Abstract
Background
Arhalofenate acid, the active acid form of arhalofenate, is a non-agonist peroxisome proliferator-activated receptor γ (PPARγ) ligand, with uricosuric activity via URAT1 inhibition. Phase II studies revealed decreased acute arthritis flares in arhalofenate-treated gout compared with allopurinol alone. Hence, we investigated the anti-inflammatory effects and mechanisms of arhalofenate and its active acid form for responses to monosodium urate (MSU) crystals.
Methods
We assessed in-vivo responses to MSU crystals in murine subcutaneous air pouches and in-vitro responses in murine bone marrow-derived macrophages (BMDMs) by enzyme-linked immunosorbent assay (ELISA), SDS-PAGE/Western blot, immunostaining, and transmission electron microscopy analyses.
Results
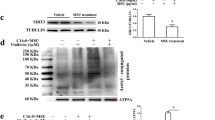
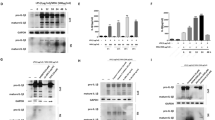
Oral administration of arhalofenate (250 mg/kg) blunted total leukocyte ingress, neutrophil influx, and air pouch fluid interleukin (IL)-1β, IL-6, and CXCL1 in response to MSU crystal injection (p < 0.05 for each). Arhalofenate acid (100 μM) attenuated MSU crystal-induced IL-1β production in BMDMs via inhibition of NLRP3 inflammasome activation. In addition, arhalofenate acid dose-dependently increased activation (as assessed by phosphorylation) of AMP-activated protein kinase (AMPK). Studying AMPKα1 knockout mice, we elucidated that AMPK mediated the anti-inflammatory effects of arhalofenate acid. Moreover, arhalofenate acid attenuated the capacity of MSU crystals to suppress AMPK activity, regulated expression of multiple downstream AMPK targets that modulate mitochondrial function and oxidative stress, preserved intact mitochondrial cristae and volume density, and promoted anti-inflammatory autophagy flux in BMDMs.
Conclusions
Arhalofenate acid is anti-inflammatory and acts via AMPK activation and its downstream signaling in macrophages. These effects likely contribute to a reduction of gout flares.
Similar content being viewed by others
Background
Anti-inflammatory prevention and treatment of attacks of gouty arthritis remain challenging, in part because many patients have incomplete responses or contraindications to one or more of the primary oral anti-inflammatory therapies (colchicine, nonsteroidal anti-inflammatory drugs (NSAIDs), and corticosteroids) [1, 2]. Moreover, gout flares often increase in frequency in the initial phase of urate-lowering therapy (ULT), thereby contributing to poor adherence to ULT and lack of improvement in health-related quality of life [1, 2]. Arhalofenate is a non-agonist ligand of peroxisome proliferator-activated receptor γ (PPARγ) with weak transactivation but robust transrepression activity [3]. It was first developed as an insulin sensitizer for type 2 diabetes mellitus [3]. Subsequently, arhalofenate was demonstrated to have uricosuric activity, as an inhibitor of URAT1, organic anion transporter 4 (OAT4) and OAT10 [4]. In a recent phase II trial in gout patients, which assessed acute gout flare as the primary endpoint, arhalofenate significantly reduced the risk of acute gouty arthritis in comparison with allopurinol alone, whereas there was no significant difference compared with allopurinol in combination with prophylactic colchicine [5]. The risk for urate-lowering therapy-induced gout flares depends on the degree of serum urate lowering [2]. Hence, this study was performed to directly test and characterize the anti-inflammatory effects of arhalofenate pertinent to gout.
Acute gouty arthritis is a characteristically severe phenotypic inflammatory response to deposits of monosodium urate (MSU) crystals which induce expression of NF-κB-dependent proinflammatory cytokines including pro-interleukin (IL)-1β and multiple chemokines [6, 7]. MSU crystals also stimulate activation of the NLRP3 inflammasome, with consequent maturation and release of IL-1β [6, 7]. This is a central driver of the gouty inflammation cascade which involves recruitment and activation of phagocytes [6, 7]. Core factors that modulate activation of the NLRP3 inflammasome, and experimental gout-like inflammation, include mitochondrial function, autophagy, and AMP-activated protein kinase (AMPK) [8, 9].
