Abstract
Background
To explore whether local transplantation of mesenchymal stem cells (MSCs) in temporal muscle can promote collateral angiogenesis and to analyze its main mechanisms of promoting angiogenesis.
Methods
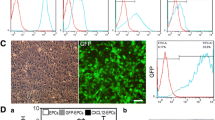
Bilateral carotid artery stenosis (BCAS) treated mice were administrated with encephalo-myo-synangiosis (EMS), and bone marrow mesenchymal stem cells (BMSCs) were transplanted into the temporal muscle near the cerebral cortex. On the 30th day after EMS, the Morris water maze, immunofluorescence, laser speckle imaging, and light sheet microscopy were performed to evaluate angiogenesis; In addition, rats with bilateral common carotid artery occlusion were also followed by EMS surgery, and BMSCs from GFP reporter rats were transplanted into the temporal muscle to observe the survival time of BMSCs. Then, the concentrated BMSC-derived conditioned medium (BMSC-CM) was used to stimulate HUVECs and BMECs for ki-67 immunocytochemistry, CCK-8, transwell and chick chorioallantoic membrane assays. Finally, the cortical tissue near the temporal muscle was extracted after EMS, and proteome profiler (angiogenesis array) as well as RT-qPCR of mRNA or miRNA was performed.
Results
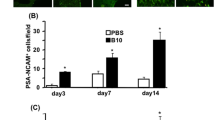
The results of the Morris water maze 30 days after BMSC transplantation in BCAS mice during the EMS operation, showed that the cognitive impairment in the BCAS + EMS + BMSC group was alleviated (P < 0.05). The results of immunofluorescence, laser speckle imaging, and light sheet microscopy showed that the number of blood vessels, blood flow and astrocytes increased in the BCAS + EMS + BMSC group (P < 0.05). The BMSCs of GFP reporter rats were applied to EMS and showed that the transplanted BMSCs could survive for up to 14 days. Then, the results of ki-67 immunocytochemistry, CCK-8 and transwell assays showed that the concentrated BMSC-CM could promote the proliferation and migration of HUVECs and BMECs (P < 0.05). Finally, the results of proteome profiler (angiogenesis array) in the cerebral cortex showed that the several pro-angiogenesis factors (such as MMP-3, MMP-9, IGFBP-2 or IGFBP-3) were notably highly expressed in MSC transplantation group compared to others.
Conclusions
Local MSCs transplantation together with EMS surgery can promote angiogenesis and cognitive behavior in chronic brain ischemia mice. Our study illustrated that MSC local transplantation can be the potential therapeutical option for improving EMS treatment efficiency which might be translated into clinical application.
Similar content being viewed by others
Background
Moyamoya disease is a cerebrovascular disease with an unclear etiology that mainly occurs in East Asia. Its characteristic lesion is progressive stenosis at the end of the bilateral internal carotid arteries, the anterior cerebral arteries, and the beginning of the middle cerebral arteries, accompanied by abnormal vascular network formation at the skull base [1, 2]. There is still a lack of effective drug treatments for moyamoya disease, and vascular reconstruction surgery is the main treatment method. The main methods of surgery include direct bypass surgery and indirect bypass surgery [3]. Currently, most centers perform both direct and indirect bypass surgery in one surgery and combine the advantages of both, known as combined vascular reconstruction surgery [4, 5].
Encephalo-myo-synangiosis (EMS) is a widely used indirect bypass surgery for Moyamoya disease and is also a part of combined vascular reconstruction surgery [6,7,8]. The temporal muscle of the patient was dissected and covered on the cerebral hemispheric surface with insufficient perfusion in this surgery. The principle of the surgery is to induce neovascularization of the temporal muscle and supply blood to the brain to alleviate the degree of cerebral ischemia [9]. However, some of the cohort studies showed that a certain proportion of the patients who underwent EMS surgery failed to achieve the expected results. Failure to achieve the expected results of EMS surgery is potentially due to effective collateral circulation between the temporal muscle and the cerebral hemisphere not being well established [10, 11]. Therefore, promoting the collateral circulation of EMS will largely improve the outcome for these patients.
