Abstract
Heterotopic ossification (HO) is the formation of bone in non-osseous tissues, such as skeletal muscles. The HO could have a genetic or a non-genetic (acquired) background, that is, it could be caused by musculoskeletal trauma, such as burns, fractures, joint arthroplasty (traumatic HO), or cerebral or spinal insult (neurogenetic HO). HO formation is caused by the differentiation of stem or progenitor cells induced by local or systemic imbalances. The main factors described so far in HO induction are TGFβ1, BMPs, activin A, oncostatin M, substance P, neurotrophin-3, and WNT. In addition, dysregulation of noncoding RNAs, such as microRNA or long noncoding RNA, homeostasis may play an important role in the development of HO. For example, decreased expression of miRNA-630, which is responsible for the endothelial–mesenchymal transition, was observed in HO patients. The reduced level of miRNA-421 in patients with humeral fracture was shown to be associated with overexpression of BMP2 and a higher rate of HO occurrence. Down-regulation of miRNA-203 increased the expression of runt-related transcription factor 2 (RUNX2), a crucial regulator of osteoblast differentiation. Thus, understanding the various functions of noncoding RNAs can reveal potential targets for the prevention or treatment of HO.
Similar content being viewed by others
Introduction
Heterotopic ossification: a brief overview
Heterotopic ossification (HO) is a dysregulation of skeletal muscle homeostasis and regeneration that leads to the formation of mature bone in unusual locations. HO develops in skeletal muscles and surrounding tissues, such as fascia, tendons, skin, and subcutis. Most lesions are small and clinically irrelevant, but extensive HO can limit patient physical functioning and quality of life [1]. The clinical manifestations vary depending on the stage of HO development. Typical clinical symptoms in the early phases are such as localized pain, tenderness, and swelling. At later stages, a limited range of motion (ROM) may affect the joint, resulting in complete ankylosis in the most severe cases [2].
Acquired heterotopic ossification
HO formation may occur due to fractures, extensive soft tissue damage, burns, amputations, and combat-related injuries. It can also be triggered by iatrogenic trauma associated with the surgical approach. Up to 90% of acetabulum fractures and 8.6% of distal humerus fractures subjected to surgical treatment result in the formation of HO [3]. It can also occur after arthroscopic procedures and after hip arthroscopy was detected in up to 46% of cases [4]. Approximately 29.9% of patients with total hip arthroplasty (THA) developed HO, but most cases were asymptomatic. However, large lesions occurring in 0.57–2.7% of patients can significantly limit surgery benefits [5]. Extensive HO can influence patient life quality assessed by patient-reported outcome measures (PROMs). It is worth mentioning that mature HO rarely causes pain. However, they substantially reduce the ROM in the affected joint [6]. Patients who were subjected to total hip arthroplasty (THA) with extensive HO lesions (Brooker III, IV) do not benefit from surgery in the ROM aspect, as compared to the preoperative status [7]. HO has been reported to significantly limit joint ROM after revision knee arthroplasty [8], burns [9], and elbow fractures [10]. However, even severe ROM-limiting HOs usually do not cause pain [11].
Another example of acquired HO is neurogenic heterotopic ossification (NHO). The NHO may form around the hip, knee, elbow, and glenohumeral joint due to the response to neuroinflammation signals and systemic changes caused by central nervous system (CNS) injury. Joint mobility problems resulting from NHO often cause nursing challenges [12]. The presence of NHO is associated with a poor functional outcome in patients after CNS injury [13]. In such cases, restricted ROM is the major problem, as pain is often absent due to sensory deficits [14]
Recently, HO has been reported in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection who require mechanical ventilation. The mechanism of HO in COVID-19 patients is unclear. However, prolonged immobilization, global inflammation, and cytokine storm are likely to be the triggering factors [15, 16].
Zhang et al. described another subtype of HO—heterotopic ossification of the tendon and ligament (HOTL). HOTL includes ossification of the posterior longitudinal ligament of the spine (OPLL) and calcific tendinitis [17]. However, usually OPLL and calcific tendinitis are not mentioned in reviews focusing on HO [1, 18, 19] but are described separately [20, 21]. OPLL can result in radiculopathy or myelopathy that causes spasticity and gait disturbances [17]. It is the most common in the cervical spine. OPLL affects approximately 0.1–4.3% of the world population, with a high prevalence in Asian populations [22]. Unlike other types of acquired HO, it does not result from trauma. The risk factors for OPLL are both genetic, e.g., COL11A2 [23] and COL6A1 polymorphisms [24], and environmental, i.e., high-sodium diet [25], obesity [26], and nonalcoholic fatty liver disease [27]. The role of ncRNAs in OPLL is broadly described in a review by Yuan et al. [28]. The calcific tendinitis occurs when repetitive microtrauma acts on the tendons, e.g., in athletes or manual workers. It is common in the rotator cuff tendons [29]. The lesions resemble incomplete ossification and do not contain mature bone but amorphous calcium deposits [30], so they should not be considered a HO subtype.
Genetic heterotopic ossification
Heterotopic ossification may be the most notable clinical characteristic of three genetic diseases: fibrodysplasia ossificans progressiva (FOP), progressive osseous heteroplasia (POH), and hereditary Albright osteodystrophy (AHO). Genetic HOs are very severe but rare conditions and belong to the so-called orphan disease family. Osteogenesis induction in FOP is caused by a mutation of the activin A receptor type 1/activin receptor-like kinase 2 gene (ACVR1/ALK2; in most cases R206H), which encodes the bone morphogenetic protein (BMP) receptor, type I. POH and AHO are caused by mutations in the GNAS1 gene, influencing Wingless-related integration site (WNT) and Hedgehog signaling (HH), the key controllers of skeletal maturation and regeneration [31,32,33]. The prevalence of FOP ranges from 0.4 to 65 cases per 10,000 [34] and the prevalence of AHO is 7.2 cases per million [35]. The epidemiology of POH remains unknown, as less than 60 cases have been reported, so far [36]. Among ossifications of genetic origin, the most severe are those occurring in patients suffering from FOP. FOP is characterized by periodic exacerbations with localized painful soft tissue inflammation that leads to the development of HO in muscles, joints, tendons, and ligaments. Over time, it leads to severe joint limitations and loss of ROM. Most FOP patients are wheelchair-bound in the third decade of life [37]; in addition, they report emotional problems, such as anxiety, depression, or irritability. The severity of pain associated with FOP significantly influences emotional health and overall quality of life [38].
Possible mechanism of heterotopic ossification
Although many research projects have been devoted to the description of HO, including histological descriptions of the lesions, their progression, and risk factors, the exact molecular processes remain unknown [39]. Patients with HO are most likely to have global changes at the multi-omics level [40], including genetic [41,42,43], epigenetic [44], transcriptomic, proteomic [45, 46], and metabolic processes [47]. In particular, both genetic HO and acquired HO have already been represented in animal models. The existence of an animal model that satisfies human disease conditions is essential for a detailed explanation of the molecular and cellular mechanisms responsible for disease progression and the preclinical evaluation of potential promising therapeutic tools [31, 39].
Many lines of evidence indicate that the development of HO in skeletal muscle may be the result of pathological differentiation of stem or progenitor cells present in skeletal muscle [39]. However, the identity of these cells is not yet clear. Animal studies suggest that progenitor cells responsible for pathological osteogenesis may differ depending on the HO subtype. Research involving mouse models indicates that endothelial cells, mesenchymal cells, pericytes, tendon, and other connective tissue cells, or circulating stem cells may be the source of HO precursors [48, 49]. In both acquired and genetic HO, the differentiation of precursor cells is initiated by inflammatory cells, including lymphocytes, macrophages, and mast cells [50, 51]. It is accompanied by the release of cytokines and growth factors by immune cells including interleukin 1β (IL-1β), interleukin 6 (IL-6), oncostatin M (OSM), neurotrophin-3 (NT-3), activin A, BMP, transforming growth factor β (TGFβ), and substance P (SP) [50,51,52]. Differentiation of precursor cells leading to the formation of HO is complemented by increased translation of proteomic biomarkers of HO, such as alkaline phosphatase (ALP), osteocalcin (OCN), osteopontin (OPN/SSP1), and bone sialoprotein (BSP) [45].
The HO can form as any other bone through endochondral or intramembranous ossification. Both processes occur during mammalian skeletal development and bone remodeling during fracture healing [53]. Endochondral ossification occurs in cartilage models of long bones when hypertrophic chondrocytes produce an extracellular matrix that is mineralized in the ossification centers [54]. HO develops through endochondral osteogenesis, which is preceded by infiltration and migration of lymphocytes, fibroproliferation, neovascularity, and cartilage formation [55]. Similarly, in FOP the HO develops with endochondral ossification. Impaired osteochondrogenesis in FOP results not only in extraskeletal bone formation, but also in growth plate dysplasia, early osteoarthritis, and joint deformation [56]. The flare-ups in FOP are accompanied by an elevation of serum cartilage-derived retinoic acid-sensitive protein (CD-RAP), which is a biomarker of chondrogenesis [57]. Opposing to FOP [58] POH ossifications are formed by an intramembranous process in which mesenchymal cells differentiate directly into osteoblasts and form ossification centers [59]. During embryogenesis Intramembranous ossification contributes to the development of the skull, mandible, and middle part of the clavicle [60], and is also responsible for pathological bony bridge formation in growth plate injuries [104]. It is currently being investigated in phase 2 in adult FOP patients [105]. Palovarotene, a retinoic acid receptor gamma (RARγ) agonist, previously used in patients with emphysema [106], can inhibit the chondrogenesis phase in HO formation. Palovarotene influences BMP/SMAD-dependent pathway [107] and NF-κB signaling [108] preventing HO formation via endochondral ossification. In a trauma-induced HO rat model. Palovarotene inhibited HO formation and caused down-regulation of chondrogenesis biomarkers, i.e., SRY-box transcription factor 9 (SOX9) and osteogenesis biomarkers (OCN, RUNX2) [109]. It was already tested in adult FOP patients, and a phase 3 clinical trial phase 3 is ongoing (MOVE) [110]. The ongoing clinical trials of FOP drugs are summarized in Table 1.
