Abstract
Background
Few anthelminthics are currently available, manifesting the urgent need for new treatment options. In vitro profiling of current anthelminthics against larval and adult stage helminths displayed varying effects on closely related worm species and between life stages of the same species. Conversely, limited research has been performed on the egg stage of human hookworms, and the effects of investigational compounds on the egg stage are not routinely assessed.
Methods
We profiled the development and hatching of Heligmosomoides polygyrus, Ancylostoma duodenale and Necator americanus eggs isolated from rodent faeces in liquid media with various nutrient levels, osmolar concentrations, and acidities in dependence on incubation temperature and light exposure. Incubation conditions were optimised to allow the study of drug effect on immature and embryonated eggs. We analysed concentration-effect relationships of commercially available anthelminthics over 72 h.
Results
Rapid embryonation and hatching were observed at room temperature with and without light exposure without nutrient supplementation in a wide range of acidities. Hookworms hatched optimally at room temperature in PBS achieving > 75% hatching over 34 h. Developmental delays were seen when eggs were stored at 4 °C with no effect on viability. Similar delays were also seen with increased osmolar concentrations resulting in decreased viability. Benzimidazole anthelminthics effectively reduced the viability and prevented hatching of hookworm eggs, with albendazole and thiabendazole eliciting particularly potent effects at EC50 values below 1 µM. Macrolide anthelminthics as well as emodepside, oxantel pamoate, and pyrantel pamoate were inactive while monepantel, levamisole, and tribendimidine displayed varied potencies among the hookworm species.
Conclusion
The presented egg-hatching assay will complement ongoing anthelminthic drug discovery and allow a full characterisation of drug activity against all life stages. In the development and application of the egg-hatching assay, good accordance was observed between the three hookworm species evaluated. Marketed anthelminthics show differences of drug action compared to larval and adult stages highlighting the importance of profiling drug activity against all life stages.
Graphical Abstract

Similar content being viewed by others
Background
The human hookworms Necator americanus and Ancylostoma duodenale infect about 470 million people worldwide and are primarily endemic in poverty-stricken areas of tropical and sub-tropical climates [1, 2]. Infection initiated by skin penetration leads to invasion of the lungs and intestine, resulting in painful lung and intestinal manifestations and anemia [1,2,3]. Within the host, adult hookworms release thousands of eggs daily, which exit through faeces and continue the life cycle and cause subsequent infections [1]. Currently, mass drug administration (MDA) is fundamental to control infection and reduce morbidity [1, 4]; however, only few chemotherapeutic agents are currently available against these helminths [5, 6]. The standard therapy used for treatment includes drugs derived from anthelminthics for veterinary use, primarily albendazole and mebendazole of the benzimidazole family [5]. Monotherapies administered at single doses display only moderate cure rates and efficacy varies over studied regions [7]. Additionally, pharmacotherapy does not prevent reinfection [8] and no licensed vaccines are currently available [9, 10]. Since there is an urgent need for new treatment options, and anthelminthic drug discovery is underfunded and neglected [4, 6, 11], it is essential to optimise drug discovery.
Current methods in helminth drug discovery overview a variety of phenotypic [12,13,14] and motility [14,15,16,17,18] based assays that have been developed and advanced to detect nematocidal activity primarily using larval and adult stages [10]. However, only few studies published incorporate the use of egg hatching to determine ovicidal abilities in drug discovery, omitting a part of the life cycle [10, 19]. Nevertheless, similar methodologies have been of use for over 40 years in the detection of helminth strains resistant to treatment [20,21,22]. The establishment of an egg-hatching assay platform for human hookworms harbors the potential to detect cryptic ovicidal effects of extant compounds, complementing anthelminthic drug screening.
The aim of our study was to enable the complete characterisation of anthelminthics against all life stages of hookworms by targeting the understudied egg stage. We developed an in vitro assay for drug evaluation against the egg stage through optimisation of laboratory conditions of hookworm egg maturation and hatching of Heligosomoides polygyrus, Ancylostoma ceylanicum, and N. americanus. We evaluated both unembryonated and embryonated eggs to consider drug sensitivity differences based on egg maturity. The anthelminthics assessed in this study included key benzimidazoles and macrolides and further commercially available anthelminthics, namely monepantel, levamisole, tribendimidine, emodepside, oxantel pamoate, and pyrantel pamoate. Full characterisation of the potency of broadly employed anthelminthics will help answer why drug action differs over life stages and in translation between host species as well as test whether this assay could be integrated in hookworm drug discovery [23].