Mesenchymal stem cells (MSCs) are multipotent cells that can differentiate into various cell types, including endothelial cells, chondrocytes, myocytes, adipocytes, and even glial cells [12, 13]. Furthermore, an increasing number of studies have suggested that MSCs exhibit an active treatment effect against various disorders, including inflammation, neuron degeneration diseases, cancers, ischemia diseases, etc. [14,15,16,17]. Studying the underlying mechanisms of this omnipotent therapeutic effect has become a hot topic. Some research has shown that MSCs may release many cytokine factors and noncoding RNAs, such as miRNAs and lncRNAs, which can effectively promote angiogenesis [18, 38]. These studies suggest that MSC treatment alone results in significant treatment efficacy in animal models. Moreover, several clinical trials around the world have demonstrated the safety of MSC transplantation in brain ischemia [39]. In recent decades, EMS surgery has become popular and has resulted in superior curative effects in treating chronic brain ischemia, such as Moyamoya disease and middle cerebral artery stenosis [40, 41]. However, there is a lack of studies evaluating the effect of MSC transplantation in combination with surgery. Our present study provides evidence that local MSC transplantation remarkably improved post-EMS neovascularization.
Transplantation of stem cells can be done using either freshly prepared cells or cryopreserved cells, but cryopreservation can in some cases adversely affect adult stem cell (and stem cell-containing) populations, which may lead to therapeutic failure [42], therefore, freshly prepared stem cells were used in our study for the experiments.
The mechanisms by which MSCs promote neovascularization comprise multiple signals [43,44,45]. Due to their differentiation potential, MSCs are able to directly differentiate into cells participating in neovascularization, including pericytes, endothelial cells, and smooth muscle cells [46], which have been verified in the treatment of a variety of diseases. Numerous circumstances, such as hypoxia or inflammation, initiate MSC differentiation [47]. In our current study, MSCs were unlikely to differentiate into these vascular cells but were likely to secrete functional factors that promote angiogenesis. According to the our array result, multiple pro-angiogenesis such as MMP-3, MMP-9, IGFBP-2 or IGFBP-3 were expressed at much higher level in the MSC transplantation group. Indeed, Matrix metalloproteinases (MMPs) are proteases that exerts pro-angiogenesis effect by degradation of the vascular basement membrane, participating in extracellular matrix remodeling and releasing of angiogenic mediators [48]. Also, numerous studies revealed that Insulin-like growth factor binding proteins (IGFBPs), such as IGFBP-2, exert angiogenic function in both IGF and IGFR1 dependent pathways [49]. Our findings suggested the potential angiogenic mechanisms of MSC transplantation, while more detail investigations are needed to identify the major effectors. Moreover, previous studies revealed that MSCs release numerous proangiogenic growth factors, such as VEGF and PDGF [50]. In addition, MSC exosomal noncoding RNAs, such as miRNAs or circRNAs, were also shown to regulate endothelial cell gene expression, which facilitates vascular generation [51, 52]. Therefore, we also evaluated the gene expression of known pro-angiogenesis factors. Indeed, MSC or MSC supernatant transplantation notably increases VEGF, and TGF expression. Despite the well-known VEGF and TGFβ, our observation revealed TGFα as the most predominant upregulated factor after BMSC-CM treatment, indicating its crucial effect on neovascularization. Transforming growth factor alpha (TGFα) belongs to the TGF superfamily, and its structure is more similar to that of the EGF family [53, 54].There are no overwhelming investigations on the pro-angiogenesis impact of TGFα, while several studies have demonstrated its important role in stem cell self-renewal and differentiation.
Current strategies for MSC treatment are predominantly cell transplantation, which comes from the umbilical cord, bone marrow, or adipose tissue [55, 56]. Ethical problems or unpredicted tumorigenesis of MSC transplantation hinder the clinical application of MSC treatment [57]. Our study provides evidence that the supernatant of MSCs exhibits a pro-angiogenesis effect equivalent to that of cell transplantation in EMS, which further avoids the malpractice of cell transplantation. Furthermore, implementation of the supernatant application is easy to accomplish during EMS by injecting it onto temporal muscle [58]. Further studies are needed to examine whether transdermal injection to the temporalis muscle after EMS is applicable.