Most studies focus on the prevention of HO, and when mature lesions have already formed, the only treatment method is surgical excision [70]. An innovative approach uses osteoclasts, cells responsible for bone remodeling, against mature HO. Osteoclasts were modified with tetracycline, which has a high affinity for bone hydroxyapatite. Artificially engineered osteoclasts with a high affinity for calcified bone capable of HO resorption were successful in treating already formed lesions in tenotomy, intramuscular, and genetic HO mouse models [111].
In recent years, great progress has been made in genetic HO treatment [32, 88, 101]. Recently (January 2022), Health Canada approved palovarotene (Sohonos) as the first drug for Fibrodysplasia Ossificans Progressiva (FOP) [112]. For acquired HO, NSAIDs and RT are still the methods of choice [69]. Emerging evidence indicates the importance of noncoding RNA in the pathogenesis of HO. It suggests that a clinically relevant noncoding RNA signature may be detected in patients with certain risk factors and could be used to predict or prevent HO [113].
Advances in ncRNA therapy
Noncoding RNAs (ncRNAs) do not encode proteins but play an important role in many biological processes, such as the regulation of gene expression, RNA processing, or protein synthesis. The group of ncRNAs includes, for example, ribosomal RNAs (rRNAs) and transfer RNAs (tRNAs) involved in protein translation, small nucleolar RNAs (snoRNAs) regulating rRNA biogenesis, and small nuclear RNAs (snRNAs) participating in mRNA splicing (broadly reviewed in [117, 118]). Recently, the role of ncRNAs, such as small interference RNA (siRNA), has been intensively investigated in the context of diseases related to the musculoskeletal system [119]. Moreover, ncRNAs have the potential to be applied in the therapy [120]. Here, we focus on two groups of ncRNAs, i.e., microRNAs (miRNAs) and long noncoding RNAs (lncRNAs), which were described to be involved in the formation and progression of HO.
microRNA (miRNA)
MicroRNAs are short RNA molecules that consist of approximately 18–30 nucleotides and play an important role in posttranscriptional RNA silencing. Mature miRNA binds to the 3’untranslated region (3’UTR) of its target mRNA, which leads to destabilization or degradation of the mRNA. Regardless of the mechanism of action, the effect of miRNA activity is to prevent protein synthesis coded by target mRNA. Although most of the data underline the inhibitory properties of miRNAs, it should be mentioned that some studies showed that miRNA activity can also lead to up-regulation of gene expression [121,122,123,124,125,126,127]. This activation involves argonaute RISC catalytic component 2 gene (AGO2) and fragile X mental retardation-related protein 1 gene (FXR1) instead of GW182 and was observed in quiescent (G0) somatic cells and frog oocytes [128, 129]. miR-10a can serve as an example of miRNA-dependent up-regulation of gene expression. Its binding to 5’UTR increased the translation of mRNAs encoding ribosomal proteins during amino acid starvation of mouse embryonic stem cells [130].
The biogenesis of miRNA is quite complex and involves many cellular mechanisms (Fig. 1.). The primary miRNA transcript (pri-miRNA) is a long molecule with a characteristic local hairpin structure within which a mature miRNA sequence is present. Pri-miRNA is cleaved by the endonuclease DROSHA, which, together with the RNA binding protein Di George syndrome critical-related gene 8 (DGCR8), form the microprocessor complex [131,132,133,134]. Crop** of the pri-miRNA leads to the formation of pre-miRNA. However, not all miRNA biogenesis involves the Microprocessor complex. Some miRNAs are encoded in so-called mirtrons present in pre-mRNA introns [135,136,137,138]. Thus, these pre-miRNAs are generated during pre-mRNA splicing. Furthermore, pre-miRNAs are exported from the nucleus to the cytoplasm by exportin 5 (XPO5)/RanGTP complex [139,140,141]. In the cytoplasm, pre-miRNA is further processed by other endonuclease DICER, which results in the formation of a miRNA duplex [142,143,144]. Although both miRNA strands can be functional, during biogenesis one of them is degraded and the other forms an RNA-induced silencing complex (RISC) together with AGO and GW182 proteins [145,146,147,148,149]. After forming the RISC complex, some specific regions of the miRNA structure can be distinguished. Among them, the most important is the “seed” domain, which is directly responsible for recognizing target mRNA. Binding of RISC to mRNA results in mRNA destabilization, mRNA degradation, or inhibition of translation [150]. Furthermore, multiple studies have reported that miRNAs not only act within the cell cytoplasm but also are released into extracellular fluids. These extracellular miRNAs have the potential to be used as biomarkers for diverse conditions [151,152,153]. Extracellular miRNAs can be delivered to target cells and have the potential to act as autocrine, paracrine, and/or endocrine regulators that modulate cellular activity.
miRNA biogenesis and mechanism of action. miRNA is transcribed by POL II, leading to the generation of often polycistronic pri-miRNA. Pri-miRNA is cleaved by the microprocessor complex which consists of DROSHA and DGCR8 and results in the release of small hairpin-shaped RNA, called pre-miRNA. The pre-miRNA is exported to the cytoplasm by the XPO5/RanGTP complex and cleaved by DICER. DICER cleavage results in the release of a miRNA duplex. One of the miRNA strands becomes degraded, and the other forms a RISC complex with proteins from the AGO and GW182 families. Mature miRNA acts in RISC, and its activity leads to mRNA degradation, mRNA destabilization by deadenylation and decap**, or inhibition of translation. The figure was created for this article; it is not based on any previously published image
Long noncoding RNA (lncRNA)
Long noncoding RNAs are a very heterogeneous group of ncRNAs, generated via pathways similar to mRNAs and characterized by a minimum size of 200 nucleotides (Fig. 2). In fact, the length of the molecule is the only common feature of all lncRNAs. These transcripts play diverse roles within the cell, acting in both the nucleus and the cytoplasm. However, three main mechanisms of lncRNA activity can be distinguished [117, 118]. In the nucleus, lncRNAs were shown to be involved in the regulation of chromosome structure and mediating chromatin remodeling by recruiting histone-modifying complexes such as histone acetylases or deacetylases (HDACs) (e.g., [154,155,156]; reviewed in [157]). Furthermore, lncRNAs can directly affect gene expression by binding to enhancer sites or transcription factors [158,159,160]. lncRNAs acting in the cytoplasm are responsible for posttranscriptional regulation of gene expression by modulating the accessibility or stability of mRNA [117, 118]. Finally, cytoplasmic lncRNAs can directly bind to and inhibit miRNA-dependent gene silencing by functioning as a competing RNA [161, 162]. Therefore, lncRNAs appear to be important regulators of many processes, from the organization of chromosome and chromatin remodeling to the posttranscriptional regulation of gene expression in the cytoplasm.
Mechanisms of lncRNA action. Nuclear lncRNAs regulate gene expression by: A regulating chromatin remodeling; B recruiting chromatin-modifying complexes (such as HDACs); C regulating transcription factor activity. Furthermore, cytoplasmic lncRNAs impact mRNA processing through: D regulating mRNA stability; E regulating translation; F acting as competitors for miRNAs; and G protein function by binding proteins or peptides. The figure was created for this article; it is not based on any previously published image
miRNAs and lncRNAs as potential therapeutics
Noncoding RNAs represent very promising tools for the treatment of various diseases [163]. Eleven RNA-based therapeutics have already been approved by the FDA and/or the European Medicines Agency (EMA) [163]. These therapeutics are small interfering RNAs (siRNAs) or antisense oligonucleotides (ASO). They are used, for example, in the therapy of spinal muscular atrophy, Duchene muscular dystrophy, or homozygous familial hypercholesterolemia [163]. Moreover, numerous RNAs are clinically tested. Some of the RNA-based therapies (phase 2 or 3 clinical trials) involve miRNA. However, no lncRNA-based therapy has been available to the clinic, so far [163]. The function of miRNA-based therapeutics relies on restoring or depleting the miRNA or inhibiting their interactions with targets. Few types of miRNA-based molecules are currently being investigated as therapeutics. First, miRNA mimics that have the same sequence as endogenous miRNA and mimic their function. Currently, one molecule, i.e., miR29 mimic, is tested in clinical trials for the treatment of pathological skin fibrosis (NCT02603224, nct03601052). The second type of molecules are antagomiRs that are antisense to specific miRNAs and prevent their interactions with targets. Two such molecules are currently being investigated in clinical trials, these are anti-miR103/107 (NCT020612662 and NCT02826525) and anti-miR122 (NCT01646489, NCT01727934, NCT01872936, and NCT01200420). They are tested in the treatment of type 2 diabetes and hepatitis C virus infection, respectively. Many other miRNA- or lncRNA-based therapeutics are also studied, for example, in the treatment of resistance to cancer therapy [164] and other diseases [165].
miRNAs and lncRNAs as potential biomarkers
Several HO biomarkers have been proposed to date, but there is no consensus which of them should be used in clinical practice [166]. Physiological bone turnover indicators based on protein levels are not specific and can change due to other health conditions [167]. Thus, ncRNAs are a promising new group of biomarkers that can be easily isolated from all body fluids and were suggested to be specific markers in many other diseases [168,169,170,171,172]. However, before ncRNA could be widely used as a diagnostic tool, the problem of low expression levels, instability [172], and sequencing costs [208] had to be overcome. Once the mechanism of the complex ncRNA interactions will be uncovered, validation studies and the establishment of cutoff values are needed to enable the application of ncRNA as reliable HO biomarkers. In addition, their specificity as biomarkers should also be investigated in other diseases and reproducible measurement methods should be implemented.
