Abstract
Background
Neuropsychiatric involvement in systemic lupus erythematosus (SLE) is a common clinical manifestation. In SLE patients, cerebral function is a more sensitive predictor of central nervous system damage, and abnormalities in cerebral function may be apparent before substantial neuropsychiatric symptoms occur. The 5-hydroxynyptamine(5-HT) system has the ability to interact with the majority of the neurochemical systems in the central nervous system (CNS), influencing brain function. Serotonin transporter gene-linked polymorphic region (5-HTTLPR) is an essential element of the 5-HT system gene polymorphism and is directly related to the control of 5-hydroxytryptamine transporter (5-HTT)gene expression. The relationship between 5-HTTLPR and functional brain measurements in SLE patients requires more investigation because it is one of the most attractive imaging genetics targets for shedding light on the pathophysiology of neuropsychiatric lupus.
Methods
Resting-state functional magnetic resonance imaging (rs-fMRI) images were collected from 51 SLE patients without obvious neuropsychiatric manifestations and 44 healthy volunteers. Regional homogeneity (ReHo), amplitude of low-frequency fluctuations (ALFF), and fractional amplitude of low-frequency fluctuations (fALFF) were selected as indicators for evaluating brain function. In accordance with the Anatomical Automatic Labeling template, the gray matter was divided into 116 regions. The mean ReHo value, mean ALFF value, and mean fALFF value of each brain region were extracted. 5-HTTLPR genotypes of all research objects were tested by polymerase chain reaction and agarose gel electrophoresis. Two-way analysis of covariance was used to investigate whether there is an interaction effect between SLE disease status and 5-HTTLPR genotype on resting-state brain function.
Results
In SLE patients with S/S homozygosity, there were notably lower mean ReHo, mean ALFF, and mean fALFF values observed in the right parietal, inferior angular gyrus, and the right paracentral lobule compared to healthy controls. However, this distinction was not evident among carriers of the L allele. Within the S/S genotype, SLE patients exhibited decreased mean ReHo in the left posterior cingulate gyrus, reduced mean fALFF in the left caudate nucleus, and diminished mean ALFF in the left temporal pole: superior temporal gyrus, in contrast to the HC group. Conversely, no such differences were discerned among carriers of the L allele. Notably, among L allele carriers, SLE patients displayed a higher mean ReHo value in the right hippocampus compared to the HC group, while demonstrating a lower mean ALFF value in the left medial and paracingulate gyrus in contrast to the HC group. Conversely, these differences were not apparent among S/S homozygotes.
Conclusions
Brain function in the right parietal and inferior angular gyrus and the right paracentral lobule is affected by the interaction effect of SLE disease status and 5-HTTLPR genotype.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is an autoimmune disease that causes production of many autoantibodies due to aberrant immune system activation, culminating in the numerous organ damage [1]. One of the most typically damaged organs in SLE is the nervous system, and the accompanying central and peripheral nervous system dysfunction is known as neuropsychiatric systemic lupus erythematosus (NPSLE). According to published research, it affects between 12 and 95% of SLE patients [2]. The vast diversity of presentations of NPSLE, from typical headaches, cognitive abnormalities, and mood disorders to unusual manifestations such as Guillain–Barre syndrome and autonomic dysfunction, is one of the obstacles doctors frequently confront in diagnosing and managing patients with NPSLE [3]. In SLE patients, cerebral function is a more sensitive predictor of central nervous system damage, and abnormalities in cerebral function may be apparent before substantial neuropsychiatric symptoms occur.
Resting-state functional magnetic resonance imaging (rs-fMRI) is a noninvasive imaging technique that uses a blood oxygenation level-dependent (BOLD) signal to investigate brain function in a variety of central nervous system (CNS) diseases such as Alzheimer’s disease, Parkinson’s disease, depression, schizophrenia, and others [4,5,6,7]. Increasing amounts of study have used functional magnetic resonance imaging (fMRI)to investigate alterations in brain function in SLE patients and have revealed brain functional abnormalities even in non-NPSLE patients [8, 1.