In summary, our present study revealed improvement of neovascularization and cognitive recovery in chronic brain ischemia post-EMS by using MSC as well as supernatant treatment, which provides a novel combination of therapeutic methods for the clinical treatment of chronic brain ischemia.
In addition, a limitation in the study was the randomization of the experimental animals into sham-operated and "model" groups during experimental grou**, which did not make sense because it automatically prevented allocation concealment. Moreover, the model used in the study only mimics vascular cognitive impairment to a certain extent, and we will use a combined model of vascular cognitive impairment for more in-depth exploration in future studies [59, 60].
Conclusions
In conclusion, transplantation of MSCs during EMS can promote angiogenesis, and concentrated BMSC-CM can also achieve similar effects. Our study illustrated that MSC locally transplantation can be potential therapeutical options for improving EMS treatment efficiency which might be translated into clinical application.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- MSC:
-
Mesenchymal stem cells
- BCAS:
-
Bilateral carotid artery stenosis
- EMS:
-
Encephalo-myo-synangiosis
- BCCAO:
-
Bilateral common carotid artery occlusion
- CCH:
-
Chronic cerebral hypoperfusion
- CCA:
-
Common carotid arteries
References
Fujimura M, et al. 2021 Japanese guidelines for the management of moyamoya disease: guidelines from the research committee on moyamoya disease and Japan Stroke Society. Neurol Med Chir (Tokyo). 2022;62:165–70. https://doi.org/10.2176/jns-nmc.2021-0382.
Bao XY, Duan L. Chinese expert consensus on the treatment of MMD. Chin Neurosurg J. 2023;9:5. https://doi.org/10.1186/s41016-023-00318-3.
Guey S, Tournier-Lasserve E, Herve D, Kossorotoff M. Moyamoya disease and syndromes: from genetics to clinical management. Appl Clin Genet. 2015;8:49–68. https://doi.org/10.2147/TACG.S42772.
Araki Y, et al. Surgical designs of revascularization for moyamoya disease: 15 years of experience in a single center. World Neurosurg. 2020;139:e325–34. https://doi.org/10.1016/j.wneu.2020.03.217.
Moussouttas M, Rybinnik I. A critical appraisal of bypass surgery in moyamoya disease. Ther Adv Neurol Disord. 2020;13:1756286420921092. https://doi.org/10.1177/1756286420921092.
Kobayashi K, Takeuchi S, Tsuchida T, Ito J. Encephalo-myo-synangiosis (EMS) in moyamoya disease. Neurol Med Chir. 1981;21:1229–38.
Jang D-K, et al. Bypass surgery versus medical treatment for symptomatic moyamoya disease in adults. J Neurosurg. 2016;127:492–502. https://doi.org/10.3171/2016.8.JNS152875.
Takeuchi S, et al. Treatment of moyamoya disease by temporal muscle graft “encephalo-myo-synangiosis.” Childs Brain. 1983;10(1):1–15.
Kuroda S, Houkin K. Bypass surgery for moyamoya disease: concept and essence of surgical techniques. Neurol Medico Chir. 2012;52:287–94. https://doi.org/10.2176/nmc.52.287.
Sahoo SS, Suri A, Bansal S, Devarajan SLJ, Sharma BS. Outcome of revascularization in moyamoya disease: evaluation of a new angiographic scoring system. Asian J Neurosurg. 2015;10:252–9. https://doi.org/10.4103/1793-5482.162681.
Wang M, et al. preoperative collateral perfusion using arterial spin labeling: a predictor of surgical collaterals in moyamoya angiopathy. Front Neurosci. 2022;16:839485. https://doi.org/10.3389/fnins.2022.839485.
Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. https://doi.org/10.1126/science.284.5411.143.
Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. https://doi.org/10.1038/nature00870.
