Abstract
Background
Critically ill children suffer from impaired physical/neurocognitive development 2 years later. Glucocorticoid treatment alters DNA methylation within the hypothalamus–pituitary–adrenal (HPA) axis which may impair normal brain development, cognition and behaviour. We tested the hypothesis that paediatric-intensive-care-unit (PICU) patients, sex- and age-dependently, show long-term abnormal DNA methylation within the HPA-axis layers, possibly aggravated by glucocorticoid treatment in the PICU, which may contribute to the long-term developmental impairments.
Results
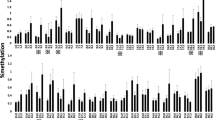
In a pre-planned secondary analysis of the multicentre PEPaNIC-RCT and its 2-year follow-up, we identified differentially methylated positions and differentially methylated regions within HPA-axis genes in buccal mucosa DNA from 818 former PICU patients 2 years after PICU admission (n = 608 no glucocorticoid treatment; n = 210 glucocorticoid treatment) versus 392 healthy children and assessed interaction with sex and age, role of glucocorticoid treatment in the PICU and associations with long-term developmental impairments. Adjusting for technical variation and baseline risk factors and correcting for multiple testing (false discovery rate < 0.05), former PICU patients showed abnormal DNA methylation of 26 CpG sites (within CRHR1, POMC, MC2R, NR3C1, FKBP5, HSD11B1, SRD5A1, AKR1D1, DUSP1, TSC22D3 and TNF) and three DNA regions (within AVP, TSC22D3 and TNF) that were mostly hypomethylated. These abnormalities were sex-independent and only partially age-dependent. Abnormal methylation of three CpG sites within FKBP5 and one CpG site within SRD5A1 and AKR1D1 was partly attributable to glucocorticoid treatment during PICU stay. Finally, abnormal methylation within FKBP5 and AKR1D1 was most robustly associated with long-term impaired development.
Conclusions
Two years after critical illness in children, abnormal methylation within HPA-axis genes was present, predominantly within FKBP5 and AKR1D1, partly attributable to glucocorticoid treatment in the PICU, and explaining part of the long-term developmental impairments. These data call for caution regarding liberal glucocorticoid use in the PICU.
Similar content being viewed by others
Background
Critical illness in children, which requires treatment in a paediatric intensive care unit (PICU), represents a form of severe physical stress, hallmarked by a whole range of (neuro)endocrine abnormalities [1,2,3,4,5]. A typical response to the severe stress of critical illness is an acute activation of the hypothalamus–pituitary–adrenal (HPA) axis [1, 6, 7]. Indeed, critically ill patients show an acute rise in total and free cortisol, lasting shorter in children than in adults [1, 8,9,10,11]. Unlike long assumed, the rise in cortisol is not driven by a rise in ACTH, as ACTH levels have been shown to be normal or low during critical illness [1, 10, 11]. Instead, the elevated cortisol levels appeared explained by low levels of its binding proteins corticosteroid-binding globulin and albumin, combined with suppressed breakdown of cortisol [1, 11]. Suppressed cortisol breakdown has been revealed by reduced expression and/or activity of the cortisol metabolising enzymes 11β-hydroxysteroid dehydrogenase (11βHSD) 2 and the A-ring reductases, and by strongly reduced cortisol plasma clearance during tracer infusion and after administration of a hydrocortisone bolus [1, 11].
Follow-up studies of children who needed PICU admission for a wide variety of surgical or medical reasons have shown that these children reveal high risk for important impairments in physical and neurocognitive development and behavioural problems years after the acute illness, also in the absence of pre-existing conditions known to affect or possibly affect development [12,13,14,15,16,17]. Other severe early-life adverse events, such as abuse or neglect occurring during several sensitive neurodevelopmental windows, have been associated with similar neurocognitive impairments and behavioural problems and with risk of psychiatric and metabolic diseases later in life [18,19,20]. Some of these associations appear to be sex- and/or age-specific [20].