The role of miRNA and lncRNA in heterotopic ossification
Dysregulation of the miRNA expression profile in HO
Recently, the role of miRNA and lncRNA has been intensively investigated to understand the background of diseases related to the musculoskeletal system [119] as well as to identify novel drug targets [120]. However, the knowledge about the role of these molecules in the formation of HO is very limited. The role of some of them has been described in the formation of HO resulting from mutations or different types of injury. The study by Ji et al., in which the level of miRNA was compared between patients asymptomatic for HO and those who developed HO, showed that miR205 and miRNA-215 were upregulated and muscle-specific miRNAs, that is, miR1, miR26a, miR133a, miR133b, miR146b, and miR206 were dysregulated [173]. miR205 was described mainly in myoepithelial cells and many cancer types [174] while miR215 was found in osteosarcoma [175]. The other ones which had expression changes were described in this study, i.e., miR1, miR26a, miR133a, miR133b, miR146b, and miR206 belong to muscle-specific miRNAs known as myomiRs. They play an important role in the regeneration of skeletal muscle, including activation, proliferation, and differentiation of muscle stem cells, i.e., satellite cells [176]. Thus, disrupted expression of myomiRs during HO development may be related to defective differentiation of myogenic cells [176].
Comparison of the miRNA profile in the serum of patients with immature HO and mature HO allowed the identification of miR630 as a factor that may be involved in the development of these pathologies [177]. The level of miR630 was significantly lower in the case of both types of HO. Thus, it was suggested that this molecule could serve as an early marker of HO formation [177]. Interestingly, miR630 and its direct target Slug, that is, a member of the Snail family of zinc finger transcription factors, was involved in the endothelial–mesenchymal transition of endothelial cells, which were suggested to be a source of cells responsible for HO formation [177]. Down-regulation of miR630 simultaneously with BMP4 and TGFβ2 treatment increased endothelial cells’ osteogenic differentiation [177].
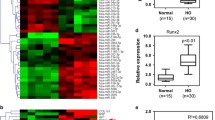
Another molecule, identified by comparison of miRNAs between the normal bone in patients and primary and mature HO, was miR203 [68]. The level of miR203 decreased in HO; simultaneously, the level of RUNX2 was increased. miR203 directly targets RUNX2 [68]. Furthermore, up-regulation of miR203 inhibited osteogenic differentiation of human osteoblasts. Interestingly, a chemically modified miRNA mimic, named agomiR203, injected into mice that underwent a tenotomy to generate a traumatic HO model, significantly decreased the development of HO, compared to control mice, i.e., injected with phosphate-buffered saline [68].
Next, miR421 is associated with cell proliferation and cancer [178, 179]. However, patients with HO show significantly lower expression of miR421 in their bone and blood, compared to patients who did not develop HO. Thus, miR421 could play a regulatory role in the HO induction [180]. It was documented that BMP2, i.e., the strongest osteogenic induction factor, was the direct target of miR421. Verification of the role of BMP2 in patients with HO induced by humeral fracture showed a significantly increased expression of BMP2 in ossified tissues and blood. Therefore, the development of HO may be related to the up-regulation of BMP2 and down-regulation of miR421 [180].
On the other hand, miR433 is related to numerous diseases. Its expression is increased in fibrotic heart disease rat models [181]. Furthermore, miR433 by targeting the RAP1a and MAPK signaling pathway, miR433 inhibits cancer cell proliferation [182]. Additionally, miR433 plays an important role in esophageal cancer and glioma [183, 184]. Bioinformatics prediction showed that miR433 is a potential upstream regulator of OPN/SPP1 [185]. OPN/SPP1 is a pro-inflammatory factor that affects the adhesion and proliferation of synovial cells [186]. OPN/SPP1 is overexpressed in the cartilage and synovium of osteoarthritis patients with osteoarthritis [187, 188]. The expression of OPN/SPP1 is regulated, among others, by microRNAs, including miR433. This molecule affects the expression of OPN/SPP1 by direct binding to the 3′‑UTR of OPN/SPP1 mRNA [189]. In the case of patients with callus and HO in patients with traumatic brain injury (TBI), the expression of OPN/SPP1 increased significantly and miR433 was reduced, leading to increased accumulation of pro-inflammatory OPN/SPP1 protein in bone tissues and negatively affecting tibial fracture healing of tibial fractures [189].
De Vasconcellos et al. analyzed the miRNA expression profile in samples of patients develo** HO and healthy individuals. HO patients demonstrated a unique molecular signature, that is, significantly upregulated miR1, miR26a, miR125b, miR133a, miR133b, and miR206 [113]. When MPCs, i.e., mesenchymal progenitor cells isolated from traumatized muscle tissue of patients, were transfected with miRNA mimics encoding selected miRNAs and induced to undergo osteogenic differentiation, the most potent osteogenic inductors were identified for myomiRs—miR1 and miR206. Next, in vitro and in silico analyses allowed the identification of SOX9, which is involved, among others, in chondrocyte differentiation, as a candidate downstream target of miR1 and miR206 miRNAs in osteogenic differentiation. Investigation of the expression level of SOX9 in samples from patients develo** HO showed that it is downregulated in samples obtained from patients develo** patients, as compared to control [113]. Thus, SOX9 expression is modulated during the development and progression of HO.
It was documented that progenitors, residing in the interstitium of skeletal muscle and expressing the platelet-derived growth factor receptor α (PDGFRα+) marker, may participate in the formation of HO [190]. Recently, an in vitro study by Zhu et al. in which human PDGFRα+ muscle cells were induced to undergo osteogenic differentiation showed that miR19b-3p could be involved in this process, as its level increases as differentiation progresses. [191]. miR19b-3p acts by indirect induction of OCN, OPN/SPP1, and RUNX2 levels [191]. Furthermore, miR19b3p down-regulates phosphate and tension homolog deleted on chromosome ten (PTEN), which is involved in the regulation of bone formation by inhibiting the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling pathway, which was shown during osteogenic differentiation of stem cells [192, 193]. Transfection of PDGFRα+ muscle cells with miR19b3p mimic or miR19b3p inhibitor resulted in a decrease in PTEN mRNA when miR19b-3p was overexpressed and increased when miR19b3p was knocked down. Thus, miR19b 3p promotes PDGFRα+ muscle cell osteogenic differentiation of PDGFR+ muscle cells by inhibiting PTEN. Importantly, inhibition of miR19b3p inhibits osteogenesis of PDGFRα+ muscle cells, suggesting that miR9b3p could be a therapeutic target in future therapy against HO. Another molecule regulating the PTEN/PI3K/AKT signaling pathway during osteogenic differentiation of in vitro cultured human primary chondrocytes was miR181a/b-1. Zheng et al. showed that stable lentiviral overexpression of miR181a/b-1 in human chondrocytes enhanced osteogenesis in vitro [194]. Furthermore, PTEN expression was lower in human chondrocytes in which miR181a/b-1 was overexpressed, and consequently, PI3K/AKT signaling was increased [194]. These findings suggest that miR181a/b could also be a target in therapies for bone conditions such as fractures or HO. Another study by Qin et al. investigated miR-17-5p targeting the ankylosis protein homolog (ANKH), a gene associated with ankylosing spondylitis (AS). The miR-17-5p inhibitor reduces heterotopic bone formation in samples from the human hip joint capsule [195].
miRNA that targets ACVR1/ALK2
miR148 was described as a probable target for the development of therapeutic agents against FOP [196]. It directly targeted and downregulated ACVR1/ALK2 mRNA and protein in HeLa cells. Furthermore, in HeLa cells, miR148 downregulated the mRNA of the inhibitor of DNA binding -1, -2, and -3 (ID-1, -2, -3), suppressing the BMP signaling pathway [196]. Point mutations of the ACVR1/ALK2 gene and their constitutive activation of the BMP signaling pathway are observed in patients with FOP. Constitutively active ACVR1/ALK2 was also shown to cause endothelial–mesenchymal transition of endothelial cells, leading to FOP lesions. However, more studies are needed to examine the role of miR148 in FOP and other HO patients. Another miRNA targeting ACVR1/ALK2 mRNA is miR208a-3p [197]. However, its role in osteoblast differentiation was studied in the mouse hind limb unloading (HLU) model. The overexpression of miR-208a-3p was inhibited, while the silencing of miR-208a-3p with antagomiR-208a-3p promoted osteoblast differentiation [197].
lncRNA and heterotopic ossification
In vitro studies using human bone marrow mesenchymal stromal cells (hMSCs) showed that lncRNA could also be involved in osteogenesis and bone formation [198]. MSCs were also considered a source of cells responsible for HO formation [39]. The overexpression of lncRNA H19 promoted the osteogenic differentiation of hMSCs in vitro [198]. Furthermore, miR675 encoded by exon 1 of the lncRNA H19 also had an osteogenic effect in hMSC [198]. lncRNA H19 and miR675 downregulated TGFβ1 leading to inhibition of SMAD3 phosphorylation inhibition [198]. On the other hand, TGFβ1 was shown to inhibit osteogenic differentiation of hMSCs. Furthermore, lncRNA H19 and miR675 negatively regulated HDAC4/5, and thus increased the expression of osteoblast markers, such as RUNX2, expression [198]. Cells overexpressing lncRNA H19 transplanted subcutaneously more efficiently formed the bone. However, the role of lncRNA H19 or miR675 was not studied in HO patients or mice models of HO formation.