Gentile P, Garcovich S. Concise review: adipose-derived stem cells (ASCs) and adipocyte-secreted exosomal microRNA (A-SE-miR) modulate cancer growth and promote wound repair. J Clin Med. 2019;8:855. https://doi.org/10.3390/jcm8060855.
Van Nguyen T-T, Vu NB, Van Pham P. Mesenchymal stem cell transplantation for ischemic diseases: mechanisms and challenges. Tissue Eng Regen Med. 2021;18:587–611. https://doi.org/10.1007/s13770-021-00334-3.
Burnham AJ, Daley-Bauer LP, Horwitz EM. Mesenchymal stromal cells in hematopoietic cell transplantation. Blood Adv. 2020;4:5877–87. https://doi.org/10.1182/bloodadvances.2020002646.
Gentile P, Sterodimas A, Pizzicannella J, Calabrese C, Garcovich S. Research progress on mesenchymal stem cells (MSCs), adipose-derived mesenchymal stem cells (AD-MSCs), drugs, and vaccines in inhibiting COVI D-19 disease. Aging Dis. 2020;11:1191–201. https://doi.org/10.14336/AD.2020.0711.
Pankajakshan D, Agrawal DK. Mesenchymal stem cell paracrine factors in vascular repair and regeneration. J Biomed Technol Res. 2014. https://doi.org/10.19104/jbtr.2014.107.
**a X, et al. Growth hormone-releasing hormone and its analogues: significance for MSCs-mediated angiogenesis. Stem Cells Int. 2016. https://doi.org/10.1155/2016/8737589.
Zhu H, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–60. https://doi.org/10.1038/nprot.2009.238.
Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–603. https://doi.org/10.1161/01.STR.0000143725.19053.60.
Ni J, Ohta H, Matsumoto K, Watanabe H. Progressive cognitive impairment following chronic cerebral hypoperfus ion induced by permanent occlusion of bilateral carotid arteries in rats. Brain Res. 1994;653:231–6. https://doi.org/10.1016/0006-8993(94)90394-8.
Hayashi T, et al. Critical role of platelet-derived growth factor-α in angiogenesis after indirect bypass in a murine moyamoya disease model. J Neurosurg. 2020;134:1535–43. https://doi.org/10.3171/2020.3.JNS193273.
Brooks PC, et al. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1995;79:1157–64. https://doi.org/10.1016/0092-8674(94)90007-8.
Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–84. https://doi.org/10.1016/j.biocel.2003.11.001.
Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–92. https://doi.org/10.1634/stemcells.2006-0709.
Hu H, et al. Exosomes derived from bone marrow mesenchymal stem cells promote angiogenesis in ischemic stroke mice via upregulation of MiR-21-5p. Biomolecules. 2022;12:883. https://doi.org/10.3390/biom12070883.
Feng D, et al. BMSC-EV-derived lncRNA NORAD facilitates migration, invasion, and angiogenesis in Osteosarcoma cells by regulating CREBBP via delivery of miR-877–3p. Oxid Med Cell Longev. 2022. https://doi.org/10.1155/2022/8825784.
Lu G-D, Cheng P, Liu T, Wang Z. BMSC-derived exosomal miR-29a promotes angiogenesis and osteogenesis. Front Cell Dev Biol. 2020;8:608521. https://doi.org/10.3389/fcell.2020.608521.
Chu M, et al. Role of MiR-126a-3p in endothelial injury in endotoxic mice. Crit Care Med. 2016;44:e639–50. https://doi.org/10.1097/CCM.0000000000001629.
Wu X-G, et al. Cancer-derived exosomal miR-221-3p promotes angiogenesis by targeting THBS2 in cervical squamous cell carcinoma. Angiogenesis. 2019;22:397–410. https://doi.org/10.1007/s10456-019-09665-1.
Lv W, Li W-Y, Xu X-Y, Jiang H, Bang OY. Bone marrow mesenchymal stem cells transplantation promotes the release of endogenous erythropoietin after ischemic stroke. Neural Regen Res. 2015;10:1265–70. https://doi.org/10.4103/1673-5374.162759.
