performed in triplicate. All measurements are shown as mean ± SEM. Statistical significance is shown as *P < 0.05
These results indicate that the cellular and biological characteristics of menstrual blood-derived cells from an infertile patient were equivalent to those from a volunteer.
Successful establishment of mechanically injured endometrium model
We performed an experiment to establish a mechanically damaged endometrium model (Fig. 4A and Additional file 9). In comparison with the sham group, endometrial damage was apparent in the injured group at days 0 and 1 (Fig. 4B, H, E). Although arrangement of endometrial epithelial and stromal cells in the injured group was repaired by day 4, their endometrial lumen looked flat (Fig. 4B, H, E). Furthermore, in the injured group, fibrotic areas were found at days 1, 4, and 7 (Fig. 4B, MT), while no fibrotic areas were observed in the sham group. In addition, endometrial thickness in the injured group was significantly thinner than in the sham group at day 4 and 7 (Fig. 4C), whereas number of glands and endometrial area in the injured group decreased at each time point, but there were no significant differences between the sham and injured groups (Fig. 4D, E). We therefore established a mechanically injured endometrium model by endometrial curettage.
Establishment of a mouse model for mechanically injured endometrium. A Procedure for endometrial injury. A mouse model for endometrial injury was established by physical endometrial scratches using a 20-gauge needle in uterine horns, inserted from a cephalic side of the uterine horns. B Comparison of endometrial histology between sham groups and injury models at different time points after the surgical procedure (n = 3 in each group). Endometrial thickness was evaluated in a sagittal section of a left uterine horn as the maximal distance from endometrial–myometrial interface to endometrial surface (shown in H&E stain of the sham group at day 0). In addition, the endometrial area was evaluated in a sagittal section of a left uterine horn as the total area of the endometrium (shown in H&E stain of the sham control at day 0). In the injured models, endometrial damage was observed by H&E staining at day 0. Further, fibrotic areas in endometrium were detected by Masson trichrome stain at day 1, 4, and 7, whereas there were no fibrotic areas observed in the sham control. C Endometrial thickness in the injured model was significantly thinner at days 4 and 7. D There was no significant difference in the number of glands at each different time point. E There was no significant difference in the endometrial area at each different time point. All measurements are shown as mean ± SEM. Statistical significance is shown as *P < 0.05 and **P < 0.01. 'ns' means 'not significant.' Black bars are 500 μm, and white bars are 200 μm
Successful establishment of an intrauterine transplantation protocol and no findings of tumorigenicity from transplanted menstrual blood-derived cells
We performed intrauterine transplantation of MenSCs to determine the intrauterine distribution of the transplanted cells (Fig. 5A, B). At day 1 after transplantation, MenSCs were detected as human vimentin- and lamin AC-positive cells at the injured sites of endometrium including endometrial stroma, showing successful transplantation into injured endometrium. At day 7 after the transplantation, we found small quantity of the cells positive for human vimentin and lamin AC in endometrial stroma; however, these could not be observed at day 30. No apparent tumor formation was observed in endometrium. These findings suggest that transplanted cells gradually disappear in the mouse uterus without local tumorigenicity, probably due to engraftment failure or immunogenicity.
Local distribution of MenSCs after intrauterine transplant. A Procedure for intrauterine transplantation of MenSCs. After endometrial scratching with a 20-gauge needle, 1.0 × 106 of menstrual blood-derived cells in 30 μl of PBS were injected into uterine horns. B Local distribution of transplanted intrauterine MenSCs at each time point (n = 3). The transplanted cells were positive for human vimentin and lamin AC at day 1 in injured endometrial lesions. Small numbers of cells were found at day 7 in the stroma, not in the epithelia. At day 30, the cells positive for human vimentin and lamin AC were no longer detected. Black bars are 200 μm, and white bars are 50 μm
To assess the systemic biodistribution and tumorigenicity of the intrauterine transplanted cells, we histologically evaluated the major organs (Additional file 10: Figure S4). There were no obvious human vimentin-positive cells and tumor formation at 1, 7, and 30 days after intrauterine transplantation. In addition, no tumor formation was observed on the peritoneum by gross findings at the different time points.