In vitro and in vivo studies using human adipose-derived stem cells (hASCs) showed that lncRNA MIAT (myocardial infarction-associated transcript), which plays an important role in signaling pathways such as Hippo, PI3K/AKT/c-MET, and WNT/β-catenin, is significantly downregulated in osteogenic differentiation of stem cells [199]. MIAT knockdown in hASC reverses the inhibition induced by tumor necrosis factor α (TNFα) of osteogenesis. However, its precise role and mechanism of action remained unknown [199]. Another factor that could be involved in HO formation is bromodomain-containing protein 4 (BRD4), that is, a regulator of gene expression involved in osteoclast differentiation. BRD4 belongs to the bromodomain and extraterminal (BET) protein family and participates in the organization of superenhancers and the regulation of oncogene expression. In the HO model, overexpression of BRD4 was associated with an increased level of mitotically associated lncRNA, i.e., Mancr [200], which role was first identified in invasive breast cancer [201]. The BRD4 acting through Mancr lncRNA increased the expression of RUNX2, OSX gene, and ALP encoding gene and, which, as a result, led to HO induction. In this study, a new BRD4-Mancr-RUNX2 pathway was identified, associated with HO signaling, was identified [200]. Nagasawa et al. hypothesized that Mancr activates RUNX2 expression by another ncRNA—miR-218 [202]. Furthermore, Liu et al. described the use of JQ1, a BRD4-specific antagonist that reduces HO formation [200]. Similarly, silencing Mancr inhibited osteogenesis [200]. Next, Hatzikotoulas et al. hypothesized that variation in the CAS20 locus that encodes lncRNA is responsible for susceptibility to HO in patients undergoing THA. Upon BMP2 causing osteogenic differentiation of MSCs, the expression of CASC20 was induced. CASC20 overexpression was related to RUNX2 and OSX activation and resulted in mineralized tissue formation [42]. The identified studies on ncRNA participating in the formation of HO are listed in Table 2.
Conclusions
Role of ncRNA in HO pathogenesis
The review sums up to date literature on the involvement of ncRNA in the processes that could underlay the HO (Fig. 3). The available data are limited and are based only on several research papers. In some of them, animal models of traumatic heterotopic ossification—Achilles tenotomy in mice [68, 200] or specific muscle/chondrocytes cell lines were used [191]. Other studies included profiling of ncRNA in humans, including post-traumatic HO [113, 173], patients after THA surgery [200] and NHO following CNS injury [189]. Of all published studies, the vast majority concerned only selected miRNA (miR-1, miR-19b-3p, miR-133a, miR-133b, miR-148a, miR-203, miR-206, miR 421, miR 433 and miR-630). Most of the ones that were previously identified by bioinformatic analysis and shown to target specific elements of the HO signaling network elements, e.g., ACVR1/ALK2 [196], BMP2 [180]. Other miRNA studies aimed at targets such as Slug, which is the regulator of endothelial–mesenchymal transition [177], OPN/SSP1 mRNA [189] or PTEN that controls osteogenic differentiation of muscle cells [90]. Only a few studies analyzed the role of lncRNA in the pathogenesis of HO (lncRNA MIAT, lncRNA Mancr, and lncRNA CASC20) [42, 199, 200]. Two studies proposed a different approach and described complex miRNA profiling and changes in trauma-induced HO [68, 113]. Importantly, it was shown which ncRNA could potentially serve as a biomarker of HO development and its severity. What is important, none of the specific ncRNA that was found to be significantly dysregulated in OPLL (miR-10a, miR-563, miR-199b, miR-182, miR-615, miR-132, lncR MALAT1, and lncR XIST) was investigated in other types of HO or its animal model [28].
Confirmed miRNA targets possibly involved in HO formation. miR-148a targets ACVR1 coding mRNA that results in a decrease in ACVR1 expression. ACVR1 is one of the BMPRs that acts as receptors for BMP. BMP binding to BMPRs results in activation of SMAD-dependent or SMAD-independent signaling pathways that leads to activation of transcription factors involved in osteogenesis, such as RUNX2 or DLX5. Other pathways potentially involved in HO formation are WNT, NF-κB or the HIF1 pathway. Activation of all these pathways leads to expression of transcription factors, such as RUNX2, DLX5, or OSX, which play a crucial role in bone formation, as well as BMPs. miR-203 is known to be a negative regulator of Runx2 translation, while miR-421 acts as a negative regulator of BMPs translation. The figure was created for this article; it is not based on any previously published image
Most studies evaluated miRNAs and their impact on bone morphogenesis signaling, including canonical BMP [180, 196] or WNT/β-catenin and MAPK pathways [68]. The expression of the osteogenesis master regulator RUNX2 [68, 113, 177, 191] or levels of well-known osteogenic biomarkers, i.e., OCN [68, 113, 177, 191], OPN/SSP1 [177, 189, 191], ALP [68, 113, 177, 191], and BSP [68] or BMP2 [180] were assessed to determine effect of the miRNAs investigated. Similarly, studies in HO identified lncRNA (MIAT, Mancr, and CASC20) that aim for OSX [42, 200] ALP [42, 199, 200], OCN [42, 199], RUNX2 [42, 199, 200] as their effectors. Until now, no ncRNA studies addressed recently described pathways involved in HO, such as HIF-1α, mTOR, or NF-κB [39].
In few identified studies, researchers successfully blocked osteogenesis or HO formation using miRNA mimics or shRNA (e.g., [177]). Other ncRNAs, i.e., siRNA against RUNX2, OSX, or SMAD, were shown to inhibit HO [203,204,205,206]. Thus, ncRNA and its intracellular regulations are a potential target for future HO therapy. Although, to date, none of the miRNA molecules are tested in human clinical trials to treat HO [207].
Future perspectives of ncRNA in HO
The roles of noncoding RNAs in the pathogenesis of HO remain largely unknown and undefined. Therefore, it is not clear would be the impact of such investigation on the progress in develo** appropriate therapies. Each of the research works identifies different RNAs which could indicate that the exact regulatory role of ncRNA in HO remains unknown. Possible research directions for the future, providing such critical but still missing information, include the comparison of the expression profile in different types of HO and normal tissues rather than the comparison of binary interactions.
The therapeutic use of ncRNA in HO requires a design of the delivery method to target cells that ensures stability and safety. One of the challenges of ncRNA therapies is the method of delivery, which is handled by the use of viral (adenovirus, lentivirus) or non-viral vectors [208]. The delivery of viral vectors is efficient, but there is a risk of immunogenicity, toxicity, and carcinogenesis [208]. Therefore, constructs, such as locked nucleic acid [209], cholesterol-conjugated miRNA, lipid particles [210], or bacterially derived minicells, could be used [211]. Another issue is the stability of ncRNA, which could be improved by chemical modifications that prevent it from nuclease [208, 212]. To avoid the immune response, chemical modifications or small molecule inhibitors of ncRNA could be used [163]. To avoid off-target effects, ncRNA can be enriched with cell-specific ligands [208]. Compared to other drugs tested in FOP such as antibodies and small molecules, ncRNA has some potential therapeutic benefits as a therapeutic target. The half-life of ncRNA drugs may be very long, which means more patient-friendly doses [213]. In addition, unlike antibodies, the ncRNA can be transferred from cell to cell by the paracrine effect in exosomes, increasing the bioavailability of the drug in the affected tissue [214]. This review has presented that miRNA and lncRNA are intensively studied in the aspect of HO in both animal models and humans. However, given the complexity of this pathological process involving multiple pathways and possible effector cells in different HO types, the knowledge about the role of ncRNAs in the formation of HO is still very limited.
Availability of data and materials
Not applicable.