Rs-fMRI acquisition
The MRI scans for all subjects were conducted by a highly experienced radiologist, utilizing the same 1.5 T MRI scanner manufactured by General Electric (Twinspeed; GE Medical Systems, Milwaukee, WI, USA). Initially, plain scanning including routine T1-weighted image and T2-weighted image was conducted to rule out any intracranial organic lesions. An echo planner imaging sequence scan was used with the following parameters: repetition time = 2000 ms, echo time = 40 ms, thickness = 5 mm with an interslice gap of 1 mm, field of view = 240 mm × 240 mm, matrix size = 64 × 64, flip angle = 90°, number of excitation = 2.00, number of layers = 24, time point = 160. The total fMRI scan time was 320 s.
The rs-fMRI data was preprocessed on a DPARSF software based on the MatlabR2016a platform. As per the Anatomical Automatic Labeling template from MNI, the gray matter in each subject's brain was segmented into 116 regions. Among these, the initial 90 regions corresponded to the brain's gray matter, while the remaining 26 regions represented the gray matter of the cerebellum. The rs-fMRI data processing toolkit REST V1.8 was used to extract the mean ReHo value, mean ALFF value, and mean fALFF value of each region.
Demographics and psychological assessment
The details of sex, age, weight, height, medical history, family medical background, personal history, and habitual hand use were recorded for both SLE patients and HC. Lupus disease activity was assessed using the SLE disease activity index-2000 version (SLEDAI-2000) [15]. We used Hamilton Depression Scale (HAMD) [16] and Hamilton Anxiety Scale (HAMA) [17] to assess levels of depression and anxiety in SLE patients. Scores on the two scales were recorded and evaluated by two systematically trained physicians and achieved good inter-examiner reliability after systematic training.
Statistical analysis
Statistical software IBM SPSS Statistics 21 (IBM Inc. Armonk, NY, USA) was used for data analysis. Quantitative data following normal distribution were expressed as \(\overline{x }\)±s, and t test was used for between-group comparison; otherwise, the quantitative data of non-normal distribution data was expressed as M (P25%, P75%), and the Mann–Whitney U test was used for between-group comparison of two groups. The chi-square test was chosen when analyzing qualitative data. When P < 0.05, the difference was considered to be statistically significant.
The mean ReHo, mean ALFF, and mean fALFF values of each brain region were used as dependent variables, whereas diagnosis of lupus (SLE vs HC) and 5-HTTLPR genotype (L allele carriers vs S allele homozygous) were used as independent variables and age as a covariate for a two-way analysis of covariance to investigate an interaction between disease status and 5-HTTLPR genotype on resting-state brain function. If the strength of the interaction was statistically significant, post hoc analysis (Bonferroni method) was used to compare the differences between subgroups. When P < 0.05, the difference was considered to be statistically significant.
Results
Hardy–Weinberg genetic equilibrium test
According to the inclusion and exclusion criteria established in this study, a total of 51 SLE patients without obvert neuropsychiatric manifestations and 44 healthy controls were included. In order to test population representativeness of the two groups, Hardy–Weinberg genetic equilibrium test was used. It showed that both groups are in line with the Hardy–Weinberg genetic equilibrium law (Table 1), indicating that both samples are representative of the population.
General information, 5-HTTLPR genotype, and allele frequency
The gender distribution, age demographics, and educational levels within both the SLE and HC groups displayed no statistically significant variations (Table 2). In the SLE cohort, 26 individuals carried the L allele (3 L/L, 23 L/S), while 25 individuals were S/S homozygotes, resulting in L allele and S allele frequencies of 28.43% and 71.57%, respectively. Within the HC group, there were 21 L allele carriers (4 L/L, 17 L/S), alongside 23 S/S homozygotes, reflecting L allele and S allele frequencies of 28.41% and 71.59%, respectively. Notably, there were no statistically significant discrepancies observed in the 5-HTTLPR genotype or allele frequencies between the SLE and HC groups (Table 2). These findings indicate a congruence in the genetic backgrounds of the 5-HTTLPR gene in both cohorts, suggesting comparability in brain functionality.
