Abstract
Background
The circadian clock is an evolutionarily conserved mechanism that exerts pervasive temporal control in stem cell behavior. This time-kee** machinery is required for orchestrating myogenic progenitor properties in regenerative myogenesis that ameliorates muscular dystrophy. Here we report a screening platform to discover circadian clock modulators that promote myogenesis and identify chlorhexidine (CHX) as a clock-activating molecule with pro-myogenic activities.
Methods
A high-throughput molecular docking pipeline was applied to identify compounds with a structural fit for a hydrophobic pocket within the key circadian transcription factor protein, Circadian Locomotor Output Cycles Kaput (CLOCK). These identified molecules were further screened for clock-modulatory activities and functional validations for pro-myogenic properties.
Results
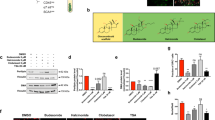
CHX was identified as a clock activator that promotes distinct aspects of myogenesis. CHX activated circadian clock that reduced cycling period length and augmented amplitude. This action was mediated by the targeted CLOCK structure via augmented interaction with heterodimer partner Bmal1, leading to enhanced CLOCK/Bmal1-controlled transcription with upregulation of core clock genes. Consistent with its clock-activating function, CHX displayed robust effects on stimulating myogenic differentiation in a clock-dependent manner. In addition, CHX augmented the proliferative and migratory activities of myoblasts.
Conclusion
Our findings demonstrate the feasibility of a screening platform to discover clock modulators with myogenic regulatory activities. Discovery of CHX as a pro-myogenic molecule could be applicable to promote regenerative capacities in ameliorating dystrophic or degenerative muscle diseases.
Similar content being viewed by others
Introduction
The circadian clock, driven by a transcriptional feed-back loop coupled with translational and posttranslational regulations, generates the ∼24-h rhythm in physiology and behavior [1, 2]. This evolutionarily conserved time-kee** mechanism imparts pervasive temporal control in diverse biological processes, including metabolic regulation, cell cycle and stem cell behavior [3,4,5,6,7,8]. Disruption of circadian clock regulation, increasingly prevalent in a modern lifestyle, leads to the development of metabolic disorders [9, 10], a myriad of cancers [11], and dysregulation of tissue remodeling [6,7,8]. Therapeutic targeting of circadian clock and its biological output pathways may have applications in metabolic disorders, cancer treatment and prevention, and dystrophic muscle diseases [6, 11,12,13,14,Plasmids and shRNA pcDNA3-BMAL1-His plasmid was purchased from Addgene (Addgene 31367). pcDNA3.0-6XMyc-Clock and pcDNA3.0-3XFLAG-Cry2 were generated by PCR amplification and sub-cloned into the pCDNA3.0–6XMyc or pCDNA3.0–3XFLAG vector separately. The primers used for PCR amplifications were: CLOCK-s: GCGGCCGCATGGTGTTTACCGTAAGC, CLOCK-as: CTCGAGTTCAGCCCTAACTTCTGCA; Cry2-s: GCGGCCGCATGGCGGCGGCTGCTGTGGT Cry2-as: CTCGAGTCAGGAGTCCTTGCTTGCT. The cysteine 267 mutation of CLOCK gene was generated using site-directed mutagenesis. The primer sequences used to generate this mutation were: CLOCK-C267A-s: TTCATCAAGGAAATGGCTACTGTTGAA, CLOCK-C267A-as: GCCATTTCCTTGATGAACTGAGGTG. The short hairpin RNAs of CLOCK used to generate stable knockdown U2OS cells were purchased from Sigma-Aldrich (TR0000018976) and cloned into the PLKO.1 puro vector. Stable clones of U2OS cells containing control or shCLOCK were generated by lentiviral transduction and stable selection. HEK293A were transfected with lentiviral packaging plasmids (psPAX2 and pMD2.G) and lentivirus vectors PLKO.1 or PLKO.1-shCLOCK using PEI Max. At Forty-eight hours after transfection, lentiviruses were collected through a 0.45-µm filter to remove cell debris. U2OS cells were then infected using the lentiviral medium supplemented with polybrene (Santa Cruz, SC-134220). Stable cell colonies were selected in the presence of 1 μg/ml puromycin at 24 h following lentiviral infection, Luciferase assays were performed as described [23, 24, 38, 58, 59]. So far, the identified Cry modulators stabilize the protein and augment its clock repressor function by inhibiting its proteosome-mediated degradation [14, 25, 60]. To date, clock-modulating compounds that directly target CLOCK and Bmal1, the key transcription activators that drive core clock transcription feed-back loop have yet to be identified. This study is the first to report a CLOCK protein-targeting molecule, CHX, with activity in promoting myogenic differentiation. We provided experimental evidence for the target specificity of CHX, with loss of its clock-modulatory activity in clock mutant and its pro-myogenic effect in clock-deficient myoblasts. Mutating CLOCK protein cysteine 267 abrogated CHX-induced CLOCK/Bmal1 association, demonstrating the targeting specificity of the