Research has suggested that stress affects development of brain regions crucial for many aspects of cognition and behaviour through increased glucocorticoid signalling that, when prolonged or excessive, can induce abnormal DNA methylation within different levels of the HPA-axis and glucocorticoid signalling [21,22,23,24]. More specifically, such DNA-methylation changes have been reported for genes encoding corticotropin-releasing hormone (CRH) and its receptor CRHR1, arginine vasopressin (AVP) and its receptor AVPR1b, pro-opiomelanocortin (POMC), corticotropin receptor (MC2R), glucocorticoid receptor GRα (NR3C1) and for the GRα-regulated chaperone protein FK506 binding protein 51 (FKBP5) [23, 25,26,27,28,58,59] and exposure to endocrine-disrupting plasticisers leaching from indwelling medical devices [60,61,62]. Also, as critically ill patients are unable to eat, artificial feeding is often provided [63]. Feeding management of critically ill children has been shown to affect the DNA methylome up until PICU discharge [41, 42]. Since prolonged or excessive stress can induce abnormal DNA methylation within different levels of the HPA-axis and glucocorticoid signalling in the context of other adverse early-life exposures [21,22,23,24, 59], the development of such abnormalities after paediatric critical illness seemed plausible. Our present findings are indeed in line with those from studies on the impact of other forms of early-life stress which revealed mostly hypermethylation in NR3C1 and hypomethylation in FKBP5 within the gene body (intron 7) and the promotor [64, 65]. Via research performed in humans in combination with experiments in cells, it has previously been shown that stress reduces methylation in the FKBP5 gene, interestingly at a CpG site which we also found to be hypomethylated, whereby FKBP5 expression was found to be upregulated [66]. Of importance in the context of critical illness, a study in mice revealed that lipopolysaccharide injection increased FKBP5 expression in the hippocampus, driving increased neuroinflammation [67]. Whether the stress-induced rise in endogenous glucocorticoids mediated this effect is currently unclear. However, in patients with Cushing syndrome, it has been shown that long standing excessive endogenous hypercortisolism induces hypomethylation in FKBP5, explaining their long-lasting psychopathological sequelae [24]. In experimental models, also exogenous hypercortisolism has shown to reduce DNA methylation in FKBP5 in neuronal cells, and in vivo this was associated with increased FKBP5 expression across several brain regions [68]. These data provided a plausible explanation for our finding that hypomethylation in the FKBP5 gene was aggravated in patients who 2 years earlier had been treated with glucocorticoids in the PICU, taking into account potential confounders affecting the need of glucocorticoid treatment. It currently remains unclear what brings about the hypomethylation, though downregulated DNA methyltransferase-1 and cleavage of the DNA backbone directly by the GRα have been suggested to play a role [39, 69,70,71]. Some of the differentially methylated CpG sites that we found within NR3C1 and FKBP5 have also been described with other forms of stress. For example, cg15910486 within the promotor/5’UTR of NR3C1 was reported to be hypermethylated in children who experienced early-life adverse events; cg23416081 and cg15929276 located within the 5’UTR region of FKBP5 have previously been shown to alter cortisol reactivity and behaviour in children; cg20813374 located in the promotor/5’UTR of FKBP5 is a known stress-related epigenetic signature previously associated with myocardial infarction and inflammation; and cg03546163 in the 5’UTR of FKBP5 was shown to be differentially methylated in the context of Cushing’s syndrome [24, 65, 66, 72].
Also glucocorticoid metabolism can be altered by early-life stress [33]. We here found that genes encoding cortisol metabolising enzymes were differently methylated in former PICU patients, more specifically for 5α- and 5β-reductase and 11β-HSD1. These changes could again be partly explained by glucocorticoid treatment during PICU stay years before. The methylation differences within HSD11B1, SRD5A1 and AKR1D1 may result in altered systemic cortisol availability and hereby affect development as was suggested by our functional outcome analysis. The HSD11B1 hypomethylation we observed in former patients appeared to some extent protective against rather than contributing to some of the developmental impairments. However, a strong harmful association was found between most functional outcome measures and the abnormal DNA methylation in AKRD1, which encodes the 5β-reductase enzyme, an association that has not been reported before.