Clinical parameters in SLE patients with different 5-HTTLPR genotypes
SLE patients were grouped according to 5-HTTLPR genotype. Because the frequency of L/L genotype is very low, this study alloted L allele carriers (L/L + L/S) into one group. The general data such as gender, age, and clinical data such as SLE disease activity and mental scale were compared between the L allele carrier group and the S/S genotype group. No significant differences in gender, age, education level, SLEDAI, HAMA, and HAMD scores were seen between the two groups (Table 3).
Gray matter function in SLE patients with different 5-HTTLPR genotypes
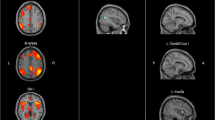
The mean ReHo value, mean ALFF value, and mean fALFF value of each gray matter area in the L allele carrier group and the S/S group in SLE patients were compared. The results showed that in SLE patients, the ReHo values of the right parietal and inferior angular gyrus, right hippocampus, and right posterior cingulate gyrus of the S/S group were lower than those of the L allele carriers; the ALFF values of the bilateral hippocampus were lower than those of the L allele carriers; the fALFF values of the right parietal and inferior angular gyrus, bilateral hippocampus, left posterior cingulate gyrus, and right lingual gyrus were lower than those of the L allele carriers (Table 4, Fig. 2).
Gray matter function in SLE patients with different 5-HTTLPR genotypes. A ReHo, Red: Parietal_Inf_R, Orange: Hippocampus_R, Kelly: Cingulum_Post_R; B ALFF, Red: Hippocampus_L, Orange: Hippocampus_R; C fALFF, Orange: Hippocampus_L, Green: Cingulum_Post_L, Red: Parietal_Inf_R, Kelly: Hippocampus_R, Blue: Lingual_R; L: left; R: right
Interaction between 5-HTTLPR gene and SLE disease state on brain function
The outcomes indicated that the mean ReHo, ALFF, and fALFF values within individual cerebellar gray matter regions remained unaffected by the interplay between SLE disease status and the 5-HTTLPR genotype. Among 90 Gy matter regions, the mean ReHo, mean ALFF, and mean fALFF values of the right parietal inferior angular gyrus and the right paracentral lobule were all affected by the interplay between SLE disease status and 5-HTTLPR genotype (Table 5, Fig. 3). Post hoc test analysis found that in the S/S genotype, the mean ReHo, mean ALFF, and mean fALFF values of the right parietal and inferior angular gyrus and right paracentral lobule of SLE patients were lower than those of the HC group, while such difference was not found among L allele carriers.
5-HTTLPR interacts with SLE disease state on gray matter function. A ReHo, Kelly: Cingulum_Post_L, Red: Parietal_Inf_R, Orange: Hippocampus_R, Green: Paracentral_Lobule_R; B ALFF, Orange: Cingulum_Mid_L, Kelly: Temporal_Pole_Sup_L, Red: Parietal_Inf_R, Green: Paracentral_Lobule_R; C fALFF, Orange: Caudate_L, Red: Parietal_Inf_R, Kelly: Paracentral_Lobule_R; L: left; R: right
In addition, the mean ReHo value of the left posterior cingulate gyrus, the mean fALFF value of the left caudate nucleus, and the mean ALFF value of the left temporal pole: superior temporal gyrus were all affected by the interaction effect of SLE disease status and 5-HTTLPR genotype (Table 5, Fig. 3). Post hoc analysis found that in the S/S genotype, the mean ReHo value of the left posterior cingulate gyrus, the mean fALFF value of the left caudate nucleus, and the mean ALFF value of the left temporal pole: superior temporal gyrus of the SLE patients were lower than those of the HC group, while such difference was not found among L allele carriers. The mean ReHo values in the right hippocampus and the mean ALFF values in the left medial and paracingulate gyrus were all affected by the interaction between SLE disease status and the 5-HTTLPR genotype (Table 5, Fig. 3). Post hoc analysis found that among the L allele carriers, the mean ReHo value of the right hippocampus of SLE patients was higher than that of the HC group, and the mean ALFF value of the left medial and paracingulate gyrus was lower than that of the HC group, while such difference was not found among the S/S homozygotes.