Abbreviations
- ACVR1/ALK2:
-
Activin A receptor type 1/activin receptor-like kinase-2
- AGO2:
-
Argonaute RISC catalytic component 2
- AHO:
-
Albright osteodystrophy
- AKT:
-
Protein kinase B
- ALP :
-
Alkaline phosphatase
- ANKH:
-
Ankylosis protein homolog
- ASO:
-
Antisense oligonucleotides
- AS:
-
Ankylosing spondylitis
- BL:
-
Baseline
- BMP:
-
Bone morphogenetic protein
- BRD4:
-
Bromodomain-containing protein 4
- BSP :
-
Bone sialoprotein
- CNS:
-
Central nervous system
- CD-RAP:
-
Cartilage-derived retinoic acid-sensitive protein
- COX:
-
Cyclooxygenase
- DAMP:
-
Damage-associated molecular pattern
- DGCR8:
-
Di George syndrome critical-related gene 8
- DLX5:
-
Distal-less homeobox 5
- EMA:
-
European Medicines Agency
- FOP:
-
Fibrodysplasia ossificans progressiva
- FXR1:
-
Fragile X mental retardation-related protein 1
- hASCs:
-
Human adipose-derived stem cells
- HDAC4/5:
-
Histone deacetylase 4/5
- HH:
-
Hedgehog
- HIF-1α :
-
Hypoxia-inducible factor 1α
- HLU:
-
Hindlimb unloading
- HMGB1:
-
High-mobility group box 1
- hMSCs:
-
Human bone marrow mesenchymal stromal cells
- HO:
-
Heterotopic ossification
- HOTL:
-
Heterotopic ossification of the tendon and ligament
- HSP:
-
Heat shock protein
- IL-1β:
-
Interleukin 1β
- IL-6:
-
Interleukin 6
- lncRNA:
-
Long noncoding RNA
- LPS:
-
Lipopolysaccharide
- Mancr:
-
Mitotically associated long noncoding RNA
- MIAT :
-
Myocardial infarction-associated transcript
- miRNA:
-
MicroRNA
- MAPK:
-
Mitogen-activated protein kinase
- MPCs :
-
Mesenchymal progenitor cells
- MVECs:
-
Microvascular endothelial cells
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin protein complex-1
- ncRNA:
-
Noncoding RNA
- NF-κB:
-
Nuclear factor-κB
- NHO:
-
Neurogenic heterotopic ossification
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- NT-3:
-
Neurotrophin-3
- OCN:
-
Osteocalcin
- OPLL:
-
Ossification of posterior longitudinal ligament of the spine
- OPN/SSP1:
-
Osteopontin
- OSM:
-
Oncostatin M
- OSX:
-
Osterix
- PAMP:
-
Pathogen-associated molecular pattern
- PET:
-
Positron emission tomography
- PI3K:
-
Phosphoinositide 3-kinase
- POH:
-
Progressive osseous heteroplasia
- POLII:
-
Polymerase II
- PROMs:
-
Patient-reported outcome measures
- pri-miRNA:
-
Primary miRNA transcript
- PTEN:
-
Phosphate and tension homolog deleted on chromosome ten
- RCT:
-
Randomized controlled trial
- RISC:
-
RNA-induced silencing complex
- ROM:
-
Range of motion
- RT:
-
Radiotherapy
- RUNX2:
-
Runt-related transcription factor 2
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- siRNA:
-
Small interfering RNA
- snoRNA:
-
Small nucleolar RNA
- snRNA:
-
Small nuclear RNA
- SOX9:
-
SRY-box transcription factor 9
- SP:
-
Substance P
- TBI:
-
Traumatic brain injury
- TGFβ:
-
Transforming growth factor β
- THA:
-
Total hip arthroplasty
- TLR:
-
Toll-like receptor
- TNFα:
-
Tumor necrosis factor α
- tRNAs:
-
Transfer RNA
- WBCT:
-
Whole-body computed tomography
- XPO5:
-
Exportin 5
References
Meyers C, et al. Heterotopic ossification: a comprehensive review. JBMR Plus. 2019;3(4):e10172.
Xu Y, et al. Heterotopic ossification: clinical features, basic researches, and mechanical stimulations. Front Cell Dev Biol. 2022. https://doi.org/10.3389/fcell.2022.770931.
Nauth A, et al. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma. 2012;26(12):684–8.
Zhou L, et al. Heterotopic ossification after arthroscopic procedures: a sco** review of the literature. Orthop J Sports Med. 2022;10(1):23259671211060040.
Rüdiger HA, et al. The impact of heterotopic ossification on self-reported outcomes after total hip arthroplasty using the direct anterior approach. J Bone Jt Surg Am. 2020;102(Suppl 2):91–8.
Neal B, et al. Incidence of heterotopic bone formation after major hip surgery. ANZ J Surg. 2002;72(11):808–21.
Pohl F, et al. The influence of heterotopic ossification on functional status of hip joint following total hip arthroplasty. Strahlenther Onkol. 2005;181(8):529–33.
Gkiatas I, et al. Heterotopic ossification negatively influences range of motion after revision total knee arthroplasty. J Arthroplast. 2021;36(8):2907–12.
Skorochod R, Nesher G, Gronovich Y. Heterotopic ossification in patients with burns: a systematic literature review. Curr Phys Med Rehabil Rep. 2022. https://doi.org/10.1007/s40141-022-00356-5.
Hong CC, et al. Clinically relevant heterotopic ossification after elbow fracture surgery: a risk factors study. Orthop Traumatol Surg Res. 2015;101(2):209–13.
Ahrengart L, Lindgren U. Functional significance of heterotopic bone formation after total hip arthroplasty. J Arthroplasty. 1989;4(2):125–31.
Wong KR, et al. Neurological heterotopic ossification: novel mechanisms, prognostic biomarkers and prophylactic therapies. Bone Res. 2020;8(1):42.
Johns JS, et al. Impact of clinically significant heterotopic ossification on functional outcome after traumatic brain injury. J Head Trauma Rehabil. 1999;14(3):269–76.
Taly AB, et al. Neurogenic heterotopic ossification: a diagnostic and therapeutic challenge in neurorehabilitation. Neurol India. 2001;49(1):37–40.
Meyer C, et al. Heterotopic ossification in COVID-19: a series of 4 cases. Ann Phys Rehabil Med. 2020;63(6):565–7.
Mezghani S, et al. Heterotopic ossification and COVID 19: imaging analysis of ten consecutive cases. Eur J Radiol. 2022;152:110336.
Zhang Q, et al. Heterotopic ossification of tendon and ligament. J Cell Mol Med. 2020;24(10):5428–37.
McCarthy EF, Sundaram M. Heterotopic ossification: a review. Skeletal Radiol. 2005;34(10):609–19.
Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37(3):129–36.
Abiola R, Rubery P, Mesfin A. Ossification of the posterior longitudinal ligament: etiology, diagnosis, and outcomes of nonoperative and operative management. Glob Spine J. 2016;6(2):195–204.
Oliva F, Via AG, Maffulli N. Calcific tendinopathy of the rotator cuff tendons. Sports Med Arthrosc Rev. 2011;19(3):237–43.
Le HV, et al. Ossification of the posterior longitudinal ligament: pathophysiology, diagnosis, and management. J Am Acad Orthop Surg. 2022. https://doi.org/10.5435/JAAOS-D-22-00049.
Maeda S, et al. Functional impact of human collagen α2 (XI) gene polymorphism in pathogenesis of ossification of the posterior longitudinal ligament of the spine. J Bone Miner Res. 2001;16(5):948–57.
Kong Q, et al. COL6A1 polymorphisms associated with ossification of the ligamentum flavum and ossification of the posterior longitudinal ligament. Spine. 2007;32(25):2834–8.
Okamoto K, et al. Dietary habits and risk of ossification of the posterior longitudinal ligaments of the spine (OPLL); findings from a case-control study in Japan*. J Bone Miner Metab. 2004;22(6):612–7.
Hirai T, et al. Prevalence and distribution of ossified lesions in the whole spine of patients with cervical ossification of the posterior longitudinal ligament a multicenter study (JOSL CT study). PLoS ONE. 2016;11(8):e0160117.
Endo T, et al. Close association between non-alcoholic fatty liver disease and ossification of the posterior longitudinal ligament of the spine. Sci Rep. 2021;11(1):17412.
Yuan X, Shi L, Chen Y. Non-coding RNAs in ossification of spinal ligament. Eur Spine J. 2021;30(4):801–8.
Darrieutort-Laffite C, Blanchard F, Le Goff B. Calcific tendonitis of the rotator cuff: From formation to resorption. Jt Bone Spine. 2018;85(6):687–92.
Uhthoff HK. Calcifying tendinitis, an active cell-mediated calcification. Virchows Arch A Pathol Anat Histol. 1975;366(1):51–8.
Cappato S, et al. Genetic and acquired heterotopic ossification: a translational tale of mice and men. Biomedicines. 2020;8(12):611.
De Brasi D, et al. Fibrodysplasia ossificans progressiva: a challenging diagnosis. Genes (Basel). 2021;12(8):1187.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92.
Liljesthröm M, Pignolo RJ, Kaplan FS. Epidemiology of the global fibrodysplasia ossificans progressiva (FOP) community. J Rare Dis Res Treat. 2020;5(2):31–6.
Kapoor S, et al. Albright’s hereditary osteodystrophy. Indian J Pediatr. 2006;73:153–6.
Mantovani G, et al. Diagnosis and management of pseudohypoparathyroidism and related disorders: first international consensus statement. Nat Rev Endocrinol. 2018;14(8):476–500.
Rocke DM, et al. Age- and joint-specific risk of initial heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1994;301:243–8.
Peng K, et al. Longitudinal evaluation of pain, flare-up, and emotional health in fibrodysplasia ossificans progressiva: analyses of the international FOP registry. JBMR Plus. 2019;3(8):e10181.