Wang J-W, et al. Transplantation with hypoxia-preconditioned mesenchymal stem cells sup presses brain injury caused by cardiac arrest-induced global cerebral ischemia in rats. J Neurosci Res. 2017;95:2059–70. https://doi.org/10.1002/jnr.24025.
Chen K-H, et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2017;7:74537–56. https://doi.org/10.18632/oncotarget.12902.
Lee J, et al. Efficacy of intravenous mesenchymal stem cells for motor recovery after ischemic stroke: a neuroimaging study. Stroke. 2022;53:20–8. https://doi.org/10.1161/STROKEAHA.121.034505.
Zhang Y, Yao H. Potential therapeutic mechanisms and tracking of transplanted stem cells: implications for stroke treatment. Stem Cells Int. 2017;2017:2707082. https://doi.org/10.1155/2017/2707082.
Pischiutta F, Sammali E, Parolini O, Carswell HVO, Zanier ER. Placenta-derived cells for acute brain injury. Cell Transpl. 2018;27:151–67. https://doi.org/10.1177/0963689717732992.
Savitz SI. Develo** cellular therapies for stroke. Stroke. 2018;46:2026–31. https://doi.org/10.1161/STROKEAHA.115.007149.
Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178. https://doi.org/10.1186/s12974-019-1571-8.
Wang K-C, Phi JH, Lee JY, Kim S-K, Cho B-K. Indirect revascularization surgery for moyamoya disease in children and its special considerations. Korean J Pediatr. 2012;55:408–13. https://doi.org/10.3345/kjp.2012.55.11.408.
Choi IJ, Cho SJ, Chang JC, Park SQ, Park HK. Angiographic results of indirect and combined bypass surgery for adult moyamoya disease. J Cerebrovasc Endovasc Neurosurg. 2012;14:216–22. https://doi.org/10.7461/jcen.2012.14.3.216.
Weise G, et al. Transplantation of cryopreserved human umbilical cord blood mononuclear cells does not induce sustained recovery after experimental stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2014;34:e1-9. https://doi.org/10.1038/jcbfm.2013.185.
Jayaraman S, Gnanasampanthapandian D, Rajasingh J, Palaniyandi K. Stem cell-derived exosomes potential therapeutic roles in cardiovascular diseases. Front Cardiovasc Med. 2021;8:723236. https://doi.org/10.3389/fcvm.2021.723236.
Kim R, et al. Regulation of alternative macrophage activation by MSCs derived hypoxic conditioned medium, via the TGF-β1/Smad3 pathway. BMB Rep. 2020;53:600–4. https://doi.org/10.5483/BMBRep.2020.53.11.177.
Yong KW, et al. Mesenchymal stem cell therapy for ischemic tissues. Stem Cells Int. 2018. https://doi.org/10.1155/2018/8179075.
Kéramidas M, et al. The dual effect of mesenchymal stem cells on tumour growth and tumour angiogenesis. Stem Cell Res Ther. 2013;4:41. https://doi.org/10.1186/scrt195.
Mao C, et al. Extracellular vesicles from anoxia preconditioned mesenchymal stem cells alleviate myocardial ischemia/reperfusion injury. Aging (Albany NY). 2021;13:6156–70. https://doi.org/10.18632/aging.202611.
Bajbouj K, Ramakrishnan RK, Hamid Q. Role of matrix metalloproteinases in angiogenesis and its implications in asthma. J Immunol Res. 2021;2021:6645072. https://doi.org/10.1155/2021/6645072.
Slater T, Haywood NJ, Matthews C, Cheema H, Wheatcroft SB. Insulin-like growth factor binding proteins and angiogenesis: from cancer to cardiovascular disease. Cytokine Growth Factor Rev. 2019;46:28–35. https://doi.org/10.1016/j.cytogfr.2019.03.005.
Hoang DH, et al. Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and xeno-free condition. Front Mol Biosci. 2020;7:119. https://doi.org/10.3389/fmolb.2020.00119.