Methods
Animals
Female mice (NMRI strain; age 3 weeks; weight ca. 20 − 22 g) were purchased from Charles River (Sulzfeld, Germany) and male Syrian Golden hamsters were purchased from Janvier Laboratories (Le Genest-Saint-Isle, France). Rodents were kept in polycarbonate cages under environmentally controlled conditions (temperature ∼25 °C; humidity ∼70%; 12 h light:12 h dark cycle) with free access to water and food. The rodents were acclimatised for 1 week before infection.
Compounds and culture media
Abamectin, albendazole, doramectin, eprinomectin, fenbendazole, flubendazole, ivermectin, levamisole hydrochloride, mebendazole, oxfendazole, oxibendazole, pyrantel pamoate, ricobendazole, and thiabendazole were the products of Sigma-Aldrich (Buchs, Switzerland). Milbemycin oxime and moxidectin were purchased from Hangzhou Dingyan Chemical Co., Ltd. (Hangzhou, China). Tribendimidine was obtained from Shandong ** larvae within, the egg stage is particularly sensitive to benzimidazole exposure.
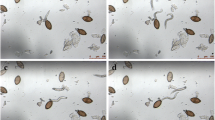
Effect of benzimidazoles, macrolides, and miscellaneous anthelminthics on unembryonated N. americanus, A. ceylanicum, and H. polygyrus and embryonated H. polygyrus eggs after 72 h of drug exposure at 100 µM. Eggs are depicted for active drugs preventing hatching, while larvae are depicted for inactive drugs
All six macrolides tested displayed no activity against the hookworm eggs (all EC50 values > 100 µM). Complete hatching was observed among these compounds after 72 h (Fig. 3). Macrolides feature much larger structures than the benzimidazoles (Additional file 1: Fig S9b), revealing a possible steric cause for the lack of ovicidal activity. In contrast to the benzimidazoles the protective barrier of the shell may be preventing entry of the drug and exposure of the develo** larvae within. Additionally and in contrast to hookworm larvae susceptible to macrolides [23, 30], the develo** larvae within the egg likely do not depend on the feeding and motility facilitated by the macrolide target glutamate and GABA channels [30].
Among the miscellaneous anthelminthic compounds tested, monepantel, levamisole, and tribendimidine displayed varied potencies among the hookworm species. Monepantel has been previously described with activity only against A. ceylanicum L3 [23], correlating with the highest potency observed among A. ceylanicum eggs. High potency was also observed against embryonated H. polygyrus eggs, although no activity was displayed against the unembryonated eggs. Lower potency of monepantel was also observed against N. americanus eggs, not seen previously against its L3 or adult stages [23]. Levamisole and tribendimidine act on the same receptor [31] presenting overall high potencies among hookworm L3 and adult parasites [23]. This activity was also seen among the hookworm eggs with overall lower potency of tribendimidine compared to levamisole. These three active compounds did not display the level of embryonic deterioration observed among the active benzimidazoles as many remaining unhatched eggs exhibited clear embryonation (Fig. 3). Emodepside, oxantel pamoate, and pyrantel pamoate displayed no activity against the hookworm eggs (all EC50 values > 100 µM) with complete hatching observed by 72 h (Fig. 3). Further research is needed to describe the lack of activity seen with these compounds which may be of interest in explaining the difference in activity seen compared to compounds such as levamisole and tribendimidine found active with similar mechanisms of action [32].
Conclusion
In summary, we developed and utilised egg-hatching assays to broaden the understanding of anthelminthic drug activity on hookworm egg maturation and hatching. Hookworms hatched optimally at room temperature in PBS achieving > 75% hatching over 34 h. Storing eggs at 4 °C proved beneficial to study drug effect on embryonated eggs by delaying maturation and hatching while preserving egg viability with > 95% hatching observed within 2 weeks or when returned to room temperature.
The in vitro assessment of the hookworm eggs showed further differences of drug action compared to larval and adult stages, displaying a changed sensitivity to the tested compounds within the eggshell. The benzimidazoles displayed the strongest potency against the egg stage, in particular thiabendazole and albendazole with EC50 values < 1 µM. Conversely, the macrolides displayed no activity although previously activity was seen against adults and particular high potency against larvae (23). Emodepside, oxantel pamoate, and pyrantel pamoate were inactive while monepantel, levamisole, and tribendimidine displayed varied potencies among the hookworm species. The activity discrepancies of currently profiled anthelminthics observed between life stages highlight the importance of profiling drug activity against all life stages, including the egg-stage model, in drug discovery for human hookworms. Likewise, we would not recommend screening compounds against the egg stage solely, even though this might be the most cost-effective method. Also, in adapting the developed assay from H. polygyrus to N. americanus and A. ceylanicum, assay conditions and determinants did not need to be altered, displaying good accordance among the three parasite models. Additionally, the resulting data from the assays were predominantly similar among the three species, further verifying H. polygyrus and A. ceylanicum as excellent models in hookworm drug discovery.