Pulik Ł, et al. The survey of cells responsible for heterotopic ossification development in skeletal muscles-human and mouse models. Cells. 2020;9(6):1324.
Hasin Y, Seldin M, Lusis A. Multi-omics approaches to disease. Genome Biol. 2017;18(1):83.
Mitchell EJ, et al. The genetics of heterotopic ossification: insight into the bone remodeling pathway. J Orthop Trauma. 2010;24(9):530–3.
Hatzikotoulas K et al. Genome-wide association and functional analyses identify CASC20 and KIF26B as target loci in heterotopic ossification. bioRxiv, 2019; 845958.
Yang Z, et al. Potential genes and pathways associated with heterotopic ossification derived from analyses of gene expression profiles. J Orthop Surg Res. 2021;16(1):499.
Logan NJ, et al. Demethylation of ITGAV accelerates osteogenic differentiation in a blast-induced heterotopic ossification in vitro cell culture model. Bone. 2018;117:149–60.
Edsberg LE, et al. A survey of proteomic biomarkers for heterotopic ossification in blood serum. J Orthop Surg Res. 2017;12(1):69–69.
Wei Z, et al. Comparative proteomic analysis identifies differentially expressed proteins and reveals potential mechanisms of traumatic heterotopic ossification progression. J Orthop Transl. 2022;34:42–59.
Wang L, et al. Could heterotopic ossification be prevented by varying dietary n-3/n-6 polyunsaturated fatty acid ratio: a novel perspective to its treatment? Med Hypotheses. 2013;80(1):57–60.
Lees-Shepard JB, Goldhamer DJ. Stem cells and heterotopic ossification: lessons from animal models. Bone. 2018;109:178–86.
Lounev VY, et al. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Jt Surg Am Vol. 2009;91(3):652–63.
Huang Y, et al. Macrophages in heterotopic ossification: from mechanisms to therapy. npj Regen Med. 2021;6(1):70.
Convente MR, et al. The immunological contribution to heterotopic ossification disorders. Curr Osteoporos Rep. 2015;13(2):116–24.
Tseng HW, et al. Interleukin-1 is overexpressed in injured muscles following spinal cord injury and promotes neurogenic heterotopic ossification. J Bone Miner Res. 2022;37(3):531–46.
Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42(6):551–5.
Mackie EJ, et al. Endochondral ossification: how cartilage is converted into bone in the develo** skeleton. Int J Biochem Cell Biol. 2008;40(1):46–62.
Foley KL, et al. Histopathology of periarticular non-hereditary heterotopic ossification. Bone. 2018;109:65–70.
Culbert AL, et al. Alk2 regulates early chondrogenic fate in fibrodysplasia ossificans progressiva heterotopic endochondral ossification. Stem Cells. 2014;32(5):1289–300.
Lindborg CM, et al. Cartilage-derived retinoic acid-sensitive protein (CD-RAP): a stage-specific biomarker of heterotopic endochondral ossification (HEO) in fibrodysplasia ossificans progressiva (FOP). Bone. 2018;109:153–7.
Kaplan FS, et al. The histopathology of fibrodysplasia ossificans progressive. An endochondral process. J Bone Jt Surg Am. 1993;75(2):220–30.
Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000;15(11):2084–94.
DeSesso JM, Scialli AR. Bone development in laboratory mammals used in developmental toxicity studies. Birth Defects Res. 2018;110(15):1157–87.
**an CJ, et al. Intramembranous ossification mechanism for bone bridge formation at the growth plate cartilage injury site. J Orthop Res. 2004;22(2):417–26.
Barruet E, et al. NF-κB/MAPK activation underlies ACVR1-mediated inflammation in human heterotopic ossification. JCI Insight. 2018;3(22):e122958.
Huang Y, Wang X, Lin H. The hypoxic microenvironment: a driving force for heterotopic ossification progression. Cell Commun Signal. 2020;18(1):20.
Wu J, et al. BMP and mTOR signaling in heterotopic ossification: does their crosstalk provide therapeutic opportunities? J Cell Biochem. 2019;120(8):12108–22.
Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122(20):3589–94.
Kan C, et al. Conserved signaling pathways underlying heterotopic ossification. Bone. 2018;109:43–8.
Wang S, et al. Identification of the biomarkers and pathological process of heterotopic ossification: weighted gene co-expression network analysis. Front Endocrinol. 2020;11:581768–581768.
Tu B, et al. miR-203 inhibits the traumatic heterotopic ossification by targeting Runx2. Cell Death Dis. 2016;7(10):e2436–e2436.
Migliorini F, et al. NSAIDs for prophylaxis for heterotopic ossification after total hip arthroplasty: a Bayesian network meta-analysis. Calcif Tissue Int. 2021;108(2):196–206.
Łęgosz P, et al. Challenges of heterotopic ossification-molecular background and current treatment strategies. Clin Exp Pharmacol Physiol. 2018;45(12):1229–35.
Takada Y, et al. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappaB activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene. 2004;23(57):9247–58.
Pakos EE, Ioannidis JP. Radiotherapy versus nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004;60(3):888–95.
Patel AB, et al. Radiation therapy prophylaxis for heterotopic ossification in non-hip sites. J Cancer Ther. 2018;9(1):1–8.
Sautter-Bihl ML, Liebermeister E, Nanassy A. Radiotherapy as a local treatment option for heterotopic ossifications in patients with spinal cord injury. Spinal Cord. 2000;38(1):33–6.
Łęgosz P, et al. Heterotopic ossification: a challenging complication of total hip arthroplasty: risk factors, diagnosis, prophylaxis, and treatment. Biomed Res Int. 2019;2019:3860142.
Milakovic M, et al. Radiotherapy for the prophylaxis of heterotopic ossification: a systematic review and meta-analysis of randomized controlled trials. Radiother Oncol. 2015;116(1):4–9.
Hu Z-H, et al. Radiotherapy for the prophylaxis of heterotopic ossification after total hip arthroplasty: a systematic review and meta-analysis of randomized controlled trails. Med Dosim. 2021;46(1):65–73.
Galietta E, et al. Prophylactic radiotherapy of hip heterotopic ossification: a narrative mini review. In Vivo. 2022;36(2):533–42.
Rosenberg DM, et al. Radiation-induced sarcoma after heterotopic ossification prophylaxis: a case report. JBJS Case Connect. 2019;9(4):e0146.
Farris MK, et al. Osteosarcoma following single fraction radiation prophylaxis for heterotopic ossification. Radiat Oncol. 2012;7(1):1–6.
Mourad WF, et al. Radiation-induced sarcoma following radiation prophylaxis of heterotopic ossification. Pract Radiat Oncol. 2012;2(2):151–4.
Hanna M, Farid YR, Finn HA. Low-dose preoperative unshielded radiation is effective in heterotopic ossification prophylaxis and does not affect porous fixation in total hip arthroplasty at 2 years minimum follow-up: a radiographic study. JAAOS: J Am Acad Orthop Surg. 2022;30(5):223–8.
Shapira J, et al. Efficacy of NSAIDs versus radiotherapy for heterotopic ossification prophylaxis following total hip arthroplasty in high-risk patients: a systematic review and meta-analysis. HIP Int. 2021;32(5):576–90.
Vavken P, Dorotka R. Economic evaluation of NSAID and radiation to prevent heterotopic ossification after hip surgery. Arch Orthop Trauma Surg. 2011;131(9):1309–15.
Wang Y, et al. Irradiation alters the differentiation potential of bone marrow mesenchymal stem cells. Mol Med Rep. 2016;13(1):213–23.
Felix-Ilemhenbhio F, et al. Pathophysiology and emerging molecular therapeutic targets in heterotopic ossification. Int J Mol Sci. 2022;23(13):6983.
Vanhoutte F, et al. Pharmacokinetics and pharmacodynamics of garetosmab (Anti-Activin A): results from a first-in-human phase 1 study. J Clin Pharmacol. 2020;60(11):1424–31.
Dahir KM, et al. Garetosmab Reduces flare-ups in patients with fibrodysplasia ossificans progressiva. J Endocr Soc. 2021;5(Supplement_1):A251–2.
Ventura F, et al. Challenges and opportunities for drug repositioning in fibrodysplasia ossificans progressiva. Biomedicines. 2021;9(2):213.
The Examination of Safety and Efficacy of Garetosmab Versus Placebo Administered Intravenously (IV) in Adult Participants With Fibrodysplasia Ossificans Progressiva (OPTIMA). ClinicalTrials.gov identifier: NCT05394116. Updated May 27, 2022. https://ClinicalTrials.gov/show/NCT05394116. Accessed 23 July 2022.
Study of Single-Ascending Doses of DS-6016a in Healthy Japanese. ClinicalTrials.gov identifier: NCT04818398. Updated January 10, 2022. https://ClinicalTrials.gov/show/NCT04818398. Accessed 23 July 2022.
Rooney L, Jones C. Recent advances in ALK2 inhibitors. ACS Omega. 2021;6(32):20729–34.
Ordonez C, et al. Administration of KER-047, a novel ALK2 Inhibitor, elicited robust and sustained increases in serum iron in healthy participants. Blood. 2020;136(Supplement 1):13–13.
Sabir AH, Cole T. The evolving therapeutic landscape of genetic skeletal disorders. Orphanet J Rare Dis. 2019;14(1):300.