compound. Future experiments are needed to determine the binding affinity and specificity of CHX with CLOCK. Nonetheless, by testing CHX activity using CLOCK mutant protein, Bmal1-deficient myoblasts and CLOCK knockdown U2OS cells, current findings indicate that CHX activity in stimulating clock gene induction, myogenic differentiation and proliferation requires a functional clock. CHX is a cationic bisbiguanide molecule capable of binding to the bacterial cell wall with broad bacteriostatic or bactericidal activity [61]. It is used as a topical skin antiseptic agent and in mouth wash due to its anti-plaque and anti-gingivitis activity [62, 63]. CHX binds to proteins on skin and mucous membranes with limited systemic absorption. Potentially due to its chemical properties, we found that CHX, at concentrations higher than 1–2 µM, exhibited toxicity, although distinct cell types showed variable sensitivity to CHX concentration and duration of treatment. Dose response analysis of bioluminescence activity using Per2-Luc U2OS cells revealed a broader nontoxic concentration range (0.2–2 μM) than functional assays of pro-myogenic activity in C2C12 myoblasts (0.2–0.5 µM). We confined experiments within the lower concentration range to avoid adverse effects. This contrasts with other studies for clock modulator screening that typically used 0.5–10 μM [50] or investigation of biological functions of clock-targeting compounds [24]. Nonetheless, CHX could have clock-independent off-target effects of CHX, particularly as related to pro-myogenic activities in myoblasts. Despite its significant pro-myogenic action, the observed clock-modulatory effects of CHX on period lengthening were relatively moderate. This could be due to its mechanism of action by promoting CLOCK interaction with Bmal1. As our results revealed that CHX augmented the affinity of these interacting partners, there could be a limit posed by the binding kinetics of these proteins in vivo, and thus, not as efficacious as inhibitors that disrupt endogenous interaction. In line with this, the clock-inhibitory molecules we discovered from this screen were more potent in inhibiting Bmal1/CLOCK interaction and altering period length (unpublished data). These observations highlight the need for optimization of CHX chemical structure in order to develop new efficacious clock-activating molecules with pro-myogenic activities, while minimizing toxicity and off-target effects. Optimization of this lead compound may lead to development of novel agents for dystrophic muscle disease or metabolic disease applications. Current pharmacological modulators of circadian clock components are directed toward metabolic diseases and cancer [14, 19]. Epidemiological investigations and experimental studies have provided compelling evidence that disruption of clock regulation leads to the development of obesity and insulin resistance [64]. We reported that loss of Bmal1 resulted in obesity [37], and chronic shiftwork induced adipose tissue expansion with inflammation [ In summary, our study is the first report to demonstrate the feasibility of a screening pipeline for discovering circadian clock modulators by targeting the CLOCK protein that has myogenic regulatory properties. The discovery of CHX as a pro-myogenic molecule targeting the clock modulation may have therapeutic applications in muscular dystrophy or related muscle remodeling processes involving muscle stem cells.Generation of stable cell lines
Transient transfection luciferase assay
Conclusions
Availability of data and materials
All data generated and analyzed during this study are included in this published article and associated Supplementary Information files.
Abbreviations
- CLOCK:
-
Circadian Locomotor Output Cycles Kaput
- Bmal1:
-
Brain and Muscle Arnt-like Protein 1
- PAS:
-
Per-Arnt-Sim
- ROR:
-
RAR-related Orphan Receptor
- CHX:
-
Chlorhexidine
- MyHC:
-
Myosin heavy chain
References
Partch CL, Green CB, Takahashi JS. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014;24(2):90–9.
Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164–79.
Lamia KA. Ticking time bombs: connections between circadian clocks and cancer. F1000Research. 2017;6:1910.
Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008–15.
Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307–20.
Chatterjee S, Ma K. Circadian clock regulation of skeletal muscle growth and repair. F1000Research. 2016;5:1549.
Benitah SA, Welz PS. Circadian regulation of adult stem cell homeostasis and aging. Cell Stem Cell. 2020;26(6):817–31.
Janich P, Pascual G, Merlos-Suarez A, Batlle E, Ripperger J, Albrecht U, et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature. 2011;480(7376):209–14.
Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15(2):75–89.
Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–54.
Sulli G, Lam MTY, Panda S. Interplay between circadian clock and cancer: new frontiers for cancer treatment. Trends Cancer. 2019;5(8):475–94.