Discussion
There have been comparatively few studies investigating 5-HTTLPR in the context of SLE. In our study, there were no significant differences observed in the genotype and allele frequency of 5-HTTLPR between the SLE and HC groups. This aligns with the research findings reported by Li et al. [18]. This suggests that there isn't a direct association between genetic variations in 5-HTTLPR and susceptibility to SLE. Furthermore, our investigation found no correlation between genetic variations in 5-HTTLPR, SLE disease activity, depression, or anxiety. Xu et al. revealed that in their study, the average HAMD score among S/S homozygotes in SLE patients was higher than that among L allele carriers [19]. Additionally, within the SLE group, individuals experiencing depression exhibited a higher frequency of the S allele and S/S genotype compared to those without depression [19]. Despite these disparities with our study’s findings, the limited sample size in our investigation suggests the need for a re-evaluation of any conclusions. Confirmatory support through a larger follow-up study is essential.
The observed variance in gray matter functionality between L allele carriers and S/S homozygotes among SLE patients was primarily identified in the right inferior parietal angular gyrus and bilateral hippocampus. The S/S group exhibited poorer brain function indices across all affected brain areas compared to the L allele-carrying group. Our hypothesis attributes these findings to the variance in content and concentration of 5-HT within the synaptic cleft between S/S homozygotes and L allele carriers, given the S allele's association with reduced 5-HTT gene transcription and the L allele's association with increased 5-HTT gene transcription. The interaction between SLE illness severity and the 5-HTTLPR genotype resulted in alterations only in the resting-state brain function markers of specific gray matter brain regions. This interaction notably influenced the mean ReHo, mean ALFF, and mean fALFF values in the right parietal and inferior angular gyrus, along with the right paracentral lobule. This interaction effect highlighted that in individuals with the S/S genotype, the mean ReHo, mean ALFF, and mean fALFF values in the right parietal and inferior angular gyrus, as well as the right paracentral lobule, were lower among SLE patients compared to healthy controls. However, no such discrepancy was observed in L allele carriers. The functional roles of these regions are notable—the parieto-inferior angular gyrus primarily contributes to self-perception, executive functioning, and the integration of emotional and sensory information from facial stimuli [20]. On the other hand, the paracentral lobule governs motor and sensory innervation of the contralateral lower extremity [21], and it plays a role in motor performance, pain processing, and perception [22]. Abnormal paracentral lobular activity has been noted in individuals experiencing chronic pain, impacting sensory pain recognition or assessment [23]. Importantly, there is a lack of research exploring the relationship between the parietal and inferior angular gyrus, paracentral lobules, and 5-HTTLPR gene polymorphisms. This study highlighted the influence of the interplay between SLE disease state and 5-HTTLPR genotype on the three indices of brain function specifically in the right inferior parietal angular gyrus and the right paracentral lobule. It suggests that the 5-HTTLPR genotype modulates the impact of SLE disease on the brain function of these regions—specifically, individuals with the S/S genotype demonstrate a more pronounced effect of SLE disease on the brain function of these areas.
Moreover, the interplay between the SLE disease state and the 5-HTTLPR genotype influenced the mean ReHo value of the left posterior cingulate gyrus, the mean fALFF value of the left caudate nucleus, and the mean ALFF value of the left temporal pole: superior temporal gyrus. This interaction indicated that in individuals with the S/S genotype, the mean ReHo, fALFF, and ALFF values of these regions among SLE patients were lower compared to those of the HC group. Conversely, such differences were not observed among L allele carriers. Research has previously highlighted the significance of these brain regions. The posterior cingulate gyrus, known for its high metabolic activity in the resting state, is associated with self-evaluation [24], attention [25], formation of self-thoughts [26], and episodic memory, among other cognitive functions [27]. Similarly, the caudate nucleus, a component of the striatum, plays a crucial role in brain activity, particularly in cognitive function. Our findings indicate that the impact of SLE disease on brain function in the left posterior cingulate gyrus, left caudate nucleus, left temporal pole, and superior temporal gyrus was modulated by the 5-HTTLPR genotype. Specifically, individuals with the S/S genotype demonstrated a more pronounced effect of SLE disease on brain function in these regions.