Study to Assess the Efficacy and Safety of 2 Dosage Regimens of Oral IPN60130 for the Treatment of Fibrodysplasia Ossificans Progressiva (FOP) (FALKON). ClinicalTrials.gov identifier: NCT05039515. Updated July 6, 2022. https://ClinicalTrials.gov/show/NCT05039515. Accessed 23 July 2022.
To Assess the Efficacy, Safety, and Tolerability of INCB000928 in Participants With Fibrodysplasia Ossificans Progressiva (PROGRESS). ClinicalTrials.gov identifier: NCT05090891. Updated June 13, 2022. Available from: https://ClinicalTrials.gov/show/NCT05090891. Accessed 23 July 2022.
Calpe S, et al. Comparison of newly developed anti-bone morphogenetic protein 4 llama-derived antibodies with commercially available BMP4 inhibitors. MAbs. 2016;8(4):678–88.
Meng X, Wang H, Hao J, Recent progress in drug development for fibrodysplasia ossificans progressiva. Mol Cell Biochem 2022.
Mundy C, et al. Activin A promotes the development of acquired heterotopic ossification and is an effective target for disease attenuation in mice. Sci Signal. 2021;14(669):536.
Saracatinib Trial TO Prevent FOP (STOPFOP). ClinicalTrials.gov identifier: NCT04307953. Updated July 22, 2022. https://ClinicalTrials.gov/show/NCT04307953. Accessed 23 July 2022.
Kitoh H. Clinical aspects and current therapeutic approaches for FOP. Biomedicines. 2020;8(9):325.
Williams E, et al. Saracatinib is an efficacious clinical candidate for fibrodysplasia ossificans progressiva. JCI Insight. 2021;6(8):e95042.
Hino K, et al. Activin-A enhances mTOR signaling to promote aberrant chondrogenesis in fibrodysplasia ossificans progressiva. J Clin Investig. 2017;127:3339–52.
Maekawa H, et al. Prophylactic treatment of rapamycin ameliorates naturally develo** and episode -induced heterotopic ossification in mice expressing human mutant ACVR1. Orphanet J Rare Dis. 2020;15(1):122.
Multicenter randomized double-blind comparison test followed by open-label continuous administration test of NPC-12T for Fibrodysplasia Ossificans Progressiva. UMIN-CTR identifier: UMIN000028429. Updated February 2, 2022. https://center6.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000032495. Accessed 23 July 2022.
Hind M, Stinchcombe S. Palovarotene, a novel retinoic acid receptor gamma agonist for the treatment of emphysema. Curr Opin Investig Drugs. 2009;10(11):1243–50.
Shimono K, et al. Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists. Nat Med. 2011;17(4):454–60.
Huang J, et al. Palovarotene inhibits the NF-κB signalling pathway to prevent heterotopic ossification. Clin Exp Pharmacol Physiol. 2022;49(8):881–92.
Huang J, et al. Palovarotene can attenuate heterotopic ossification induced by tendon stem cells by downregulating the synergistic effects of smad and NF-<i>κ</i>B signaling pathway following stimulation of the inflammatory microenvironment. Stem Cells Int. 2022;2022:1560943.
An Efficacy and Safety Study of Palovarotene for the Treatment of Fibrodysplasia Ossificans Progressiva (MOVE). ClinicalTrials.gov identifier: NCT03312634. Updated March 15, 2022. https://ClinicalTrials.gov/show/NCT03312634. Accessed 23 July 2022.
** W, et al. Engineered osteoclasts as living treatment materials for heterotopic ossification therapy. Nat Commun. 2021;12(1):6327.
Hoy SM. Palovarotene: first approval. Drugs. 2022;82(6):711–6.
de Vasconcellos JF, et al. A microRNA signature for impaired wound-healing and ectopic bone formation in humans. J Bone Jt Surg Am. 2020;102(21):1891–9.
An Open-Label Extension Study of Palovarotene Treatment in Fibrodysplasia Ossificans Progressiva (FOP). ClinicalTrials.gov identifier: NCT02279095. Updated February 17, 2022. https://ClinicalTrials.gov/show/NCT02279095. Accessed 23 July 2022/
An Open-Label Extension Study of Palovarotene to Prevent Heterotopic Ossification in People With Fibrodysplasia Ossificans Progressiva (FOP) in France. ClinicalTrials.gov identifier: NCT02979769. Updated February 17, 2022. https://ClinicalTrials.gov/show/NCT02979769. Accessed 23 July 2022.
A Rollover Study to Further Evaluate the Safety and Efficacy of Palovarotene Capsules in Male and Female Participants Aged ≥14 Years With Fibrodysplasia Ossificans Progressiva (FOP) Who Have Completed the Relevant Parent Studies (PIVOINE). ClinicalTrials.gov identifier: NCT05027802. Updated July 1, 2022. https://ClinicalTrials.gov/show/NCT05027802. Accessed 23 July 2022.
Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94.
Hombach S, Kretz M. Non-coding RNAs: classification, biology and functioning. Adv Exp Med Biol. 2016;937:3–17.
Zhang S, et al. The risks of miRNA therapeutics: In a drug target perspective. Drug Des Dev Ther. 2021;15:721–33.
You C, et al. ODNA: a manually curated database of noncoding RNAs associated with orthopedics. Database (Oxford). 2019. https://doi.org/10.1093/database/baz126.
Bartel DP. Metazoan MicroRNAs. Cell. 2018;173(1):20–51.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
O’Brien J, et al. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol. 2018;9:402.
Dexheimer PJ, Cochella L. MicroRNAs: from mechanism to organism. Front Cell Dev Biol. 2020;8:409.
Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 2019;20(1):21–37.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24.
Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet. 2015;16(7):421–33.
Bukhari SIA, et al. A specialized mechanism of translation mediated by FXR1a-associated microRNP in cellular quiescence. Mol Cell. 2016;61(5):760–73.
Truesdell SS, et al. MicroRNA-mediated mRNA translation activation in quiescent cells and oocytes involves recruitment of a nuclear microRNP. Sci Rep. 2012;2(1):842.
Ørom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008;30(4):460–71.
Gregory RI, et al. The microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432(7014):235–40.
Denli AM, et al. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432(7014):231–5.
Lee Y, et al. The nuclear RNase III drosha initiates microRNA processing. Nature. 2003;425(6956):415–9.
Han J, et al. The drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18(24):3016–27.
Stavast CJ, Erkeland SJ. The non-canonical aspects of microRNAs: many roads to gene regulation. Cells. 2019;8(11):1465.
Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature. 2007;448(7149):83–6.
Okamura K, et al. The mirtron pathway generates microRNA-class regulatory RNAs in drosophila. Cell. 2007;130(1):89–100.
Berezikov E, et al. Mammalian mirtron genes. Mol Cell. 2007;28(2):328–36.
Okada C, et al. A high-resolution structure of the pre-microRNA nuclear export machinery. Science. 2009;326(5957):1275–9.
Lund E, et al. Nuclear export of microRNA precursors. Science. 2004;303(5654):95–8.
Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA (New York, NY). 2004;10(2):185–91.
Guo L, Lu Z. The fate of miRNA* strand through evolutionary analysis: implication for degradation as merely carrier strand or potential regulatory molecule? PLoS ONE. 2010;5(6):e11387.
Yang J-S, et al. Widespread regulatory activity of vertebrate microRNA* species. RNA (New York, NY). 2011;17(2):312–26.
Yang X, et al. Both mature miR-17-5p and passenger strand miR-17-3p target TIMP3 and induce prostate tumor growth and invasion. Nucl Acids Res. 2013;41(21):9688–704.
Pratt AJ, MacRae IJ. The RNA-induced silencing complex: a versatile gene-silencing machine. J Biol Chem. 2009;284(27):17897–901.
Hutvágner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297(5589):2056–60.
Hammond SM, et al. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature. 2000;404(6775):293–6.
Wang Y, et al. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature. 2008;456(7224):921–6.
Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110.
Wee LM, et al. Argonaute divides Its RNA guide into domains with distinct functions and RNA-binding properties. Cell. 2012;151(5):1055–67.
Israeli D, et al. Circulating miRNAs are generic and versatile therapeutic monitoring biomarkers in muscular dystrophies. Sci Rep. 2016;6(1):28097.
Cacchiarelli D, et al. miRNAs as serum biomarkers for duchenne muscular dystrophy. EMBO Mol Med. 2011;3(5):258–65.
Vignier N, et al. Distinctive serum miRNA profile in mouse models of striated muscular pathologies. PLoS ONE. 2013;8(2):e55281.
McHugh CA, et al. The xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature. 2015;521(7551):232–6.
Wang X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454(7200):126–30.
Wang KC, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472(7341):120–4.
Clemson CM, et al. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell. 2009;33(6):717–26.
Atianand MK, et al. A long noncoding RNA lincRNA-EPS Acts as a transcriptional brake to restrain inflammation. Cell. 2016;165(7):1672–85.
Martianov I, et al. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445(7128):666–70.
Feng J, et al. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20(11):1470–84.
Wang G-Q, et al. Sirt1 AS lncRNA interacts with its mRNA to inhibit muscle formation by attenuating function of miR-34a. Sci Rep. 2016;6(1):21865.
Cesana M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–69.
Winkle M, et al. Noncoding RNA therapeutics: challenges and potential solutions. Nat Rev Drug Discov. 2021;20(8):629–51.