He B, Chen Z. Molecular targets for small-molecule modulators of circadian clocks. Curr Drug Metab. 2016;17(5):503–12.
Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z, et al. Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov. 2019;9(11):1556–73.
Lee JW, Hirota T, Kumar A, Kim NJ, Irle S, Kay SA. Development of small-molecule cryptochrome stabilizer derivatives as modulators of the circadian clock. ChemMedChem. 2015;10(9):1489–97.
Gao H, **ong X, Lin Y, Chatterjee S, Ma K. The clock regulator Bmal1 protects against muscular dystrophy. Exp Cell Res. 2020;397(1): 112348.
**ong X, Gao H, Lin Y, Yechoor V, Ma K. Inhibition of Rev-erbalpha ameliorates muscular dystrophy. Exp Cell Res. 2021;406(2): 112766.
Gloston GF, Yoo SH, Chen ZJ. Clock-enhancing small molecules and potential applications in chronic diseases and aging. Front Neurol. 2017;8:100.
Sulli G, Manoogian ENC, Taub PR, Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci. 2018;39(9):812–27.
Battaglin F, Chan P, Pan Y, Soni S, Qu M, Spiller ER, et al. Clocking cancer: the circadian clock as a target in cancer therapy. Oncogene. 2021;40(18):3187–200.
Huang N, Chelliah Y, Shan Y, Taylor CA, Yoo SH, Partch C, et al. Crystal structure of the heterodimeric CLOCK:BMAL1 transcriptional activator complex. Science. 2012;337(6091):189–94.
Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251–60.
Stratmann M, Schibler U. REV-ERBs: more than the sum of the individual parts. Cell Metab. 2012;15(6):791–3.
Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature. 2018;553(7688):351–5.
Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485(7396):62–8.
Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, et al. Identification of small molecule activators of cryptochrome. Science. 2012;337(6098):1094–7.
Janich P, Toufighi K, Solanas G, Luis NM, Minkwitz S, Serrano L, et al. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013;13(6):745–53.
Solanas G, Peixoto FO, Perdiguero E, Jardi M, Ruiz-Bonilla V, Datta D, et al. Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell. 2017;170(4):678–92.
Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, et al. CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proc Natl Acad Sci USA. 2010;107(44):19090–5.
Casanova-Vallve N, Duglan D, Vaughan ME, Pariollaud M, Handzlik MK, Fan W, et al. Daily running enhances molecular and physiological circadian rhythms in skeletal muscle. Mol Metab. 2022;61: 101504.
Yin H, Li W, Chatterjee S, **ong X, Saha P, Yechoor V, et al. Metabolic-sensing of the skeletal muscle clock coordinates fuel oxidation. FASEB J. 2020;34(5):6613–27.
Sato S, Basse AL, Schonke M, Chen S, Samad M, Altintas A, et al. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab. 2019;30(1):92–110.
Chatterjee S, Nam D, Guo B, Kim JM, Winnier GE, Lee J, et al. Brain and muscle Arnt-like 1 is a key regulator of myogenesis. J Cell Sci. 2013;126(Pt 10):2213–24.
Chatterjee S, Yin H, Li W, Lee J, Yechoor VK, Ma K. The nuclear receptor and clock repressor Rev-erbalpha suppresses myogenesis. Sci Rep. 2019;9(1):4585.
Katoku-Kikyo N, Paatela E, Houtz DL, Lee B, Munson D, Wang X, et al. Per1/Per2-Igf2 axis-mediated circadian regulation of myogenic differentiation. J Cell Biol. 2021;220(7):e202101057.
Lowe M, Lage J, Paatela E, Munson D, Hostager R, Yuan C, et al. Cry2 is critical for circadian regulation of myogenic differentiation by Bclaf1-mediated mRNA stabilization of cyclin D1 and Tmem176b. Cell Rep. 2018;22(8):2118–32.
Chatterjee S, Yin H, Nam D, Li Y, Ma K. Brain and muscle Arnt-like 1 promotes skeletal muscle regeneration through satellite cell expansion. Exp Cell Res. 2015;331(1):200–10.
Guo B, Chatterjee S, Li L, Kim JM, Lee J, Yechoor VK, et al. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012;26(8):3453–63.
Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov. 2014;13(3):197–216.
He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016;23(4):610–21.
Nohara K, Mallampalli V, Nemkov T, Wirianto M, Yang J, Ye Y, et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat Commun. 2019;10(1):3923.
Liu W, Zhou M, Li Z, Li H, Polaczek P, Dai H, et al. A selective small molecule DNA2 inhibitor for sensitization of human cancer cells to chemotherapy. EBioMedicine. 2016;6:73–86.
Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38(1):79–96.
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, et al. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem. 2004;47(7):1750–9.