Our investigation further demonstrated that the interaction between SLE disease status and the 5-HTTLPR genotype affected the mean ReHo values in the right hippocampus and the mean ALFF values in the left medial and paracingulate gyrus. This interaction implies that among SLE patients, those carrying the L allele exhibit heightened mean ReHo values in the right hippocampus and reduced mean ALFF values in the left medial and paracentral cingulate gyrus compared to healthy controls. Conversely, individuals with the S/S genotype do not display such distinctions. The limbic system, encompassing the hippocampus and cingulate gyrus, plays a pivotal role in emotion regulation and cognitive function. Notably, depressive, anxious, and cognitive symptoms are prevalent among SLE patients, potentially attributed to the functional impairment of adjacent brain regions caused by the disease. Our study highlighted that the 5-HTTLPR genotype of the subjects influenced the impact of SLE disease on the brain function indices of the hippocampus and cingulate gyrus. Specifically, individuals with the L/S or L/L genotype demonstrated a more pronounced effect of SLE disease on the brain function of these regions.
Conclusions
In conclusion, our study sheds light on the intricate relationship between SLE, the 5-HTTLPR genotype, and brain function. By uncovering specific brain regions affected by this interaction, we highlight the potential influence of genetic variations on neurological manifestations in SLE patients. However, our findings are tempered by limitations such as sample size constraints, cross-sectional design, and the exclusive focus on the 5-HTTLPR genotype. Future research endeavors should encompass larger, longitudinal studies integrating diverse genetic and environmental factors. Addressing these limitations could provide a more comprehensive understanding of how genetic variations impact brain function in the context of SLE, ultimately guiding more targeted interventions and therapeutic approaches for affected individuals.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available due to the protection of individuals’ privacy but are available from the corresponding author on reasonable request.
Abbreviations
- ALFF:
-
Amplitude of low-frequency fluctuations
- ACR:
-
American College of Rheumatology
- BOLD:
-
Blood oxygenation level-dependent
- CNS:
-
Central nervous system
- fALFF:
-
Fractional amplitude of low-frequency fluctuations
- fMRI:
-
Functional magnetic resonance imaging
- HC:
-
Healthy controls
- HAMD:
-
Hamilton Depression Scale
- HAMA:
-
Hamilton Anxiety Scale
- MRI:
-
Magnetic resonance imaging
- NPSLE:
-
Neuropsychiatric lupus erythematosus
- ReHo:
-
Regional homogeneity
- rs-fMRI:
-
Resting-state functional magnetic resonance imaging
- SLE:
-
Systemic Lupus Erythematosus
- SLEDAI-2000:
-
SLE disease activity index-2000 version
- 5-HT:
-
5-Hydroxytryptamine
- 5-HTT:
-
5-Hydroxytryptamine transporter
- 5-HTTLPR:
-
Serotonin transporter gene-linked polymorphic region
References
Kiriakidou M, Ching CL. Systemic lupus erythematosus. Ann Intern Med. 2020;172(11):Itc81-itc96.
Schwartz N, Stock AD, Putterman C. Neuropsychiatric lupus: new mechanistic insights and future treatment directions. Nat Rev Rheumatol. 2019;15(3):137–52.
Unterman A, Nolte JE, Boaz M, et al. Neuropsychiatric syndromes in systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum. 2011;41(1):1–11.
delEtoile J, Adeli H. Graph theory and brain connectivity in alzheimer’s disease. Neuroscientist. 2017;23(6):616–26.
Engels G, Vlaar A, McCoy B, et al. Dynamic functional connectivity and symptoms of parkinson’s disease: a resting-state fMRI study. Front Aging Neurosci. 2018;10:388.
Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28–38.
Lottman KK, Gawne TJ, Kraguljac NV, et al. Examining resting-state functional connectivity in first-episode schizophrenia with 7T fMRI and MEG. NeuroImage Clin. 2019;24:101959.
Zhang XD, Jiang XL, Cheng Z, et al. Decreased coupling between functional connectivity density and amplitude of low frequency fluctuation in non-neuropsychiatric systemic lupus erythematosus: a resting-stage functional MRI study. Mol Neurobiol. 2017;54(7):5225–35.