Continuing application of this egg-hatching assay will aid in enabling further drug discovery by identifying ovicidal abilities of known anthelminthics and potential drug candidates.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Ghodeif A, Jain H. Hookworm. Treasure Island (FL): StatPearls Publishing; 2022. p. 2022.
Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet. 2018;391:252–65.
Loukas A, Hotez PJ, Diemert D, Yazdanbakhsh M, McCarthy JS, Correa-Oliveira R, et al. Hookworm infection. Nature Rev Dis Primers. 2016;2:16088.
World Health Organization. Research priorities for helminth infections: technical report of the TDR disease reference group on helminth infections. Geneva: World Health Organization; 2012. p. 2012.
Campbell S, Soman-Faulkner K. Antiparasitic Drugs. Treasure Island: StatPearls Publishing; 2022.
Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–48.
Moser W, Schindler C, Keiser J. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ. 2017;358:j4307.
Albonico M, Wright V, Ramsan M, Haji HJ, Taylor M, Savioli L, et al. Development of the egg hatch assay for detection of anthelminthic resistance in human hookworms. Int J Parasitol. 2005;35:803–11.
Adegnika AA, de Vries SG, Zinsou FJ, Honkepehedji YJ, Dejon Agobé JC, Vodonou KG, et al. Safety and immunogenicity of co-administered hookworm vaccine candidates Na-GST-1 and Na-APR-1 in Gabonese adults: a randomised, controlled, double-blind, phase 1 dose-escalation trial. Lancet Infect Dis. 2021;21:275–85.
Herath H, Taki AC, Rostami A, Jabbar A, Keiser J, Geary TG, et al. Whole-organism phenotypic screening methods used in early-phase anthelmintic drug discovery. Biotechnol Adv. 2022;57:107937.
Lombardo FC, Pasche V, Panic G, Endriss Y, Keiser J. Life cycle maintenance and drug-sensitivity assays for early drug discovery in Schistosoma mansoni. Nat Protoc. 2019;14:461–81.
Abriola L, Hoyer D, Caffrey CR, Williams DL, Yoshino TP, Vermeire JJ. Development and optimization of a high-throughput screening method utilizing Ancylostoma ceylanicum egg hatching to identify novel anthelmintics. PLoS ONE. 2019;14:e0217019.
Partridge FA, Brown AE, Buckingham SD, Willis NJ, Wynne GM, Forman R, et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int J Parasitol Drugs Drug Resist. 2018;8:8–21.
Silbereisen A, Tritten L, Keiser J. Exploration of novel in vitro assays to study drugs against Trichuris spp. J Microbiol Methods. 2011;87:169–75.
Weeks JC, Roberts WM, Robinson KJ, Keaney M, Vermeire JJ, Urban JF Jr, et al. Microfluidic platform for electrophysiological recordings from host-stage hookworm and Ascaris suum larvae: a new tool for anthelmintic research. Int J Parasitol Drugs Drug Resist. 2016;6:314–28.
Kotze AC, Clifford S, O’Grady J, Behnke JM, McCarthy JS. An in vitro larval motility assay to determine anthelmintic sensitivity for human hookworm and Strongyloides species. Am J Trop Med Hyg. 2004;71:608–16.
Smout MJ, Kotze AC, McCarthy JS, Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl Trop Dis. 2010;4:e885.
Taki AC, Byrne JJ, Wang T, Sleebs BE, Nguyen N, Hall RS, et al. High-Throughput Phenotypic Assay to Screen for Anthelmintic Activity on Haemonchus contortus. Pharmaceuticals. 2021. https://doi.org/10.3390/ph14070616.
Abedon ST, Kuhl SJ, Blasdel BG, Kutter EM. Phage treatment of human infections. Bacteriophage. 2011;1:66–85.
De Clercq D, Sacko M, Behnke J, Gilbert F, Dorny P, Vercruysse J. Failure of mebendazole in treatment of human hookworm infections in the southern region of Mali. Am J Trop Med Hyg. 1997;57:25–30.
von Samson-Himmelstjerna G, Coles GC, Jackson F, Bauer C, Borgsteede F, Cirak VY, et al. Standardization of the egg hatch test for the detection of benzimidazole resistance in parasitic nematodes. Parasitol Res. 2009;105:825–34.