Chen B, et al. Targeting non-coding RNAs to overcome cancer therapy resistance. Signal Transduct Target Ther. 2022;7(1):121.
Mercer TR, Munro T, Mattick JS. The potential of long noncoding RNA therapies. Trends Pharmacol Sci. 2022;43(4):269–80.
Kazezian Z, Bull AMJ. A review of the biomarkers and in vivo models for the diagnosis and treatment of heterotopic ossification following blast and trauma-induced injuries. Bone. 2021;143:115765.
Łęgosz P, et al. The use of type I collagen cross-linked C-telopeptide (CTX-1) as a biomarker associated with the formation of periprosthetic ossifications following total hip joint arthroplasty. Ann Clin Lab Sci. 2018;48(2):183–90.
Sharma GG, et al. Non-coding RNA biomarkers in pancreatic ductal adenocarcinoma. Semin Cancer Biol. 2021;75:153–68.
Gong K, et al. Comprehensive analysis of lncRNA biomarkers in kidney renal clear cell carcinoma by lncRNA-mediated ceRNA network. PLoS ONE. 2021;16(6):e0252452.
Hu Y, et al. Comprehensive analysis of lncRNA-mRNAs co-expression network identifies potential lncRNA biomarkers in cutaneous squamous cell carcinoma. BMC Genom. 2022;23(1):274.
Joilin G, et al. An overview of MicroRNAs as biomarkers of ALS. Front Neurol. 2019;10:186.
Borga C, Meeran SM, Fassan M. Non-coding RNAs, a real next-gen class of biomarkers? Noncoding RNA Res. 2019;4(3):80–1.
Ji Y et al. Finding Molecular Targets of Heterotopic Ossification Following War Trauma using microRNA. in ORS Annual Meeting. 2012.
Chauhan N, et al. miR-205: a potential biomedicine for cancer therapy. Cells. 2020;9(9):1957.
Vychytilova-Faltejskova P, Slaby O. MicroRNA-215: from biology to theranostic applications. Mol Aspects Med. 2019;70:72–89.
McCarthy JJ. The MyomiR network in skeletal muscle plasticity. Exerc Sport Sci Rev. 2011;39(3):150–4.
Sun Y, et al. MiR-630 inhibits endothelial-mesenchymal transition by targeting slug in traumatic heterotopic ossification. Sci Rep. 2016;6(1):22729.
Jiang Z, et al. Increased expression of miR-421 in human gastric carcinoma and its clinical association. J Gastroenterol. 2010;45(1):17–23.
Zhou H, et al. MiR-421 is a functional marker of circulating tumor cells in gastric cancer patients. Biomarkers. 2012;17(2):104–10.
Ju C, et al. Regulatory effect of miR-421 on humeral fracture and heterotopic ossification in elderly patients. Exp Ther Med. 2019;17(3):1903–11.
Tao L, et al. Crucial role of miR-433 in regulating cardiac fibrosis. Theranostics. 2016;6(12):2068–83.
Zhang T, et al. miR-433 inhibits breast cancer cell growth via the MAPK signaling pathway by targeting Rap1a. Int J Biol Sci. 2018;14(6):622–32.
Shi Q, et al. MiR-433-3p inhibits proliferation and invasion of esophageal squamous cell carcinoma by targeting GRB2. Cell Physiol Biochem. 2018;46(5):2187–96.
Sun S, et al. MiR-433-3p suppresses cell growth and enhances chemosensitivity by targeting CREB in human glioma. Oncotarget. 2017;8(3):5057–68.
Jiang K, Teng GD, Chen YQ. MicroRNA-23 suppresses osteogenic differentiation of human bone marrow mesenchymal stem cells by targeting the MEF2C-mediated MAPK signaling pathway. J Gene Med. 2020;22(10):e3216.
Chen G, et al. Role of osteopontin in synovial Th17 differentiation in rheumatoid arthritis. Arthr Rheum. 2010;62(10):2900–8.
Gao SG, et al. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthr Cartil. 2010;18(1):82–7.
Gattorno M, et al. Synovial expression of osteopontin correlates with angiogenesis in juvenile idiopathic arthritis. Rheumatology (Oxford). 2004;43(9):1091–6.
Han Z, et al. Relationship between miRNA-433 and SPP1 in the presence of fracture and traumatic brain injury. Exp Ther Med. 2021;22(3):928–928.
Wosczyna MN, et al. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27(5):1004–17.
Zhu Y, et al. MiR-19b-3p regulates osteogenic differentiation of PDGFRα(+) muscle cells by specifically targeting PTEN. Cell Biol Int. 2019;43(5):565–73.
Huang J, et al. The role of COX-2 in mediating the effect of PTEN on BMP9 induced osteogenic differentiation in mouse embryonic fibroblasts. Biomaterials. 2014;35(36):9649–59.
Zhao C, et al. miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol. 2015;12(3):343–53.
Zheng H, et al. MicroRNA-181a/b-1 over-expression enhances osteogenesis by modulating PTEN/PI3K/AKT signaling and mitochondrial metabolism. Bone. 2019;123:92–102.
Qin X, et al. miR-17-5p regulates heterotopic ossification by targeting ANKH in ankylosing spondylitis. Mol Ther Nucl Acids. 2019;18:696–707.
Song H, et al. ACVR1, a therapeutic target of fibrodysplasia ossificans progressiva, is negatively regulated by miR-148a. Int J Mol Sci. 2012;13(2):2063–77.
Arfat Y, et al. miR-208a-3p suppresses osteoblast differentiation and inhibits bone formation by targeting ACVR1. Mol Ther Nucl Acids. 2018;11:323–36.
Huang Y, et al. Long noncoding RNA H19 promotes osteoblast differentiation via TGF-β1/Smad3/HDAC signaling pathway by deriving miR-675. Stem Cells. 2015;33(12):3481–92.
** C, et al. Long non-coding RNA MIAT knockdown promotes osteogenic differentiation of human adipose-derived stem cells. Cell Biol Int. 2017;41(1):33–41.
Liu L, et al. BRD4 promotes heterotopic ossification through upregulation of LncRNA MANCR. Bone Jt Res. 2021;10(10):668–76.
Tracy KM, et al. Mitotically-associated lncRNA (MANCR) affects genomic stability and cell division in aggressive breast cancer. Mol Cancer Res. 2018;16(4):587–98.
Nagasawa M, et al. Long non-coding RNA MANCR is a target of BET bromodomain protein BRD4 and plays a critical role in cellular migration and invasion abilities of prostate cancer. Biochem Biophys Res Commun. 2020;526(1):128–34.
Lin L, et al. Adenovirus-mediated transfer of siRNA against Runx2/Cbfa1 inhibits the formation of heterotopic ossification in animal model. Biochem Biophys Res Commun. 2006;349(2):564–72.
Mishra S, et al. Delivery of siRNA silencing Runx2 using a multifunctional polymer-lipid nanoparticle inhibits osteogenesis in a cell culture model of heterotopic ossification. Integr Biol (Camb). 2012;4(12):1498–507.
Xue T, et al. Non-virus-mediated transfer of siRNAs against Runx2 and Smad4 inhibit heterotopic ossification in rats. Gene Ther. 2010;17(3):370–9.
Shrivats AR, Hollinger JO. The delivery and evaluation of RNAi therapeutics for heterotopic ossification pathologies. In: Vunjak-Novakovic G, Turksen K, editors. Biomimetics and stem cells methods and protocols. New York: Springer; 2014. p. 149–60.
Chakraborty C, et al. Therapeutic advances of miRNAs: a preclinical and clinical update. J Adv Res. 2021;28:127–38.
Dasgupta I, Chatterjee A. Recent advances in miRNA delivery systems. Methods Protoc. 2021;4(1):10.
Elmén J, et al. Antagonism of microRNA-122 in mice by systemically administered LNA-antimiR leads to up-regulation of a large set of predicted target mRNAs in the liver. Nucl Acids Res. 2008;36(4):1153–62.
Hong DS et al. MRX34, a liposomal miR-34 mimic, in patients with advanced solid tumors: final dose-escalation results from a first-in-human phase I trial of microRNA therapy. 2016, American Society of Clinical Oncology.
van Zandwijk N, et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: a first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017;18(10):1386–96.
Hueso M, et al. ncRNAs in therapeutics: challenges and limitations in nucleic acid-based drug delivery. Int J Mol Sci. 2021;22(21):11596.
Huang C-K, Kafert-Kasting S, Thum T. Preclinical and clinical development of noncoding RNA therapeutics for cardiovascular disease. Circ Res. 2020;126(5):663–78.
Dragomir M, Chen B, Calin GA. Exosomal lncRNAs as new players in cell-to-cell communication. Transl Cancer Res. 2017;7(Suppl 2):S243–52.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ŁP contributed to conceptualization; BM, IG, EB, ŁP, and AS contributed to writing—original draft preparation; BM, IG, EB, MAC, PŁ, PŁ, and AS contributed to writing, review, and editing; BM visualized the study; EB and ŁP supervised the study. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pulik, Ł., Mierzejewski, B., Sibilska, A. et al. The role of miRNA and lncRNA in heterotopic ossification pathogenesis. Stem Cell Res Ther 13, 523 (2022). https://doi.org/10.1186/s13287-022-03213-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-03213-3