Menstrual blood-derived cells from an infertile patient repair injured endometrium without apparent toxicological concerns
To evaluate therapeutic effect of infertile patient-derived MenSCs on injured endometrium, we performed histopathological and RT-qPCR analysis of mice uteri at day 7 after intrauterine transplantation. The uterus in the MenSC group looked thicker, like the sham group on gross examination, whereas that in the injured group looked atrophied and congested (Fig. 6A). Uterine weight in the MenSC group was slightly heavier compared to the injured group, without a statistically significant difference (Fig. 6B). The endometrial thickness in the MenSC group was significantly thicker than the injured group (Fig. 6C). The number of glands in the MenSC group slightly increased, compared to the injured group (Fig. 6D). The endometrial area in the MenSC group was significantly larger than the injured model (Fig. 6E). Further, the endometrial fibrotic area in the MenSC group was significantly decreased compared to the injured group (Fig. 6F). We performed immunohistochemistry to estimate cell proliferation and neovascularization by using an antibody to Ki-67 and CD34, respectively. The Ki-67-positive cells increased in the MenSC group, compared to the injured groups, without a statistically significant difference (Fig. 6A, G). CD34-positive microvessels significantly increased in the MenSC group, compared to the sham and injured group (Fig. 6A, H).
Regenerative effect of intrauterine MenSCs transplanted into injured endometrium. A Gross appearance and histological findings of endometrium in the sham, injured, and MenSC groups at day 7 (n = 4 in each group). Although the uteri of the injured group were atrophied and congested, those of the MenSC group exhibited similar gross appearance to the sham group, that is, neither atrophied nor congested. B There was no significant difference of uterine weights between the groups. C Significant recovery in endometrial thickness was observed in the MenSC group, compared to the injured group. D Number of glands in the MenSC group slightly increased, compared to the injured group (P = 0.10). E The endometrial area in the MenSC group was significantly larger than the injured group (P < 0.05). F Endometrial fibrotic area between the injured and MenSC groups. Endometrial fibrotic area in the MenSC group significantly decreased, compared to the injured group (P < 0.05). G Number of Ki-67 positive cells was slightly upregulated in the MenSC group, compared to the sham and injured group (P = 0.19). H Number of CD34-positive microvessels in the MenSC group significantly increased, compared to the injured group (P < 0.01). I–L Gene expression levels of VEGF, FGF1, FGF2, and EGF in each group. The expression levels of VEGF, FGF1, FGF2, and EGF in the MenSC group significantly decreased, compared to the sham group. The expression levels of VEGF and EGF in the MenSC group significantly decreased, compared to the injured group (P < 0.01). The experiment was performed in triplicate. Gene expression in the controls is regarded as equal to 1.0. All measurements are shown in mean ± SEM. Statistical significance is shown as *P < 0.05 and **P < 0.01. 'ns' means 'not significant.' Black bars are 500 μm, and white bars are 50 μm
We then analyzed gene expression of whole uterine tissue in each group. The mRNA expression levels of VEGF, FGF-1, FGF-2, and EGF in the MenSC group decreased, compared to the sham and injured groups. The gene expression levels for VEGF and EGF statistically significantly decreased in the MenSC group, compared to the sham and injured groups (Fig. 6I–L).
We also performed physical and blood sample examinations on each group. Between day 0 and 7 after transplantation, total body and major organ weights were not significantly different between the groups (Additional file 11: Table S6). Likewise, the blood cell counts and biochemical analysis showed no significant changes among the groups (Additional file 12: Table S7).
These results imply that intrauterine transplantation of infertile patient-derived MenSCs repairs endometrial injury and prevents fibrosis through promotion of endometrial cell proliferation and neovascularization without apparent toxicological concerns.
Fertility testing
To further evaluate the therapeutic efficacy of the intrauterine transplantation of infertile patient-derived MenSCs for fertility, we observed the gestational period and the number of delivered litters in each group (Fig. 7A). All mice in the study with vaginal plugs got pregnant and delivered pups. The gestational period was not significantly different among the sham, injured, and MenSC groups (18.9 ± 0.4, 19.9 ± 0.24, and 19.3 ± 0.2 days, respectively [mean ± SEM]; P = 0.09). However, the number of pups in the injured group significantly decreased in comparison with the sham group (7.2 ± 1.4 vs 11.6 ± 0.5 [mean ± SEM]; P < 0.05). Furthermore, the average number of pups in the MenSC group increased compared to the injured group by almost 2 pups, although the difference was not statistically significant (9.1 ± 1.0 vs 7.2 ± 1.4 [mean ± SEM]; P = 0.28) (Fig. 7B). These results show that the intrauterine transplantation of infertile patient-derived MenSCs partially ameliorates fertility in the mice with injured endometrium.