We also observed altered DNA methylation in former patients within the genes encoding CRH receptor, ACTH and its receptor MC2R, and three GR-regulated proteins, DUSP1, GILZ and TNFα, which has also been reported for other conditions of early-life stress [26, 28,20, 73]. Higher vulnerability to illness-induced DNA-methylation changes in older than in younger children, in particular from age of adrenarche onwards and into puberty, has previously been shown by our group [74].
This study has some strengths and weaknesses to highlight. The large sample size and the multicentre, prospective study design with predefined long-term assessments of former PICU patients and healthy children were strengths. In addition, our methodology applying tenfold cross-validation over 100 iterations reduced the odds of findings by chance and reduced the impact of outliers. Our study also has some limitations. First, we have studied DNA methylation in buccal mucosa, whereas the HPA-axis links well-defined brain structures with the adrenal cortex via hormonal regulation. Studying the DNA-methylation markers of stress is obviously not possible in the physiological tissues and organs where it exerts its effects through the HPA-axis. It was thus for pragmatic reasons that we analysed buccal mucosa, as these epithelial cells are accessible to clinical research. These cells are also exposed to stress hormones, but we do not know whether the methylation changes observed in these cells reflect those supposed to occur in the HPA-axis itself. This difficulty has been encountered in other studies, where blood cells or buccal cells have been used as proxies to physiological tissues [23,24,25, 29, 31, 40,41,42,43, 66]. Second, due to lack of a sample before PICU admission, we cannot discriminate between pre-existing abnormal methylation and abnormal methylation induced by the critical illness. Third, although we preferentially recruited siblings and relatives of the patients to the control group, extended with unrelated children from the same geographical area and adjusted as much as possible for baseline risk factors, we cannot exclude residual confounding by genetic background and environment. Fourth, the treatment with glucocorticoids during PICU stay had not been randomised, arguing for caution when interpreting these results. Indeed, specific conditions triggering the need for glucocorticoid treatment theoretically may confound these results. However, with the extensive adjustment for risk factors which also included type, severity and duration of illness we aimed to reduce as much as possible the impact of this limitation. Nevertheless, we cannot exclude that there may be some residual unmeasured confounding in these and the other multivariable analyses. Finally, we were not able to assess what effect the abnormal DNA methylation might have on gene transcription, nor on cortisol or ACTH levels, as we did not have the samples for these analyses. However, differential methylation in the order of magnitude as observed in the present study was associated with differential gene expression in our earlier study on DNA methylation in muscle of adult critically ill patients and controls [75].
Conclusions
Two years after critical illness in children, buccal mucosa DNA revealed abnormal methylation of CpG sites within genes of the HPA-axis, most extensively within the FKBP5 and AKR1D1 genes, which occurred largely independent of sex and age. In addition, glucocorticoid treatment while in the PICU was found to be associated with an aggravation of the methylation changes in FKBP5 and AKR1D1 detected 2 years later. The observed abnormal DNA methylation within these genes in former PICU patients statistically explained part of the long-term physical and neurocognitive developmental impairments. These findings call for attention regarding safety of a liberal glucocorticoid use in the PICU in the absence of strong underlying evidence of benefit.
Availability of data and materials
Data sharing is offered under the format of collaborative projects. Proposals can be directed to the corresponding author.
