Abstract
Background
Outbreaks of Aedes-borne arboviral diseases are becoming rampant in Africa. In Ghana, there is no organized arboviral control programme with interventions restricted to mitigate outbreaks. Insecticide application is a crucial part of outbreak responses and future preventative control measures. Thus, knowledge of the resistance status and underlying mechanisms of Aedes populations is required to ensure optimal insecticide choices. The present study assessed the insecticide resistance status of Aedes aegypti populations from southern Ghana (Accra, Tema and Ada Foah) and northern Ghana (Navrongo) respectively.
Methods
Phenotypic resistance was determined with WHO susceptibility tests using Ae. aegypti collected as larvae and reared into adults. Knockdown resistance (kdr) mutations were detected using allele-specific PCR. Synergist assays were performed with piperonyl butoxide (PBO) to investigate the possible involvement of metabolic mechanisms in resistance phenotypes.
Results
Resistance to DDT was moderate to high across sites (11.3 to 75.8%) and, for the pyrethroids deltamethrin and permethrin, moderate resistance was detected (62.5 to 88.8%). The 1534C kdr and 1016I kdr alleles were common in all sites (0.65 to 1) and may be on a trajectory toward fixation. In addition, a third kdr mutant, V410L, was detected at lower frequencies (0.03 to 0.31). Pre-exposure to PBO significantly increased the susceptibility of Ae. aegypti to deltamethrin and permethrin (P < 0.001). This indicates that in addition to kdr mutants, metabolic enzymes (monooxygenases) may be involved in the resistance phenotypes observed in the Ae. aegypti populations in these sites.
Conclusion
Insecticide resistance underpinned by multiple mechanisms in Ae. aegypti indicates the need for surveillance to assist in develo** appropriate vector control strategies for arboviral disease control in Ghana.
Graphical Abstract

Similar content being viewed by others
Background
Aedes-borne arboviral diseases are a growing public health concern, but their control and prevention have received limited attention in Ghana in Africa [1]. It has been suggested that Africa could experience a shift in vector-borne diseases from malaria to arboviruses because of the effects of warming temperatures as a result of climate change [2]. Evidence for this comes from the growing number of arboviral outbreaks such as yellow fever and dengue fever reported in West Africa in the last 5 years [3,4,5,6,7]. Ghana has had a long history of yellow fever epidemics [8] with the most recent outbreak reported in October 2021 [9]. Recently, dengue virus was detected in suspected malaria and Ebola patients in Ghana [10, 11]. Furthermore, exposure to dengue and chikungunya virus has been established in Ghana via immunological surveys [12,13,14]. Despite such outbreaks and detections, there are major gaps in arboviral diseases and vector surveillance in West Africa [15].
Aedes aegypti is the main vector for yellow fever and dengue fever, whereas Aedes albopictus is an extremely invasive species and is spreading rapidly globally [16]. One or both of these vectors are commonly found in urban and suburban settings in Africa; however, their control receives limited attention [1, 17]. Control and prevention of arboviral diseases depend heavily on vector control using insecticides in combination with larval source reduction and case management. Pyrethroids are the predominant insecticides for vector control because of their low toxicity to humans and low cost. Thus, pyrethroids are commonly used for indoor and outdoor space spraying to control adult Ae. aegypti [18]. Intentional and inadvertent exposure to insecticides has caused mosquito populations to develop resistance through natural selection [19].
The spread of insecticide resistance in Aedes mosquitoes represents a major challenge for vector control strategies. Resistance of Aedes mosquitoes to insecticides has been reported in several West African countries including Burkina Faso, Cameroon, Senegal and Ghana [20,21,22]. In Ghana, resistance of Aedes aegypti to three of the classes of insecticides [pyrethroids, the organochlorine (DDT) and carbamates] recommended by WHO for vector control has been reported [23,24,25]. Mechanisms of resistance implicated in Aedes worldwide usually involve target-site mutations and metabolic detoxification [26].