Therapeutic efficacy of transplanted intrauterine menstrual blood-derived cells for fertilization. A Scheme for evaluating fertility after intrauterine transplantation of MenSCs. B Number of pups delivered in each group. Number of pups in the injured group (n = 5) significantly decreased compared to the sham group (n = 5) (P < 0.05). Number of pups in the MenSCs group (n = 10) partially increased compared to the injured group (P = 0.28). All measurements are shown in mean ± SEM. Statistical significance is shown as *P < 0.05 and **P < 0.01. 'ns' means 'not significant'
Discussion
MenSCs have been projected to be a desirable mesenchymal stem cell resource due to their easy accessibility, periodic acquisition, beneficial cost-effectiveness, high proliferative ability, low immunological rejection, and paracrine secretion of various growth and angiogenic factors [20, 28, 32, 37,38,39]. In infertility, these cells have been investigated for their potential as a novel stem-cell therapy to infertility due to thin endometrium [21,22,23]. In this study, we successfully isolated and cultured menstrual blood-derived cells from infertile patients, even from Asherman’s syndrome, and demonstrated that their cellular potential as MSCs is equivalent to those from volunteers. Further, we showed their regenerative therapeutic effect on injured endometrium of the mouse model without obvious safety concerns such as toxicity and tumorigenicity, suggesting that infertile patient-derived MenSCs can be a source for a novel autologous stem cell therapy for infertility caused by injured endometrium.
A collection method for menstrual blood-derived cells from infertile patients
Previous reports about the regenerative effect of MenSCs on injured and fibrotic organs mainly used those derived from healthy volunteers, not infertile patients [21,22,23,24,25]. In such studies, menstrual cups were used to collect menstrual blood, but these cups are not widely used in Japan and hygiene issues remain. Furthermore, little is known about whether MenSCs can be collected and isolated from menstrual blood of infertile patients, particular in endometrial infertility. Therefore, other approaches should be considered to collect MenSCs. In this study, we established an efficient method for collection of MenSCs from infertile patients fulfilling with the ISCT criteria [31] in routine pelvic examination at infertile outpatient office, even from a patient with Asherman’s syndrome. According to the protocol in this study, 10 of 16 samples were successfully cultured; on the other hand, cells could not be isolated from 6 samples. Among them, 5 of 6 samples were collected by cotton and these samples mainly contained vaginal-epithelial like cells, indicating that a syringe is preferred to cotton as a collection method. MenSCs from infertile patients may become a more acceptable stem-cell source in the future, since our simple method to collect MenSCs can be introduced in infertility clinics.
A clinically relevant mouse model with injured endometrium
Another problem is differential methods to establish animal models with injured endometrium in previous studies. The methods include intrauterine ethanol injection [13, 14, 26], intrauterine heat irradiation [27], electric coagulation [23], and introduction of lipopolysaccharide [40]. Although these protocols can successfully establish thin endometrium animal models, most intrauterine adhesions occur after intrauterine mechanical intervention in the clinic [41]; that is, these models reported in previous studies are far from the natural pathology of infertility by thin endometrium. This fact indicates that the therapeutic effect of MenSCs using these models might not be enough for acquisition of proof of concept. As other reports using a mechanically injured model indicated [15, 42, 43], the model in this study showing thinner endometrium, fibrotic lesions, and impaired fertility is clinically relevant to the pathology of infertility with injured endometrium.
Regenerative effect of menstrual blood-derived cells on injured endometrium through paracrine effects without apparent safety concerns
Recent studies show that the regenerative effect of implanted or transplanted MSCs is through a paracrine effect rather than differentiation ability [8, 44]. Conditioned media of bone marrow derived MSCs contain abundant angiogenic, immunomodulatory, and growth factors for tissue repair [45]. Additionally, MenSCs from healthy volunteers secrete high levels of angiogenic and anti-inflammatory factors [28]. However, few reports investigated the secretory capacity of infertile patient-derived MenSCs. The analysis of pathways suggests that even infertile patient-derived MenSCs possess regenerative therapeutic potential through angiogenesis and tissue repair akin to that of MSCs derived from other tissues or organs (Additional file 13: Figure S5), thereby positioning them as viable cellular therapeutic agents. It is noteworthy that there are differences in cellular potential between the samples, though. Significant endometrial repair is probably due to neovascularization and prevention of fibrosis after intrauterine transplantation of MenSCs, without direct differentiation into endometrial epithelial or stromal cells; the regenerative potential of infertile-patient derived MenSCs is based on their paracrine effect. Performing more comprehensive investigations such as functional gain and loss assays on the paracrine factors derived from MenSCs will elucidate the fundamental mechanisms behind their therapeutic effects.