Abbreviations
- 11βHSD:
-
11β-Hydroxysteroid dehydrogenase
- ANXA1:
-
Annexin A1
- AVP:
-
Arginine vasopressin
- AVPR1b:
-
Arginine vasopressin receptor 1b
- CRH:
-
Corticotropin-releasing hormone
- CRHR1:
-
Corticotropin-releasing hormone receptor 1
- DMP:
-
Differentially methylated position
- DMR:
-
Differentially methylated region
- DUSP1:
-
Dual-specificity phosphatase-1
- FDR:
-
False discovery rate
- FKBP5:
-
FK506 binding protein 51
- GILZ:
-
Glucocorticoid-induced leucine zipper
- GRE:
-
Glucocorticoid-responsive element
- ICU:
-
Intensive care unit
- MC2R:
-
Corticotropin receptor
- NR3C1:
-
Glucocorticoid receptor GRα
- PCSK1:
-
Pro-protein convertase-1
- PeLOD:
-
Paediatric logistic organ dysfunction score
- PEPaNIC:
-
Paediatric Early versus Late Parenteral Nutrition in Intensive Care Unit
- PICU:
-
Paediatric intensive care unit
- PIM3:
-
Paediatric index of mortality 3 score
- POMC:
-
Pro-opiomelanocortin
- RCT:
-
Randomised controlled trial
- STRONGkids:
-
Screening Tool for Risk on Nutritional Status and Growth for kids
- TNF:
-
Tumour necrosis factor-α
- UTR:
-
Untranslated region
References
Jacobs A, Derese I, Vander Perre S, Wouters PJ, Verbruggen S, Billen J, et al. Dynamics and prognostic value of the hypothalamus–pituitary–adrenal axis responses to pediatric critical illness and association with corticosteroid treatment: a prospective observational study. Intensive Care Med. 2020;46:70–81.
Jacobs A, Derese I, Vander Perre S, van Puffelen E, Verstraete S, Pauwels L, et al. Non-thyroidal illness syndrome in critically ill children: prognostic value and impact of nutritional management. Thyroid. 2019;29:480–92.
Gielen M, Mesotten D, Brugts M, Coopmans W, Van Herck E, Vanhorebeek I, et al. Effect of intensive insulin therapy on the somatotropic axis of critically ill children. J Clin Endocrinol Metab. 2011;96:2558–66.
Van Dyck L, Derese I, Vander Perre S, Wouters PJ, Casaer MP, Hermans G, et al. The GH axis in relation to accepting an early macronutrient deficit and outcome of critically ill patients. J Clin Endocrinol Metab. 2019;104:5507–18.
Gardelis JG, Hatzis TD, Stamogiannou LN, Dona AA, Fotinou AD, Brestas PS, Constantopoulos AG. Activity of the growth hormone/insulin-like growth factor-I axis in critically ill children. J Pediatr Endocrinol Metab. 2005;18:363–72.
Teblick A, Peeters B, Langouche L, Van den Berghe G. Adrenal function and dysfunction in critically ill patients. Nat Rev Endocrinol. 2019;15:417–27.
Langouche L, Téblick A, Gunst J, Van den Berghe G. The hypothalamus-pituitary-adrenocortical response to critical illness: a concept in need of revision. Endocr Rev. 2023;44:1096–106.
Poomthavorn P, Lertbunrian R, Preutthipan A, Sriphrapradang A, Khlairit P, Mahachoklertwattana P. Serum free cortisol index, free cortisol, and total cortisol in critically ill children. Intensive Care Med. 2009;35:1281–5.
Aydin BK, Demirkol D, Bas F, Turkoglu U, Kumral A, Karabocuoglu M, et al. Evaluation of endocrine function in children admitted to pediatric intensive care unit. Pediatr Int. 2014;56:349–53.
Peeters B, Meersseman P, Vander Perre S, Wouters PJ, Vanmarcke D, Debaveye Y, et al. Adrenocortical function during prolonged critical illness and beyond: a prospective observational study. Intensive Care Med. 2018;44:1720–9.
Boonen E, Vervenne H, Meersseman P, Andrew R, Mortier L, Declercq PE, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368:1477–88.
Verstraete S, Verbruggen SC, Hordijk JA, Vanhorebeek I, Dulfer K, Guiza F, et al. Long-term developmental effects of withholding parenteral nutrition for 1 week in the paediatric intensive care unit: a 2-year follow-up of the PEPaNIC international, randomised, controlled trial. Lancet Respir Med. 2019;7:141–53.
Jacobs A, Dulfer K, Eveleens RD, Hordijk J, Van Cleemput H, Verlinden I, et al. Long-term developmental effect of withholding parenteral nutrition in paediatric intensive care units: a 4-year follow-up of the PEPaNIC randomised controlled trial. Lancet Child Adolesc Health. 2020;4:503–14.