Many target site knockdown resistance (kdr) mutations have been identified as resistance markers in Aedes mosquitoes globally [26,27,28,29]. So far, three kdr mutations have been detected in African Aedes populations, V410L, V1016I and F1534C [28, 30]. In Ghana, two of these mutations (V1016I and F1534C) have been found to cause resistance to pyrethroids [24, 28], with F1534C being the most common [31]. The kdr mutation V410L causes reduced sensitivity to pyrethroids [32] and was recently reported in Ae. aegypti from Burkina Faso and Cote d’Ivoire [33, 34].
The involvement of detoxification enzymes in resistance has been established by several studies in Africa, commonly via the use of synergists which elevate insecticide mortality [22, 35,36,37,38]. Piperonyl butoxide (PBO) is a synergist that primarily inhibits the cytochrome P450 monooxygenase superfamily of enzymes, members of which are frequently implicated in the metabolism of insecticides (especially pyrethroids) in mosquitoes [26]. Nets containing PBO-insecticide combinations (PBO nets) are now commonly distributed for malaria control, with demonstrated efficacy against Anopheles vector populations [39]. PBO has also been found to restore the susceptibility of several African Aedes populations to insecticides [20, 40, 41].
There is a need for a more effective arboviral vector control programme in response to the emergence of arboviral diseases in Africa. Surveillance of insecticide resistance in the target vector population is important to ensure rational choices for vector control strategies. Currently, there is a paucity of data on the insecticide resistance and mechanisms in Aedes mosquitoes in Ghana and Africa as a whole [1]. Here, we investigated the insecticide resistance status and mechanisms of Ae. aegypti mosquitoes in southern and northern Ghana to provide information for control.
Materials and methods
Study Sites
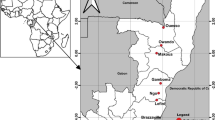
The study was carried out in four sites in the southern and northern parts of Ghana, from which larval collections were made during the rainy and dry seasons from June 2019 to January 2020. The sites were Korle Bu, Accra (5° 33’ N, 0° 12’ W), Tema (5°40′0″N, 0°0′0″E), Ada Foah (5°47′N, 0°38′E) and Navrongo (10°53′5″N, 01°05′25″W) (Fig. 1).
Korle Bu, Tema and Ada are situated in the Greater Accra region in the southern part of Ghana. These sites are urban areas with an abundance of Aedes breeding sites and Aedes mosquitoes, which may increase the risk of arboviral transmission [25]. Tema is home to Ghana’s largest seaport where car tyres are imported, thus facilitating the importation of Aedes mosquitoes including invasive species such as Ae. albopictus. Navrongo is a town in the Sahel savannah zone of Northern Ghana, with a high risk of arboviral transmission due to its proximity to neighbouring Burkina Faso where recent dengue outbreaks have occurred [42].
Larval Collection and Rearing
Immature forms of Aedes mosquitoes were collected from their breeding habitats—mainly abandoned car tyres, discarded containers and cans—within each of the study sites. Aedes larvae sampled were transported to the insectary at the Department of Medical Microbiology, University of Ghana Medical School, Accra, where they were raised to adults under stable conditions (temperature: 25 ± 2 °C, 80 ± 4% relative humidity). The larvae were fed on TetraMin Baby fish food (Tetra Werke, Melle, Germany). Emerged adults were fed on a 10% sugar solution until use in WHO susceptibility bioassays or synergist bioassays.
Adult susceptibility testing
Susceptibility tests using WHO tubes were conducted according to the WHO protocol [43] to determine phenotypic resistance. Three- to 5-day-old female mosquitoes were exposed to papers impregnated with the pyrethroids permethrin (0.75%) and deltamethrin (0.05%), DDT (4%), the organophosphate pirimiphos-methyl (0.25%) and the carbamate bendiocarb (0.1%). Though these doses are not the recommended doses for evaluating the susceptibility of Aedes mosquitoes, they are the most commonly used [20, 25, 26]. These doses were used in the absence of WHO-recommended doses for Aedes mosquitoes at the time of the bioassay, which are currently 0.03%, 0.25% and 0.21% for deltamethrin, permethrin and pirimiphos-methyl respectively [44].