Decreased expression of VEGFA, FGF-1, FGF-2, and EGF in the uteri of mice in the MenSCs group is compatible with a therapeutic effect of MenSCs on decrease of VEGFA expression and relative increase of CD34-positive vessels in a diabetic wound repair model [46]. Adipose tissue-derived MSCs have no effect in expression of FGF-1/2, EGF, and Angiopoietin-1/2 [13]. Likewise, intravenous injection of umbilical cord-derived MSCs has no effect in expression of inflammatory factors including VEGF [47]. Based on these reports, suppression of these cytokines occurs probably due to several reasons such as factors from MenSCs, MSC source, or the experimental procedures used in each report.
We herewith emphasize that intrauterine transplantation of MenSCs has a positive therapeutical potential on injured endometrium and impaired fertility. Single-cell transcriptomic analysis showed that the underlying pathology of Asherman’s syndrome is deficiency and senescence of endometrial perivascular cells [48, 49] which are a main component of MenSCs [44]. Intrauterine transplantation of autologous MenSCs is a rational approach for treating thin endometrium. Our experimental procedure, MenSC transplantation on the same day of the curettage, indicates their prophylactic efficacy on the injured endometrium. Autologous intrauterine transplantation of MenSCs may not only be a curative therapy for Asherman’s syndrome but a prophylactic therapy for patients with injured endometrium after endometrial curettage, hysteroscopic myomectomy, and endometrial ablation.
Intrauterine transplantation of MenSCs appears to have no safety concerns such as toxicity and tumorigenicity [29]. Another study reports no safety concerns on immunogenicity and tumorigenicity after intraperitoneal and intravenous transplantations of MenSCs [28]. According to both reports, transplantation of MenSCs shows no apparent safety concerns. Since these studies only evaluate short-term complications, future studies should look for evidence of long-term complications.
Future inspection of the regenerative therapy for injured endometrium using menstrual blood-derived cells
Several studies of phase I clinical trials using transplantation of autologous MSCs for severe Asherman’s syndrome have been reported: one for intra-artery transplantation of bone marrow-derived stem cells [50] and two for intrauterine transplantation of MenSCs [51, 52]. These trials showed favorable results: recovery of endometrial thickness and successful pregnancy. A clinical trial using autologous MenSC transplantation will possibly be a more effective and safer treatment for injured endometrium. However, unsolved problems still remain: what quantities of cells are most effective, how many rounds of MenSCs transplantation are required, and when is the best timing for transplantation after intrauterine interventions. The effectiveness of prophylactic transplantation of MenSCs has not yet been investigated in clinical trials. In addition, some reports indicate that intrauterine transplantation of bio-engineered scaffold-embedded MSCs [5, 53] or extracellular vesicles such as exosomes from MSCs [6, 30, 54] is preferred to MSC administration alone. Further investigation to determine the most effective therapeutic protocol using infertile patient-derived MenSCs on injured endometrium will be needed.
Conclusion
In conclusion, our study demonstrates that autologous intrauterine transplantation of infertile patient-derived MenSCs exerts their regenerative capacity as a curative and prophylactic treatment for injured endometrium without apparent safety concerns. MenSCs are an attractive and novel cell source for autologous regenerative therapy for injured endometrium. Further safety examination using good laboratory practice is needed before clinical trials.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Our datasets for the proteomics analysis generated and analyzed during the current study are available in the jPSOT repository (https://repository.jpostdb.org/) [ID: JPST002324] and the ProteomeXchange (https://www.proteomexchange.org/) [ID: PXD045424].