Watson RS, Choong K, Colville G, Crow S, Dervan LA, Hopkins RO, Knoester H, Pollack MM, Rennick J, Curley MAQ. Life after critical illness in children-toward an understanding of pediatric post-intensive care syndrome. J Pediatr. 2018;198:16–24.
Watson RS, Beers SR, Asaro LA, Burns C, Koh MJ, Perry MA, Angus DC, Wypij D, Curley MAQ, RESTORE-Cognition Investigators. Association of acute respiratory failure in early childhood with long-term neurocognitive outcomes. JAMA. 2022;327:836–45.
Hordijk JA, Verbruggen SC, Buysse CM, Utens EM, Joosten KF, Dulfer K. Neurocognitive functioning and health-related quality of life of children after pediatric intensive care admission: a systematic review. Qual Life Res. 2022;31:2601–14.
Perry-Eaddy M, Dervan LA, Manning JC, Watson RS, Curley MAQ. Pediatric critical care outcomes: state of the science. Crit Care Clin. 2023;39:309–26.
Facchi JC, Lima TAL, Oliveira LR, Costermani HO, Miranda GDS, de Oliveira JC. Perinatal programming of metabolic diseases: the role of glucocorticoids. Metabolism. 2020;104:154047.
Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–9.
van Bodegom M, Homberg JR, Henckens M. Modulation of the hypothalamic-pituitary-adrenal axis by early life stress exposure. Front Cell Neurosci. 2017;11:87.
Featherstone RE, Gifford RL, Crown LM, Amirfathi F, Alaniz JP, Yi J, et al. Early life social instability stress causes lasting cognitive decrement and elevated hippocampal stress-related gene expression. Exp Neurol. 2022;354:114099.
Fitzgerald E, Sinton MC, Wernig-Zorc S, Morton NM, Holmes MC, Boardman JP, et al. Altered hypothalamic DNA methylation and stress-induced hyperactivity following early life stress. Epigenetics Chromatin. 2021;14:31.
Yehuda R, Daskalakis NP, Bierer LM, Bader HN, Klengel T, Holsboer F, et al. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol Psychiatry. 2016;80:372–80.
Glad CA, Andersson-Assarsson JC, Berglund P, Bergthorsdottir R, Ragnarsson O, Johannsson G. Reduced DNA methylation and psychopathology following endogenous hypercortisolism: a genome-wide study. Sci Rep. 2017;7:44445.
Wiechmann T, Roh S, Sauer S, Czamara D, Arloth J, Kodel M, et al. Identification of dynamic glucocorticoid-induced methylation changes at the FKBP5 locus. Clin Epigenetics. 2019;11:83.
Lewis CR, Breitenstein RS, Henderson A, Sowards HA, Piras IS, Huentelman MJ, et al. Harsh parenting predicts novel HPA receptor gene methylation and NR3C1 methylation predicts cortisol daily slope in middle childhood. Cell Mol Neurobiol. 2021;41:783–93.
Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–66.
Wu Y, Patchev AV, Daniel G, Almeida OF, Spengler D. Early-life stress reduces DNA methylation of the Pomc gene in male mice. Endocrinology. 2014;155:1751–62.
Palma-Gudiel H, Prather AA, Lin J, Oxendine JD, Guintivano J, **a K, et al. HPA axis regulation and epigenetic programming of immune-related genes in chronically stressed and non-stressed mid-life women. Brain Behav Immun. 2021;92:49–56.
Tyrka AR, Ridout KK, Parade SH. Childhood adversity and epigenetic regulation of glucocorticoid signaling genes: associations in children and adults. Dev Psychopathol. 2016;28:1319–31.
Kertes DA, Kamin HS, Hughes DA, Rodney NC, Bhatt S, Mulligan CJ. Prenatal maternal stress predicts methylation of genes regulating the hypothalamic-pituitary-adrenocortical system in mothers and newborns in the democratic republic of Congo. Child Dev. 2016;87:61–72.