Liu S, Cheng Y, **e Z, et al. A conscious resting state fMRI study in SLE patients without major neuropsychiatric manifestations. Front Psych. 2018;9:677.
Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137–41.
Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400.
De Deurwaerdere P, Di Giovanni G. 5-HT interaction with other neurotransmitters: an overview. Prog Brain Res. 2021;259:1–5.
Han KM, Choi S, Kim A, et al. The effects of 5-HTTLPR and BDNF Val66Met polymorphisms on neurostructural changes in major depressive disorder. Psychiatry Res Neuroimaging. 2018;273:25–34.
Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725.
Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Leucht S, Fennema H, Engel R, et al. What does the HAMD mean? J Affect Disord. 2013;148(2–3):243–8.
Thompson E. Hamilton rating scale for anxiety (HAM-A). Occup Med (Oxford, England). 2015;65(7):601.
Li S, Liu S, Chen F, et al. Link-Polymorphism of 5-HTT promoter region is associated with autoantibodies in patients with systemic lupus erythematosus. J Immunol Res. 2016;2016:3042726.
Xu J, Cheng YQ, Chen B, et al. Depression in systemic lupus erythematosus patients is associated with link-polymorphism but not methylation status of the 5HTT promoter region. Lupus. 2013;22(10):1001–10.
Montefinese M, Pinti P, Ambrosini E, et al. Inferior parietal lobule is sensitive to different semantic similarity relations for concrete and abstract words. Psychophysiology. 2021;58(3):e13750.
Patra A, Kaur H, Chaudhary P, et al. Morphology and morphometry of human paracentral lobule: an anatomical study with its application in neurosurgery. Asian J Neurosurg. 2021;16(2):349–54.
Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106(31):13040–5.
Catley MJ, O’Connell NE, Berryman C, et al. Is tactile acuity altered in people with chronic pain? a systematic review and meta-analysis. J Pain. 2014;15(10):985–1000.
Kanske P, Böckler A, Trautwein FM, et al. Dissecting the social brain: introducing the EmpaToM to reveal distinct neural networks and brain-behavior relations for empathy and Theory of Mind. Neuroimage. 2015;122:6–19.
Weissman DH, Roberts KC, Visscher KM, et al. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9(7):971–8.
Christoff K, Gordon AM, Smallwood J, et al. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA. 2009;106(21):8719–24.
Sestieri C, Corbetta M, Romani GL, et al. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31(12):4407–20.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (3227094,81760296, 82060259), Yunnan Province High-level health technical talents (leading talents) (L-2019004 and L-2019011), Yunnan Province Special Project for Famous Medical Talents of the “Ten Thousand Talents Program” (YNWRMY- 2018–040 and YNWR-MY-2018–041), the Funding of Yunnan Provincial Health Science and Technology Plan (2018NS0133), the Funding of Ministry of Science and Technology of Yunnan Province (2018ZF016), Yunnan Province Clinical Research Center for Skin Immune Diseases (2019ZF012), and Yunnan Province Clinical Center for Skin Immune Diseases (ZX2019-03–02).
Author information
Authors and Affiliations
Contributions
All authors were involved in the preparation of the article and approval of the version for submission. Lihua Ma, Yifan Yang, and Shu Li contributed equally and substantially to the conception, design, and drafting of the article of the study. Bibhuti Upreti was responsible for language editing. Shuang Liu, **angyu Wang, and Ru Bai substantial contributions to the acquisition of data. Yuqi Cheng and Jian Xu revised the article critically for important intellectual content and final approval of the version of the article to be published.
Authors’ information
Lihua Ma, Yifan Yang, and Shu Li contributed equally to this work. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Institutional Review Board of Kunming Medical University. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Consent was provided for the publication of magnetic resonance imaging.
Competing interests
All study authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, L., Yang, Y., Li, S. et al. Interaction of 5-HTTLPR and SLE disease status on resting-state brain function. Arthritis Res Ther 26, 38 (2024). https://doi.org/10.1186/s13075-024-03276-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-024-03276-y