Abbreviations
- MSCs:
-
Mesenchymal stem cells
- MenSCs:
-
Menstrual blood-derived stem cells
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- AA:
-
Penicillin–streptomycin amphotericin B suspension
- PBS:
-
Phosphate-buffered saline
- PS:
-
Penicillin streptomycin
- ISCT:
-
International Society for Cellular Therapy
- FABP-4:
-
Fatty acid-binding protein-4
- PFA:
-
Paraformaldehyde
- IgG:
-
Immunoglobulin G
- HRP:
-
Horseradish peroxidase
- DAB:
-
Diaminobenzidine
- CM:
-
Conditioned media
- BSA:
-
Bovine serum albumin
- LS-MS:
-
Liquid chromatography tandem mass spectrometry
- HPLC:
-
High-performance liquid chromatography
- HUVECs:
-
Human umbilical vein endothelial cells
- ECM:
-
Endothelial cell medium
- GFR:
-
Growth factor reduce
- H&E:
-
Hematoxylin and eosin
- MT:
-
Masson trichrome
- RT-qPCR:
-
Real-time quantitative polymerase chain reaction
- VEGF:
-
Vascular endothelial growth factor
- FGF:
-
Fibroblast growth factor
- EGF:
-
Epidermal growth factor
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- SEM:
-
Standard error of the mean
- SD:
-
Standard deviation
References
Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62:2–10.
Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update. 2015;21(4):411–26.
Abrao MS, Muzii L, Marana R. Anatomical causes of female infertility and their management. Int J Gynaecol Obstet. 2013;123(Suppl 2):S18-24.
Salamonsen LA, Hutchison JC, Gargett CE. Cyclical endometrial repair and regeneration. Development. 2021;148(17):dev199577.
de Miguel-Gomez L, Romeu M, Pellicer A, Cervello I. Strategies for managing Asherman’s syndrome and endometrial atrophy: since the classical experimental models to the new bioengineering approach. Mol Reprod Dev. 2021;88(8):527–43.
Gharibeh N, Aghebati-Maleki L, Madani J, Pourakbari R, Yousefi M, Ahmadian HJ. Cell-based therapy in thin endometrium and Asherman syndrome. Stem Cell Res Ther. 2022;13(1):33.
Azizi R, Aghebati-Maleki L, Nouri M, Marofi F, Negargar S, Yousefi M. Stem cell therapy in Asherman syndrome and thin endometrium: stem cell- based therapy. Biomed Pharmacother. 2018;102:333–43.
Song YT, Liu PC, Tan J, Zou CY, Li QJ, Li-Ling J, et al. Stem cell-based therapy for ameliorating intrauterine adhesion and endometrium injury. Stem Cell Res Ther. 2021;12(1):556.
Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. 2019;37(7):855–64.
Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22.
Wu X, Jiang J, Gu Z, Zhang J, Chen Y, Liu X. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. 2020;11(1):345.
Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–48.
Yotsumoto F, Iwaguro H, Harada Y, Sobajima S, Suwabe T, Miyamoto S. Adipose tissue-derived regenerative cells improve implantation of fertilized eggs in thin endometrium. Regen Med. 2020;15(7):1891–904.
Zhang L, Li Y, Dong YC, Guan CY, Tian S, Lv XD, et al. Transplantation of umbilical cord-derived mesenchymal stem cells promotes the recovery of thin endometrium in rats. Sci Rep. 2022;12(1):412.
Alawadhi F, Du H, Cakmak H, Taylor HS. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman’s syndrome. PLoS ONE. 2014;9(5):e96662.
Santamaria X, Mas A, Cervello I, Taylor H, Simon C. Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update. 2018;24(6):673–93.
Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: the first 10 years. Hum Reprod Update. 2016;22(2):137–63.
Meng X, Ichim TE, Zhong J, Rogers J, Yin Z, Jackson J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57.
Cui CH, Uyama T, Miyado K, Terai M, Kyo S, Kiyono T, et al. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell. 2007;18(5):1586–94.
Toyoda M, Cui C, Umezawa A. Myogenic transdifferentiation of menstrual blood-derived cells. Acta Myol. 2007;26(3):176–8.
Hu J, Song K, Zhang J, Zhang Y, Tan BZ. Effects of menstrual bloodderived stem cells on endometrial injury repair. Mol Med Rep. 2019;19(2):813–20.
Chen L, Guo L, Chen F, **e Y, Zhang H, Quan P, et al. Transplantation of menstrual blood-derived mesenchymal stem cells (MbMSCs) promotes the regeneration of mechanical injuried endometrium. Am J Transl Res. 2020;12(9):4941–54.
Zhang Y, Lin X, Dai Y, Hu X, Zhu H, Jiang Y, et al. Endometrial stem cells repair injured endometrium and induce angiogenesis via AKT and ERK pathways. Reproduction. 2016;152(5):389–402.
Wang Z, Wang Y, Yang T, Li J, Yang X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res Ther. 2017;8(1):11.