Jensen Pena C, Monk C, Champagne FA. Epigenetic effects of prenatal stress on 11beta-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE. 2012;7:e39791.
Yehuda R, Seckl J. Minireview: Stress-related psychiatric disorders with low cortisol levels: a metabolic hypothesis. Endocrinology. 2011;152:4496–503.
Green BB, Armstrong DA, Lesseur C, Paquette AG, Guerin DJ, Kwan LE, Marsit CJ. The role of placental 11-beta hydroxysteroid dehydrogenase type 1 and type 2 methylation on gene expression and infant birth weight. Biol Reprod. 2015;92:149.
Nagarajan S, Seddighzadeh B, Baccarelli A, Wise LA, Williams M, Shields AE. Adverse maternal exposures, methylation of glucocorticoid-related genes and perinatal outcomes: a systematic review. Epigenomics. 2016;8:925–44.
Araki R, Nishida S, Hiraki Y, Matsumoto K, Yabe T. DNA methylation of the GC box in the promoter region mediates isolation rearing-induced suppression of srd5a1 transcription in the prefrontal cortex. Neurosci Lett. 2015;606:135–9.
Munetsuna E, Yamada H, Yamazaki M, Ando Y, Mizuno G, Hattori Y, et al. Maternal high-fructose intake increases circulating corticosterone levels via decreased adrenal corticosterone clearance in adult offspring. J Nutr Biochem. 2019;67:44–50.
Li H, Liu J, Sun Y, Wang W, Weng S, **ao S, et al. N-hexane inhalation during pregnancy alters DNA promoter methylation in the ovarian granulosa cells of rat offspring. J Appl Toxicol. 2014;34:841–56.
Zannas AS, Wiechmann T, Gassen NC, Binder EB. Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology. 2016;41:261–74.
Lebow MA, Schroeder M, Tsoory M, Holzman-Karniel D, Mehta D, Ben-Dor S, et al. Glucocorticoid-induced leucine zipper “quantifies” stressors and increases male susceptibility to PTSD. Transl Psychiatry. 2019;9:178.
Güiza F, Vanhorebeek I, Verstraete S, Verlinden I, Derese I, Ingels C, et al. Effect of early parenteral nutrition during paediatric critical illness on DNA methylation as a potential mediator of impaired neurocognitive development: a pre-planned secondary analysis of the PEPaNIC international randomised controlled trial. Lancet Respir Med. 2020;8:288–303.
Verlinden I, Güiza F, Derese I, Wouters PJ, Joosten K, Verbruggen SC, et al. Time course of altered DNA methylation evoked by critical illness and by early administration of parenteral nutrition in the paediatric ICU. Clin Epigenetics. 2020;12:155.
Coppens G, Vanhorebeek I, Verlinden I, Derese I, Wouters PJ, Joosten KF, et al. Assessment of aberrant DNA methylation two years after paediatric critical illness: a pre-planned secondary analysis of the international PEPaNIC trial. Epigenetics. 2023;18:2146966.
Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, et al. Early versus Late parenteral nutrition in critically ill children. N Engl J Med. 2016;374:1111–22.
Team RC. R: a language and environment for statistical computing R foundation for statistical computing. 2019. https://www.r-project.org.
LICMEpigenetics Package GitHub. 2022. https://github.com/LICMLeuven/LICMEpigenetics. Accessed 23 June 2023.
Lehne B, Drong AW, Loh M, Zhang WH, Scott WR, Tan ST, et al. A coherent approach for analysis of the Illumina HumanMethylation450 BeadChip improves data quality and performance in epigenome-wide association studies. Genome Biol. 2015;16:12.
Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–75.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Peters TJ, Buckley MJ, Statham AL, Pidsley R, Samaras K, Lord RV, et al. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin. 2015;8:16.
Cinar O, Viechtbauer W. The poolr package for combining independent and dependent p values. J Stat Softw. 2022;101:1–42.
Mohamed SA, Badawi NE, AbdelRasol HA, AbdelAziz HM, Khalaf NA, Yousef RM. Impaired pancreatic β-cell function in critically ill children. Front Pediatr. 2021;9:603361.
Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–56.
Van den Berghe G, Vanhorebeek I, Langouche L, Gunst J. Our scientific journey through the ups and downs of blood glucose control in the ICU. Am J Respir Crit Care Med. 2024. https://doi.org/10.1164/rccm.202309-1696SO.
Čugalj Kern B, Trebušak Podkrajšek K, Kovač J, Šket R, Jenko Bizjan B, Tesovnik T, et al. The role of epigenetic modifications in late complications in type 1 diabetes. Genes (Basel). 2022;13:705.
Mannar V, Boro H, Patel D, Agstam S, Dalvi M, Bundela V. Epigenetics of the pathogenesis and complications of type 2 diabetes mellitus. touchREV Endocrinol. 2023;19:46–53.
Martinez ME, Stohn JP, Mutina EM, Whitten RJ, Hernandez A. Thyroid hormone elicits intergenerational epigenetic effects on adult social behavior and fetal brain expression of autism susceptibility genes. Front Neurosci. 2022;16:1055116.
Schwarzenbach H. Impact of physical activity and do** on epigenetic gene regulation. Drug Test Anal. 2011;3:682–7.
Nicolaides NC, Kanaka-Gantenbein C, Pervanidou P. Developmental neuroendocrinology of early-life sress: impact on child development and behavior. Curr Neuropharmacol. 2024;22:461–74.
Verstraete S, Vanhorebeek I, Covaci A, Güiza F, Malarvannan G, Jorens PG, et al. Circulating phthalates during critical illness in children are associated with long-term attention deficit: a study of a development and a validation cohort. Intensive Care Med. 2016;42:379–92.
Vanhorebeek I, Malarvannan G, Güiza F, Poma G, Derese I, Wouters PJ, et al. Phasing out DEHP from plastic indwelling medical devices used for intensive care: does it reduce the long-term attention deficit of critically ill children? Environ Int. 2022;158:106962.
Miura R, Ikeda-Araki A, Ishihara T, Miyake K, Miyashita C, Nakajima T, et al. Effect of prenatal exposure to phthalates on epigenome-wide DNA methylations in cord blood and implications for fetal growth: the Hokkaido Study on Environment and Children’s Health. Sci Total Environ. 2021;783:147035.
Gunst J, Casaer MP, Preiser JC, Reignier J, Van den Berghe G. Toward nutrition improving outcome of critically ill patients: how to interpret recent feeding RCTs? Crit Care. 2023;27:43.
Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41.
Cicchetti D, Handley ED. Methylation of the glucocorticoid receptor gene, nuclear receptor subfamily 3, group C, member 1 (NR3C1), in maltreated and nonmaltreated children: associations with behavioral undercontrol, emotional lability/negativity, and externalizing and internalizing symptoms. Dev Psychopathol. 2017;29:1795–806.
Zannas AS, Jia M, Hafner K, Baumert J, Wiechmann T, Pape JC, et al. Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-kappaB-driven inflammation and cardiovascular risk. Proc Natl Acad Sci U S A. 2019;116:11370–9.
Gan YL, Wang CY, He RH, Hsu PC, Yeh HH, Hsieh TH, et al. FKBP51 mediates resilience to inflammation-induced anxiety through regulation of glutamic acid decarboxylase 65 expression in mouse hippocampus. J Neuroinflamm. 2022;19:152.
Cox OH, Song HY, Garrison-Desany HM, Gadiwalla N, Carey JL, Menzies J, et al. Characterization of glucocorticoid-induced loss of DNA methylation of the stress-response gene Fkbp5 in neuronal cells. Epigenetics. 2021;16:1377–97.
Kress C, Thomassin H, Grange T. Active cytosine demethylation triggered by a nuclear receptor involves DNA strand breaks. Proc Natl Acad Sci U S A. 2006;103:11112–7.
Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406.
Yang X, Ewald ER, Huo Y, Tamashiro KL, Salvatori R, Sawa A, et al. Glucocorticoid-induced loss of DNA methylation in non-neuronal cells and potential involvement of DNMT1 in epigenetic regulation of Fkbp5. Biochem Biophys Res Commun. 2012;420:570–5.