The knockdown time was recorded every 10 min during the 60-min exposure period. Mortality was recorded after a 24-h recovery period. Alive (resistant) and dead (susceptible) mosquitoes were stored in absolute ethanol for later DNA analysis.
Morphological Species Identification
Resistant and susceptible Aedes mosquitoes from all WHO susceptibility bioassays were morphologically identified using identification keys by Huang [45].
Genoty** of kdr mutations in Aedes aegypti populations
A subsample of 332 Aedes mosquitoes that were phenotypically resistant and susceptible to insecticides deltamethrin, permethrin and DDT from the bioassay tests were randomly selected for genoty** of kdr mutations, F1534C, V1016I and V410L. A total of 172 resistant Aedes mosquitoes and 160 susceptible mosquitoes representing mosquitoes from all the four study sites were used for the genoty**. Total DNA was extracted from whole mosquitoes using the DNeasy Tissue Kit (Qiagen, USA). Pyrethroid and DDT-resistant and -susceptible Ae aegypti were genotyped for kdr mutations, F1534C, V1016I and V410L, using allele-specific PCR according to the protocols of Linns et al. [46] and Villanueva-Segura et al. [47]. Primer sequences are shown in Table 1.
Synergist assays with PBO
Piperonyl butoxide (PBO) synergist assays were performed to establish the role of cytochrome P450s in the observed resistance of Aedes mosquitoes. This synergist assay was performed using WHO tubes and papers, with four replicates of 20 female Aedes mosquitoes each pre-exposed to 4% PBO-impregnated papers for 1 h, after which the mosquitoes were immediately exposed to deltamethrin (0.05%) or permethrin (0.75%) for another 1 h. For each test, two control tubes with 20 female mosquitoes each were set up, one with PBO alone papers and the other with oil-impregnated papers. The two control tubes were included in the set-up for testing. Knockdown was recorded during the 60 min period and mortality after 24 h. The synergist assays were performed according to WHO criteria [48].
Statistical analysis
WHO insecticide susceptibility tests and PBO synergist tests were analyzed using the WHO criteria [43]. Mosquitoes were classified as susceptible if the mortality rate was between 98 and 100%; as suspected resistant if the mortality rate was between 90 and 97%; as resistant if the mortality rate was < 90% [43]. Generalised linear models with binomial link function (in SPSS 26) were used to compare bioassay mortalities for each insecticide among study sites, with overall Wald Chi-square analysis results shown and populations showing differences indicated. The Chi-square test or Fisher’s exact tests were used in determining associations between kdr mutations and phenotypes in genotypic and allelic tests, with odds ratios used to measure effect size. Probability values < 0.05 were interpreted as statistically significant.
Results
Morphological species identification of resistant and susceptible Aedes mosquitoes
A sub-sample of 409 Aedes mosquitoes obtained through random sampling from a total of 2240 mosquito samples that were used for the bioassays were used for morphological identification using taxonomic keys. All the 237 Aedes mosquitoes from Korle-bu, Tema and Accra in southern Ghana and 172 Aedes mosquitoes from northern Ghana that were morphologically identified were found to be Ae. aegypti (100%).
Phenotypic resistance
Mortality of Aedes mosquitoes to DDT was significantly lower in Tema (11.7%) than in Navrongo (38.8%), Ada (77.3%) and Accra (75%), which were similar (χ2 = 77.493, df = 3, P < 0.001). Resistance to permethrin was also detected in each site: Tema (82.5%), Accra (71.3%), Ada Foah (82.5%) and Navrongo (88.8%), though with much more limited variability (χ2 = 8.024, df = 3, P = 0.046). Mortality rates to deltamethrin were significantly lower in the population from Tema (62.5%) compared to the other sites Accra (81.3%), Ada Foah (83.8%) and Navrongo (78.8%) (χ2 = 11.826, df = 3, P = 0.008). Bendiocarb resistance was found in Tema (80%) and was significantly higher than the mortalities in Accra (97.5%) and Navrongo (93.8%) and marginally vs. Ada (90.1%), each of which is classified as suspected resistant (χ2 = 13.014, df = 3, P = 0.005). Mosquitoes were resistant to pirimiphos-methyl in Tema (85%) but showed suspected resistance in Ada Foah (93.8%) and Navrongo (97.5%) whilst being susceptible in Accra (100%), with significant but relatively moderate variation among the sites (χ2 = 7.582, df = 3, P = 0.023) (Fig. 2).