Chen X, Wu Y, Wang Y, Chen L, Zheng W, Zhou S, et al. Human menstrual blood-derived stem cells mitigate bleomycin-induced pulmonary fibrosis through anti-apoptosis and anti-inflammatory effects. Stem Cell Res Ther. 2020;11(1):477.
Shao X, Ai G, Wang L, Qin J, Li Y, Jiang H, et al. Adipose-derived stem cells transplantation improves endometrial injury repair. Zygote. 2019;27(6):367–74.
Wang X, Wang C, Cong J, Bao H, Liu X, Hao C. Regenerative potential of menstrual blood-derived stem cells and platelet-derived growth factor in endometrial injury. Med Sci Monit. 2020;26:e919251.
Liu Y, Niu R, Yang F, Yan Y, Liang S, Sun Y, et al. Biological characteristics of human menstrual blood-derived endometrial stem cells. J Cell Mol Med. 2018;22(3):1627–39.
Chang QY, Zhang SW, Li PP, Yuan ZW, Tan JC. Safety of menstrual blood-derived stromal cell transplantation in treatment of intrauterine adhesion. World J Stem Cells. 2020;12(5):368–80.
Zhang S, Chang Q, Li P, Tong X, Feng Y, Hao X, et al. Concentrated small extracellular vesicles from menstrual blood-derived stromal cells improve intrauterine adhesion, a pre-clinical study in a rat model. Nanoscale. 2021;13(15):7334–47.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Alcayaga-Miranda F, Cuenca J, Luz-Crawford P, Aguila-Díazet C, Fernandez A, Figueroa FE, et al. Characterization of menstrual stem cells: angiogenic effect, migration and hematopoietic stem cell support in comparison with bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2015;6:32.
Palstrøm NB, Rasmussen LM, Beck HC. Affinity capture enrichment versus affinity depletion: a comparison of strategies for increasing coverage of low-abundant human plasma proteins. Int J Mol Sci. 2020;21(16):5963.
Yokomizo R, Fujiki Y, Kishigami H, Kishi H, Kiyono T, Nakayama S, et al. Endometrial regeneration with endometrial epithelium: homologous orchestration with endometrial stroma as a feeder. Stem Cell Res Ther. 2021;12(1):130.
Seki T, Yanaihara N, Shapiro JS, Saito M, Tabata J, Yokomizo R, et al. Interleukin-6 as an enhancer of anti-angiogenic therapy for ovarian clear cell carcinoma. Sci Rep. 2021;11(1):7689.
Goldring CE, Duffy PA, Benvenisty N, Andrews PW, Ben-David U, Eakins R, et al. Assessing the safety of stem cell therapeutics. Cell Stem Cell. 2011;8(6):618–28.
Bozorgmehr M, Gurung S, Darzi S, Nikoo S, Kazemnejad S, Zarnani AH, et al. Endometrial and menstrual blood mesenchymal stem/stromal cells: biological properties and clinical application. Front Cell Dev Biol. 2020;8:497.
Chen L, Qu J, **ang C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res Ther. 2019;10(1):1.
Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells. 2008;26(7):1695–704.
**ao L, Song Y, Huang W, Yang S, Fu J, Feng X, et al. Expression of SOX2, NANOG and OCT4 in a mouse model of lipopolysaccharide-induced acute uterine injury and intrauterine adhesions. Reprod Biol Endocrinol. 2017;15(1):14.
Hooker AB, Lemmers M, Thurkow AL, Heymans MW, Opmeer B, Brölmann HAM, et al. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: prevalence, risk factors and long-term reproductive outcome. Hum Reprod Update. 2014;20(2):262–78.
Feng Q, Gao B, Zhao X, Huang H, Yi S, Zou L, et al. Establishment of an animal model of intrauterine adhesions after surgical abortion and curettage in pregnant rats. Ann Transl Med. 2020;8(4):56.
Li B, Zhang Q, Sun J, Lai D. Human amniotic epithelial cells improve fertility in an intrauterine adhesion mouse model. Stem Cell Res Ther. 2019;10(1):257.
Sanchez-Mata A, Gonzalez-Munoz E. Understanding menstrual blood-derived stromal/stem cells: definition and properties. Are we rushing into their therapeutic applications? iScience. 2021;24(12):103501.
McBride JD, Rodriguez-Menocal L, Guzman W, Khan A, Myer C, Liu X, et al. Proteomic analysis of bone marrow-derived mesenchymal stem cell extracellular vesicles from healthy donors: implications for proliferation, angiogenesis, Wnt signaling, and the basement membrane. Stem Cell Res Ther. 2021;12(1):328.