Mulder RH, Rijlaarsdam J, Luijk MP, Verhulst FC, Felix JF, Tiemeier H, et al. Methylation matters: FK506 binding protein 51 (FKBP5) methylation moderates the associations of FKBP5 genotype and resistant attachment with stress regulation. Dev Psychopathol. 2017;29:491–503.
Buckingham JC, John CD, Solito E, Tierney T, Flower RJ, Christian H, et al. Annexin 1, glucocorticoids, and the neuroendocrine-immune interface. Ann N Y Acad Sci. 2006;1088:396–409.
Verlinden I, Coppens G, Vanhorebeek I, Guiza F, Derese I, Wouters PJ, et al. Long-term impact of paediatric critical illness on the difference between epigenetic and chronological age in relation to physical growth. Clin Epigenetics. 2023;15:8.
Van Dyck L, Güiza F, Derese I, Pauwels L, Casaer MP, Hermans G, et al. DNA methylation alterations in muscle of critically ill patients. J Cachexia Sarcopenia Muscle. 2022;13:1731–40.
Acknowledgements
The computational resources and services used in this work were provided by the VSC (Flemish Supercomputer Centre), funded by the Research Foundation—Flanders (FWO) and the Flemish Government—department EWI. The authors acknowledge the research team members involved in the study for their help with the technical and administrative support. Furthermore, they thank the children and their parents for their willingness to participate in the study.
Funding
This work was supported by European Research Council Advanced Grants (AdvG-2012-321670 and AdvG-2017-785809) to Greet Van den Berghe; by the Methusalem program of the Flemish government (through the University of Leuven to Greet Van den Berghe and Ilse Vanhorebeek, METH14/06) and by the Institute for Science and Technology, Flanders, Belgium (through the University of Leuven to Greet Van den Berghe, IWT/110685/TBM and IWT/150181/TBM); by the Sophia Research Foundation (SSWO) to Sascha Verbruggen; by the ‘Stichting Agis Zorginnovatie’ to Sascha Verbruggen and by a European Society for Clinical Nutrition and Metabolism (ESPEN) research grant to Sascha Verbruggen.
Author information
Authors and Affiliations
Contributions
GC, IV, FG, AT and GVdB designed the study. IV, ID, PJW, KD, KFJ and SCV gathered data. GC, IV, FG and GVdB analysed the data and wrote the manuscript, which was reviewed and approved by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional review boards at each participating site approved this follow-up study (Ethische Commissie Onderzoek UZ Leuven/KU Leuven: ML8052; Medische Ethische Toetsingscommissie Erasmus MC: NL49708.078). The study was performed in accordance with the 1964 Declaration of Helsinki and its amendments. Written informed consent was obtained from the parents or legal guardians, or from the children if 18 years or older.
Consent for publication
Not applicable.
Competing interests
We declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Compiled file with all Additional information: Additional Methods describing the motivation of risk factors adjusted for in multivariable analyses, the definition of ‘Syndrome’ and a stepwise explanation of the DMRcate method for the identification of differentially methylated DNA regions, and detailed description of the outcome measures evaluated at the PEPaNIC 2-year follow-up; Additional Figures showing the CONSORT diagram of study participants, univariate boxplots of the methylation status of differentially methylated positions in former PICU patients as compared with matched healthy children, and univariate boxplots of the methylation status of the CpG sites within the regions identified as differentially methylated between former PICU patients and matched healthy children; and Additional Tables reporting on the DMP and DMR analyses for former PICU patients versus healthy children, interaction of differential methylation in former PICU patients versus healthy children with sex and age at exposure, and the analyses of differential methylation between former PICU patients who received glucocorticoids during their stay in the PICU versus those who did not.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Coppens, G., Vanhorebeek, I., Güiza, F. et al. Abnormal DNA methylation within HPA-axis genes years after paediatric critical illness. Clin Epigenet 16, 31 (2024). https://doi.org/10.1186/s13148-024-01640-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13148-024-01640-y