Lipinski CA. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov Today Technol. 2004;1(4):337–41.
Baell JB, Holloway GA. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53(7):2719–40.
Huth JR, Mendoza R, Olejniczak ET, Johnson RW, Cothron DA, Liu Y, et al. ALARM NMR: a rapid and robust experimental method to detect reactive false positives in biochemical screens. J Am Chem Soc. 2005;127(1):217–24.
Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008;4(2): e1000023.
Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101(15):5339–46.
Ramanathan C, Kathale ND, Liu D, Lee C, Freeman DA, Hogenesch JB, et al. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018;14(5): e1007369.
Chen Z, Yoo SH, Park YS, Kim KH, Wei S, Buhr E, et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci USA. 2012;109(1):101–6.
Ma K, **ao R, Tseng HT, Shan L, Fu L, Moore DD. Circadian dysregulation disrupts bile acid homeostasis. PLoS ONE. 2009;4(8): e6843.
Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, et al. A noncanonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci USA. 2005;102(7):2608–13.
Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84(1):209–38.
Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14(2):82–91.
Rudnicki MA, Le Grand F, McKinnell I, Kuang S. The molecular regulation of muscle stem cell function. Cold Spring Harb Symp Quant Biol. 2008;73:323–31.
Fu L, Kettner NM. The circadian clock in cancer development and therapy. Prog Mol Biol Transl Sci. 2013;119:221–82.
Sancar A, Van Gelder RN. Clocks, cancer, and chronochemotherapy. Science. 2021;371(6524):eabb0738.
Kojetin D, Wang Y, Kamenecka TM, Burris TP. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem Biol. 2011;6(2):131–4.
Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, et al. Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472(7344):491–4.
Miller S, Son YL, Aikawa Y, Makino E, Nagai Y, Srivastava A, et al. Isoform-selective regulation of mammalian cryptochromes. Nat Chem Biol. 2020;16(6):676–85.
Lim KS, Kam PC. Chlorhexidine–pharmacology and clinical applications. Anaesth Intensive Care. 2008;36(4):502–12.
Arduino PG, Gambino A, Cabras M, Sciannameo V, Nimot Y, Karimi D, et al. Effect of two different alcohol-free chlorhexidine formulations in mouthrinses on the immediate postoperative period for oral mucosal biopsies. J Oral Sci. 2020;62(2):202–5.
Ribeiro LG, Hashizume LN, Maltz M. The effect of different formulations of chlorhexidine in reducing levels of mutans streptococci in the oral cavity: a systematic review of the literature. J Dent. 2007;35(5):359–70.
Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015;277(5):513–27.
**ong X, Lin Y, Lee J, Paul A, Yechoor V, Figueiro M, et al. Chronic circadian shift leads to adipose tissue inflammation and fibrosis. Mol Cell Endocrinol. 2021;521: 111110.
Chatterjee S, Yin H, Nam D, Li Y, Ma K. Brain and muscle Arnt-like 1 promotes skeletal muscle regeneration through satellite cell expansion. Exp Cell Res. 2015;331(1):200–10.
Welch RD, Billon C, Valfort AC, Burris TP, Flaveny CA. Pharmacological inhibition of REV-ERB stimulates differentiation, inhibits turnover and reduces fibrosis in dystrophic muscle. Sci Rep. 2017;7(1):17142.
Acknowledgements
We thank Drs. Steve Kay and Meng Qu at the University of Southern California for sharing the luciferase reporter cell lines used in this study, and Drs. Seung-Hee Yoo and Zheng Chen at University of Texas at Houston Health Science Center for providing the Per2-luciferase plasmid. We appreciate the careful editing of the manuscript by Dr. Jeffrey S Isenberg Art Riggs Diabetes and Metabolism Research Institute of City of Hope.
Funding
KM is a faculty member supported by the NCI-designated Comprehensive Cancer Center at the City of Hope National Cancer Center. This project was supported by a grant from National Institute of Health R01DK112794, a Shared Resources Pilot Project and a Type II Diabetes Innovation Award from Arthur Riggs Diabetes and Metabolism Research Institute to KM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
TK, WL and XX: data curation and investigation, formal analysis, manuscript editing; HL and DH: data curation and investigation; KM: conceptualization, formal analysis, project administration, manuscript writing and editing, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that no competing interests exist that is relevant to the subject matter or materials included in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Supplemental Figures S1–S4, Raw Data Figures S5–S8, and Supplemental Tables 1–4.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kiperman, T., Li, W., **ong, X. et al. Targeted screening and identification of chlorhexidine as a pro-myogenic circadian clock activator. Stem Cell Res Ther 14, 190 (2023). https://doi.org/10.1186/s13287-023-03424-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-023-03424-2