Dalirfardouei R, Jamialahmadi K, Jafarian AH, Mahdipour E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J Tissue Eng Regen Med. 2019;13(4):555–68.
Zhang L, Li Y, Guan CY, Tian S, Lv XD, Li JH, et al. Therapeutic effect of human umbilical cord-derived mesenchymal stem cells on injured rat endometrium during its chronic phase. Stem Cell Res Ther. 2018;9(1):36.
Lv H, Zhao G, Jiang P, Wang H, Wang Z, Yao S, et al. Deciphering the endometrial niche of human thin endometrium at single-cell resolution. Proc Natl Acad Sci U S A. 2022;119(8):e2115912119.
Zhang X, Li Y, Chen X, ** B, Shu C, Ni W, et al. Single-cell transcriptome analysis uncovers the molecular and cellular characteristics of thin endometrium. FASEB J. 2022;36(3):e22193.
Santamaria X, Cabanillas S, Cervello I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory Asherman’s syndrome and endometrial atrophy: a pilot cohort study. Hum Reprod. 2016;31(5):1087–96.
Tan J, Li P, Wang Q, Li Y, Li X, Zhao D, et al. Autologous menstrual blood-derived stromal cells transplantation for severe Asherman’s syndrome. Hum Reprod. 2016;31(12):2723–9.
Ma H, Liu M, Li Y, Wang W, Yang K, Lu L, et al. Intrauterine transplantation of autologous menstrual blood stem cells increases endometrial thickness and pregnancy potential in patients with refractory intrauterine adhesion. J Obstet Gynaecol Res. 2020;46(11):2347–55.
Li X, Lv HF, Zhao R, Ying MF, Samuriwo AT, Zhao YZ. Recent developments in bio-scaffold materials as delivery strategies for therapeutics for endometrium regeneration. Mater Today Bio. 2021;11:100101.
Chen L, Qu J, Mei Q, Chen X, Fang Y, Chen L, et al. Small extracellular vesicles from menstrual blood-derived mesenchymal stem cells (MenSCs) as a novel therapeutic impetus in regenerative medicine. Stem Cell Res Ther. 2021;12(1):433.
Acknowledgements
We would like to express our sincere thanks to K. Miyado and H. Akutsu for fruitful discussion, to M. Ichinose for providing expert technical assistance, to C. Ketcham for English editing and proofreading, and to E. Suzuki and K. Saito for secretarial work.
Funding
This research was supported by the Jikei University Research Fund for Graduate Students; by JSPS KAKENHI Grant Nos. JP22J23244 and JP20J14152; by the Grant of National Center for Child Health and Development; by the research grant of Ferring Pharmaceutical Co., Ltd.; by the research grant of TERUMO LIFE SCIENCE FOUNDATION. The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
SH, RY, AU conceptualized the study and acquired the funding, EK, MA, TS collected the menstrual sample, SH, RY, YF, H Kishigami curated and analyzed the data and investigation (in vivo and in vitro), SH, RY, TK, AU helped in methodology, HS, AO, AU administrated the project, SH, RY helped in software and writing–original draft, H Kishi, AO, HS, AU supervised the study, H Kishigami, YF, EK, MA, TS, H Kishi, HS, AO, AU contributed to writing–review & editing. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol for the use of menstrual blood-derived cells in the present study was approved by the Institutional Review Board of National Center for Child Health and Development of Japan (approval number: 2021-025 for infertile patients [May 27th, 2021] and 89 for volunteers [November 8th, 2010]). Written informed consents for participation and publication were obtained from all participants. Use of endometrial stromal cells was approved by the Institutional Review Board of National Center for Child Health and Development of Japan (approval number: 2289 [August 27th, 2019]) and the Jikei University School of Medicine (approval number: 28-083(8326), [June, 10th, 2019]). The animal experiments were approved by the Institutional Animal Care and Use Committee of National Center for Child Health and Development (approval number: 2003-002, [April 1st, 2003]). All animal experiments were based on the 3R principle (refine, reduce, and replace), and all efforts were made to minimize animal suffering and to reduce the number of animals used.
Consent for publication
Written informed consents for participation and publication were obtained from all participants.