Mitochondrial reactive oxygen species (ROS) and oxidized mitochondrial DNA (mtDNA) promote inflammation [10,11,12], mediated by activation of NF-κB [10,11,12] and activation of the NLRP3 inflammasome via dysregulated balance between thioredoxins (TRXs) and thioredoxin-interacting protein (TXNIP) [13]. TRX1 and TRX2, mainly located in the cytoplasm and mitochondria, respectively, control cellular ROS by reduction of disulfides to thiol groups [14]. TXNIP directly binds to TRX and inhibits the reducing activity of TRX through disulfide exchange [14]. However, ROS triggers disassociation of TXNIP from TRX1, promoting direct physical interaction between TXNIP and NLRP3 that leads to activation of caspase-1 and release of mature IL-1β [13].
Autophagy mediates cellular homeostasis by degrading damaged proteins and organelles, including mitochondria [15,16,17]. Although MSU crystals promote autophagosome formation, the crystals also induce impairment of proteasomal degradation leading to accumulation of p62 [17]. As a selective autophagy receptor adaptor protein [17], p62 interacts with LC3-II to facilitate autophagic degradation [17], and also is involved in MSU crystal-induced caspase-1 activation and IL-1β release [18]. One of the major factors promoting autophagy is serine/threonine kinase AMPK [19].
AMPK is a nutritional biosensor that maintains cellular energy balance [19, 20], but nutritional excesses and other factors, including stimulation by MSU crystals and IL-1β, decrease AMPK activity [9]. Significantly, AMPK functions as an NF-κB and NLRP3 inflammasome inhibitor and promotes anti-inflammatory macrophage polarization, and markedly decreases the inflammatory response to MSU crystals in cultured macrophages [21, 22]. Moreover, AMPK transduces colchicine anti-inflammatory effects in vitro [22]. Pharmacologic AMPK activation markedly limits experimental gouty inflammation in the mouse in vivo using the subcutaneous air pouch model [22]. Conversely, MSU crystal-induced inflammation is prominently potentiated in AMPKα1 knockout (KO) mice [22].
Thiazolidinedione PPARγ agonists have been shown to cause phosphorylation and activation of AMPK [36]. Levels of p62 usually inversely correlate with autophagic degradation in later stages of autophagy [36]. Although studies have shown that p62 is increased and translocated to damaged mitochondria in NLRP3 inflammasome-activated cells, the detailed molecular mechanism on the link between the NLRP3 inflammasome and autophagy, especially mitophagy, is not yet fully understood. Interestingly, recent studies reported that, on stimulation of macrophages with NLRP3 inflammasome activators, p62, whose expression is induced via NF-κB, LC3-II, and Parkin, were recruited to the damaged mitochondria, initiating organelle clearance via mitophagy [36, 37]. This “NF-κB-p62-mitophagy” signaling axis represents a key macrophage-intrinsic regulatory mechanism that keeps NLRP3 inflammasome activation in check [37, 38]. Further studies on how arhalofenate acid controls MSU crystal-induced NLRP3 inflammation related to mitophagy will be of interest.
Conclusion
Arhalofenate acid, the active acid form of arhalofenate, exerts anti-inflammatory effects in MSU crystal-treated macrophages. These effects were mediated to a large degree by inducing AMPK activation, and at least in part by associated maintenance of mitochondrial function through activation of AMPK and its downstream signaling and preservation of mitochondrial cristae surface area, and by increasing cellular quality control by promoting autophagy. The results of this study identify basic mechanisms by which arhalofenate treatment decreases acute flares in patients with gout [5].
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- Ask1:
-
Apoptosis signaling regulating kinase 1
- BMDM:
-
Bone marrow-derived macrophage
- FBS:
-
Fetal bovine serum
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- IL:
-
Interleukin
- KO:
-
Knockout
- LAMP1:
-
Lysosomal-associated membrane protein 1
- M-CSF:
-
Macrophage colony-stimulating factor
- MSU:
-
Monosodium urate
- mtDNA:
-
Mitochondrial DNA
- NAD:
-
Nicotinamide adenine dinucleotide
- OXPHOS:
-
Oxidative phosphorylation
- PBS:
-
Phosphate-buffered saline
- PGC-1α:
-
Peroxisome proliferator-activated receptor γ co-activator 1α
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- ROS:
-
Reactive oxygen species
- SIRT1:
-
Sirtuin 1
- TEM:
-
Transmission electron microscopy
- TFAM:
-
Mitochondrial transcription factor A
- TRX:
-
Thioredoxin
- TXNIP:
-
Thioredoxin-interacting protein
References
Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and nutrition examination survey 2007–2008. Arthritis Rheum. 2011;63:3136–41.