Gurunathan S, Kang M-H, Jeyaraj M, Qasim M, Kim J-H. Review of the Isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307. https://doi.org/10.3390/cells8040307.
Hu G-W, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. https://doi.org/10.1186/scrt546.
Singh B, Coffey RJ. From wavy hair to naked proteins: the role of transforming growth factor alpha in health and disease. Semin Cell Dev Biol. 2014;28:12–21. https://doi.org/10.1016/j.semcdb.2014.03.003.
Dunn AR, et al. Insights into the physiology of TGF alpha and signaling through the EGF receptor revealed by gene targeting and acts of nature. In: Princess Takamatsu symptoms, vol 24. 1994. pp. 276–289.
Ren H, Zhang Q, Wang J, Pan R. Comparative effects of umbilical cord- and menstrual blood-derived MSCs in repairing acute lung injury. Stem Cells Int. 2018. https://doi.org/10.1155/2018/7873625.
Ding D-C, Shyu W-C, Lin S-Z. Mesenchymal stem cells. Cell Transpl. 2011;20:5–14. https://doi.org/10.3727/096368910X.
Jeong J-O, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarct ion and diabetic neuropathy. Circ Res. 2011;108:1340–7. https://doi.org/10.1161/CIRCRESAHA.110.239848.
Kusaka N, et al. Enhanced brain angiogenesis in chronic cerebral hypoperfusion after administration of plasmid human vascular endothelial growth factor in combination with indirect vasoreconstructive surgery. J Neurosurg. 2005;103:882–90. https://doi.org/10.3171/jns.2005.103.5.0882.
Hainsworth AH, et al. Translational models for vascular cognitive impairment: a review including larger species. BMC Med. 2017;15:16. https://doi.org/10.1186/s12916-017-0793-9.
Kaiser D, et al. Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: an animal model of early-stage cerebral small vessel disease. Acta Neuropathol Commun. 2014;2:169. https://doi.org/10.1186/s40478-014-0169-8.
Acknowledgements
We acknowledge Tongji Hospital Nephrology Department laboratory for gifting cells and we acknowledge support from the Tongji Hospital Neurosurgery Department staff.
Funding
The present study was supported by the Hebei Provincial Key Research Projects (grant no. 2022BCA027), Natural Science Foundation of Hubei province (grant no. 2021134), the Natural Science Foundation of Tongji Hospital (grant no. 2020JZKT651), and the Natural Science Foundation of China (grant no. 81801235). The funding body provided support in research design and quality control.
Author information
Authors and Affiliations
Contributions
HZ, TL, KS, and Sheng Wang designed and supervised the project; CXZ and YH are responsible for the extraction and identification of primary cells, and CXZ and YL are responsible for the in vivo experiments on mice and rats, CXZ and XH is responsible for the in vitro experiments, XM, CYL, and CG is responsible for the data processing, and YH and XZ is responsible for the analysis and statistics of data and the writing of articles. Only HZ and YH knew the grou** of the experiment, and the rest of the experimenters were informed of the grou** after data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental animals, cells and methods used in this experiment was prepared before the study and registered in the Ethics Committee and the animal experiments were complied with the "Animal Research: Reporting In Vivo Experiments" (ARRIVE) guidelines. Ethics approval was received from The Ethics Committee of Tongji hospital of Tongji medical college of Huazhong University of Science and Technology (title: Local transplantation of MSCs improves encephalo-myo-synangiosis-mediated collateral neovascularization in chronic brain ischemia, date: January 1, 2022, approval no. TJH-202207029).
Consent for publication
Not applicable.
Competing interests
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted. The author has no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
RT-qPCR results of angiogenic related mRNA (A) and miRNA (B) of cortex attached to the temporal muscle 30 days after EMS.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, X., Huang, Y., Liu, Y. et al. Local transplantation of mesenchymal stem cells improves encephalo-myo-synangiosis-mediated collateral neovascularization in chronic brain ischemia. Stem Cell Res Ther 14, 233 (2023). https://doi.org/10.1186/s13287-023-03465-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03465-7