Robles-Pérez D, Martínez-Pérez JM, Rojo-Vázquez FA, Martínez-Valladares M. Development of an egg hatch assay for the detection of anthelmintic resistance to albendazole in Fasciola hepatica isolated from sheep. Vet Parasitol. 2014;203:217–21.
Keiser J, Haberli C. Evaluation of Commercially Available Anthelminthics in Laboratory Models of Human Intestinal Nematode Infections. ACS Infect Dis. 2021;7:1177–85.
Biendl S, Haberli C, Keiser J. Discovery of novel antischistosomal scaffolds from the open access Pandemic Response Box. Expert Rev Anti Infect Ther. 2022;20:621–9.
Karpstein T, Pasche V, Häberli C, Scandale I, Neodo A, Keiser J. Evaluation of emodepside in laboratory models of human intestinal nematode and schistosome infections. Parasit Vectors. 2019;12:226.
Drag M, Höglund J, Nejsum P, Thamsborg SM, Enemark HL. The level of embryonation influences detection of Ostertagia ostertagi eggs by semi-quantitative PCR. Parasit Vectors. 2016;9:368.
Matsuyama H, Takahashi H, Watanabe K, Fujimaki Y, Aoki Y. The involvement of cyclic adenosine monophosphate in the control of schistosome miracidium cilia. J Parasitol. 2004;90:8–14.
Anegagrie M, Lanfri S, Aramendia AA, Scavuzzo CM, Herrador Z, Benito A, et al. Environmental characteristics around the household and their association with hookworm infection in rural communities from Bahir Dar, Amhara Region, Ethiopia. PLoS Negl Trop Dis. 2021;15:e0009466.
Queensland Government. Soil pH: The State of Queensland 1995—2022; 2013 https://www.qld.gov.au/environment/land/management/soil/soil-properties/ph-levels].
Abongwa M, Martin RJ, Robertson AP. A brief review on the mode of action of antinematodal drugs. Acta Vet. 2017;67:137–52.
Robertson AP, Puttachary S, Buxton SK, Martin RJ. Tribendimidine: mode of action and nAChR subtype selectivity in Ascaris and Oesophagostomum. PLoS Negl Trop Dis. 2015;9:e0003495.
Gerald L, Mandell JEB, Dolin R. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier; 2006.
Acknowledgements
Not applicable.
Funding
Open access funding provided by University of Basel. We gratefully acknowledge financial support from the Swiss National Science Foundation (no. 175585).
Author information
Authors and Affiliations
Contributions
SB and JK conceived the experiments. SB and EE designed the experiments. EE performed the experiments. EE and SB analysed the data and drafted the first version of the manuscript. SB and JK revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal studies were carried out in accordance with Swiss national and cantonal regulations on animal welfare at the Swiss Tropical and Public Health Institute (Allschwil, Switzerland; permission no. 520 and 2070).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Figure S1 Embryonic development stages and hatching of Necator americanus, Ancylostoma ceylanicum, and Heligmosomoides polygyrus eggs. Figure S2 Viability of Necator americanus, Ancylostoma ceylanicum, and Heligmosomoides polygyrus eggs incubated within PBS, HBSS, and RPMI at room temperature. Figure S3 Hatching of unembryonated and embryonated Heligmosomoides polygyrus eggs. Figure S4 Embryonic development of freshly isolated Heligmosomoides polygyrus eggs incubated at 4 °C. Figure S5 Hatching of Heligmosomoides polygyrus eggs at room temperature and 4 °C. Figure S6 Observed delays in hatching of embryonated Heligmosomoides polygyrus eggs within media of increasing NaCl concentrations. Figure S7 Viability of Heligmosomoides polygyrus eggs in media of increasing NaCl concentrations and various acidities at room temperature. Figure S8 Abnormal appearing embryonated Heligmosomoides polygyrus eggs observed within hyperosmolar and pH 2 media. Figure S9 Chemical structures of the evaluated anthelminthics. Figure S10 In vitro concentration-response curve and EC50 value determination among egg-hatch assays. Table S1 Compound concentration range for in vitro EC50 determination.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Easland, E., Biendl, S. & Keiser, J. Development of a hookworm egg hatching assay to determine the ovicidal effects of anthelminthics. Parasites Vectors 16, 157 (2023). https://doi.org/10.1186/s13071-023-05771-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05771-8