Keenan RT, O’Brien WR, Lee KH, Crittenden DB, Fisher MC, et al. Prevalence of contraindications and prescription of pharmacologic therapies for gout. Am J Med. 2011;124:155–63.
Gregoire FM, Zhang F, Clarke HJ, Gustafson TA, Sears DD, et al. MBX-102/JNJ39659100, a novel peroxisome proliferator-activated receptor-ligand with weak transactivation activity retains antidiabetic properties in the absence of weight gain and edema. Mol Endocrinol. 2009;23:975–88.
Lavan BE, McWherter C, Choi YJ. Arhalofenate, a novel uricosuric agent, is an inhibitor of human uric acid transporters [abstract]. Ann Rheum Dis. 2013;71(Suppl 3):450–1.
Poiley J, Steinberg AS, Choi YJ, Davis CS, Martin RL, et al. Arhalofenate flare study investigators. A randomized, double-blind, active- and placebo-controlled efficacy and safety study of arhalofenate for reducing flare in patients with gout. Arthritis Rheum. 2016;68:2027–34.
Busso N, So A. Mechanisms of inflammation in gout. Arthritis Res Ther. 2010;12:206.
Cronstein BN, Sunkureddi P. Mechanistic aspects of inflammation and clinical management of inflammation in acute gouty arthritis. J Clin Rheumatol. 2013;19:19–29.
Cleophas MC, Crişan TO, Joosten LA. Factors modulating the inflammatory response in acute gouty arthritis. Curr Opin Rheumatol. 2017;29:163–70.
Terkeltaub R. What makes gouty inflammation so variable? BMC Med. 2017;15:158.
Yu JW, Lee MS. Mitochondria and the NLRP3 inflammasome: physiological and pathological relevance. Arch Pharm Res. 2016;39:1503–18.
Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends Mol Med. 2015;21:193–201.
Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5.
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–40.
Yoshihara E, Masaki S, Matsuo Y, Chen Z, Tian H, et al. Thioredoxin/TXNIP: redoxisome as a redox switch for the pathogenesis of diseases. Front Immunol. 2014;4:514.
** HS, Suh HW, Kim SJ, Jo EK. Mitochondrial control of innate immunity and inflammation. Immune Netw. 2017;17:77–88.
Okamoto K, Kondo-Okamoto N. Mitochondria and autophagy: critical interplay between the two homeostats. Biochim Biophys Acta. 2012;1820:595–600.
Rodgers MA, Bowman JW, Liang Q, Jung JU. Regulation where autophagy intersects the inflammasome. Antioxid Redox Signal. 2014;20:495–506.
Choe JY, Jung HY, Park KY, Kim SK. Enhanced p62 expression through impaired proteasomal degradation is involved in caspase-1 activation in monosodium urate crystal-induced interleukin-1b expression. Rheumatology (Oxford). 2014;53:1043–53.
Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. 2017;45:31–7.
Salminen A, Kaarniranta K. AMP-activated protein kinase (AMPK) controls the aging process via an integrated signaling network. Ageing Res Rev. 2012;11:230–41.
Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl). 2011;89:667–76.
Wang Y, Viollet B, Terkeltaub R, Liu-Bryan R. AMP-activated protein kinase suppresses urate crystal-induced inflammation and transduces colchicine effects in macrophages. Ann Rheum Dis. 2016;75:286–94.
Zhang J, Zhang Y, **ao F, Liu Y, Wang J, et al. The peroxisome proliferator-activated receptor γ agonist pioglitazone prevents NF-κB activation in cisplatin nephrotoxicity through the reduction of p65 acetylation via the AMPK-SIRT1/p300 pathway. Biochem Pharmacol. 2016;101:100–11.
Osman I, Segar L. Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling. Biochem Pharmacol. 2016;101:54–70.
Morrison A, Yan X, Tong C, Li J. Acute rosiglitazone treatment is cardioprotective against ischemia-reperfusion injury by modulating AMPK, Akt, and JNK signaling in nondiabetic mice. Am J Physiol Heart Circ Physiol. 2011;301:H895–902.
Fernandez-Marcos PJ, Auwerx J. Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr. 2011;93:884S–90.
Pasqua T, Mahata S, Bandyopadhyay GK, Biswas A, Perkins GA, et al. Impact of chromogranin A on catecholamine storage, catecholamine granule morphology, and chromaffin cell energy metabolism in vivo. Cell Tissue Res. 2016;363:693–712.
Shaked M, Ketzinel-Gilad M, Cerasi E, Kaiser N, Leibowitz G. AMP-activated protein kinase (AMPK) mediates nutrient regulation of thioredoxin-interacting protein (TXNIP) in pancreatic beta-cells. PLoS One. 2011;6:e28804.