Competing interests
AU is a co-researcher with MTI Ltd., Terumo Corp., BONAC Corp., Kaneka Corp., CellSeed Inc., ROHTO Pharmaceutical Ltd., SEKISUI MEDICAL Ltd., Metcela Inc., PhoenixBio Ltd., Dai Nippon Printing Ltd. AU is a stockholder of TMU Science Ltd., Morikuni Ltd., and Japan Tissue Engineering Ltd. AU is the associate editor of this journal but was not involved in the peer review or decision making of this manuscript. The other authors declare that there is no conflict of interest regarding the work described herein.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
A collection method of MenSCs from infertile patients. Collection method for menstrual blood samples. Samples were collected into 15-ml tubes with a syringe (A) or a 150-ml bottle with a cotton (B), containing DMEM/2% FBS/1% AA. The sample was centrifuged at 1000 rpm for 5 min (C). After centrifugation, supernatant was removed and 1 ml of DMEM/2% FBS/1% AA was added. Then, 10 ml of RBC lysis buffer was added and gently pipetted several times. After pipetting, the sample was incubated at 4°C for 12 min (D). After incubation, a sample was gently pipetted several times and centrifuged at 1000 rpm for 5 min again. Supernatant was removed, washed with 5 ml of PBS, and centrifuged at 1000 rpm for 5 min. This process was done several times to remove RBC until the supernatant looked transparent (E).
Additional file 2: Table S1.
List of antibodies for immunohistochemistry and flow-cytometry
Additional file 3: Table S2.
List of primer sequences for quantitative reverse transcription polymerase chain reaction
Additional file 4: Table S3.
Patient characteristics of samples with unsuccessful primary culture of menstrual-blood derived cells
Additional file 5: Table S4.
Flow cytometric analysis for mesenchymal stem cell markers
Additional file 6: Figure S2.
Proliferative capacity of primary and freeze-thawed MenSCs from infertile patients. (A) Phase-contrast photomicrographs of primary and freeze-thawed MenSCs from infertile patients at different time points. (B) MenSCs (1.0 × 105) at passage 4–7 were plated into 6-well plates with DMEM/10% FBS (n = 3 in each group). Cells were counted at day 1, 3, 5, and 7. Proliferative capacity was not significantly decreased until day 7 even after the freeze-thawed procedure. Statistical significance is shown as *P <0.05 and **P <0.01. 'ns' means 'not significant.' Black bars are 500 μm.
Additional file 7: Figure S3.
Proteomics analysis of CM from volunteer-derived and infertile patient-derived MenSCs. (A) Quantitative scatterplot indicated that proteins contained in CM from volunteer-derived and infertile patient-derived MenSCs were similar to each other. (B) Number of 'unique proteins' was similar in the volunteer-derived and infertile patient-derived CM in the categories of 'biological process,' 'cellular component,' and 'molecular function.' (C) Venn diagram showing that total 51 proteins were common between volunteer-derived and infertile patient-derived CM. (D) The 51 proteins were primarily categorized into subgroups of 'biological process' such as 'cellular process,' 'biological regulation,' 'developmental process,' 'multicellular organismal process,' 'response to stimulus,' 'localization,' and 'biological adhesion.'
Additional file 8: Table S5.
List of common proteins in the CM from volunteer-derived and infertile patient-derived menstrual blood-derived cells
Additional file 9. Movie of intrauterine curettage
Additional file 10: Figure S4.
Systemic biodistribution of intrauterine transplanted MenSCs in major organs. Histopathological findings of major organs at day 1, 7, and 30 after intrauterine transplantation of MenSCs (n = 3 in each group). There were no human vimentin-positive cells and tumor formation in brain, heart, lungs, liver, spleen, pancreas, kidneys, adrenal glands, and ovaries. Black bars are 500 μm.
Additional file 11: Table S6.
Body and organ weights of the mice in each group
Additional file 12: Table S7.
Blood sample test of the mice in each group
Additional file 13: Figure S5.
Pathway analysis using Reactome Pathway Database. Pathways were analyzed for the shared proteins in the conditioned media between infertile patient-derived and volunteer-derived MenSCs using Reactome Pathway Database. The conditioned media contained proteins associated with pathways for cell growth and neovascularization. P < 0.05 was defined as statistically significance.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hosoya, S., Yokomizo, R., Kishigami, H. et al. Novel therapeutic strategies for injured endometrium: intrauterine transplantation of menstrual blood‑derived cells from infertile patients. Stem Cell Res Ther 14, 297 (2023). https://doi.org/10.1186/s13287-023-03524-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03524-z