Gao K, Chi Y, Sun W, Takeda M, Yao J. 5'-AMP-activated protein kinase attenuates adriamycin-induced oxidative podocyte injury through thioredoxin-mediated suppression of the apoptosis signal-regulating kinase 1-P38 signaling pathway. Mol Pharmacol. 2014;85:460–71.
Lee WH, Kim SG. AMPK-dependent metabolic regulation by PPAR agonists. PPAR Res. 2010;2010. https://doi.org/10.1155/2010/549101.
Huang LS, Cobessi D, Tung EY, Berry EA. Binding of the respiratory chain inhibitor antimycin to the mitochondrial BC1 complex: a new crystal structure reveals an altered intramolecular hydrogen-bonding pattern. J Mol Biol. 2005;351:573–97.
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–25.
Hou X, Song J, Li XN, Zhang L, Wang X, et al. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010;396:199–205.
Chai TF, Hong SY, He H, Zheng L, Hagen T, et al. A potential mechanism of metformin-mediated regulation of glucose homeostasis: inhibition of thioredoxin-interacting protein (TXNIP) gene expression. Cell Signal. 2012;24:1700–5.
Kim MJ, Yoon JH, Ryu JH. Mitophagy: a balance regulator of NLRP3 inflammasome activation. BMB Rep. 2016;49:529–35.
Lazarou M. Kee** the immune system in check: a role for mitophagy. Immunol Cell Biol. 2015;93:3–10.
Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, et al. NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164:896–910.
Zhong Z, Sanchez-Lopez E, Karin M. Autophagy, NLRP3 inflammasome and auto-inflammatory/immune diseases. Clin Exp Rheumatol. 2016;34:12–6.
Funding
The study was supported by the Department of Veterans Affairs Merit Review grants I01BX002234 (to RLB) and I01BX001660 (to RT), and NIH grant P50 AR060772-6 Project 1 (to RT).
Availability of data and materials
The data analyzed during the study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
CM, RT, and RLB conceived of and designed the study. YJC, RLS, SKM, and RLB acquired the data and performed data analysis. All authors contributed to data interpretation. CM, RT, and RLB wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The handing of mice and experimental procedures were in accordance with requirements of the Institutional Animal Care and Use Committee and this study was granted permission by the CymaBay Research Oversight Committee.
Consent for publication
Not applicable.
Competing interests
RT has received research support jointly from Ardea/Astra-Zeneca and Ironwood, and has received payment as a consultant to SOBI, Selecta, and Horizon, and has a consulting agreement with CymaBay Therapeutics, Inc. RLB has received research funding from by CymaBay Therapeutics, Inc. The remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
A-769662 activated AMPK downstream targets involved in regulation of mitochondrial function. BMDMs were treated with direct AMPK activator A-769662 (100 μM) for 1 h before being stimulated with MSU crystals (0.2 mg/mL) for 6 or 18 h in RPMI containing 1% FBS. Western blot analysis was carried out to examine phosphorylation and expression of AMPKα, expression of SIRT1, PGC-1α, and TFAM, and expression of TXN1, TXN2, and TXNIP from 18-h treatment cells (A), and expression of LC3 and p62 from 6-h treatment cells (B). Data shown in A and B are representative of three individual experiments. (PDF 753 kb)
Additional file 2:
Arhalofenate acid promoted autophagy flux. BMDMs were treated with arhalofenate acid (100 μM) for 1 h before being stimulated with MSU crystals (0.2 mg/mL) for 6 h in RPMI containing 1% FBS. Immunofluorescence microscopy was carried out to visually identify p62 puncta (green) and lysosomes (LAMP1, red), and determine co-localization (yellow) of p62 and LAMP1 (A, 63×). The numbers of yellow punctae per cell were counted and presented in a graph (B). Data in A are representative of three individual experiments. Data in B are the mean ± SD of 200 cells examined for each condition. The p values represent comparisons between none and MSU crystals alone, or between MSU crystals alone and MSU crystals plus arhalofenate acid. (PDF 38585 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
McWherter, C., Choi, YJ., Serrano, R.L. et al. Arhalofenate acid inhibits monosodium urate crystal-induced inflammatory responses through activation of AMP-activated protein kinase (AMPK) signaling. Arthritis Res Ther 20, 204 (2018). https://doi.org/10.1186/s13075-018-1699-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-018-1699-4