Genoty** of kdr-resistant mutations and their association with phenotypic resistance
A subset of 332 Ae. aegypti obtained from the phenotypic assays were genotyped for the F1534C, V1016I and V410L kdr mutations. The genotypes and allele frequencies of each kdr mutation are shown in Table 2. The 1534C kdr mutation was detected with a high allelic frequency of 1 in the pyrethroid and DDT-resistant mosquitoes and 0.65 to 1 in the susceptible group. No significant association was observed between the presence of F1534C mutation and resistant phenotypes (Table 3). The V1016L mutation was also detected in all the sites with allelic frequencies ranging from 0.87 to 0.97 in the resistant group and 0.65 to 0.91 in the susceptible group. The V1016I mutation was significantly associated with permethrin resistance (OR = 13.2, 95% CI = 2.8–122, P < 0.001) (Table 3).
The predominant genotype was the homozygote mutant genotype for the 1534C and 1016I mutation (Table 2). The allele frequency for the V410L kdr mutation varied between 0 and 0.38 depending on the insecticide, collection site and whether dead or alive (Table 2). There was no significant association between the V410L mutation and mortality with either insecticide pooled across study sites, whilst for pooled insecticides, there was a significant association only in Navrongo (Table 3).
Triple-locus kdr frequencies and phenotypic associations
Ten genotypes were observed out of a total of 27 possible genotype combinations across the three kdr loci in the 332 mosquitoes genotyped (Fig. 3). The most common tri-locus genotype detected across all sites was the homozygote mutant for F1534C (CC) and V1016I (II) combined with the homozygote wild type for V410L (VV). This tri-loci genotype (CC/II/VV) was detected in 128 (74.4%) resistant and 87 (54.4%) susceptible Ae. aegypti mosquitoes across all the sites. The triple homozygote mutant CC/II/LL was present in 25 (14.5%) resistant and 8 (5%) susceptible Ae. aegypti mosquitoes (Fig. 3).
Frequencies of tri-loci genotypes for the VGSC mutations in phenotyped Aedes aegypti mosquitoes. Each tri-locus genotyped is named according to the genotypic composition at each kdr mutation following the order 410 (VV, VL or LL)/1016 (VV, VI or II)/1534 (FF, FC or CC). VV, wild type (susceptible); VL, heterozygotes; LL, mutant (resistant); VI, heterozygotes; II, mutant (resistant); FF, wild type (susceptible); FC, heterozygotes; CC, mutant (resistant)
Of the three most common tri-locus genotypes, CC/II/VV and CC/II/LL were significantly associated with permethrin resistance with a fivefold (OR = 5.96, 95% CI = 2.6–13.7, P < 0.001) and sevenfold (OR = 7.02, 95% CI = 1.3–68.5, P < 0.05) greater likelihood of resistance respectively (Table 4). No significant association with deltamethrin resistance was observed in the tri-loci genotypes, CC/II/VV, CC/II/LL and CC/VV/LL (P > 0.05) (Table 4).
To analyse the relationship between the number of kdr alleles across the three loci and resistance phenotypes for each insecticide, three categories were created based on comparable frequencies of each: 1–3 kdr alleles; 4 kdr alleles; 5–6 kdr alleles. Generalised linear model analysis revealed a strong relationship between the number of kdr alleles and survival to permethrin, with 5–6 kdr alleles conferring significantly greater resistance than both 1–3 alleles (OR = 114.3, P < 0.001) and 4 kdr alleles (OR = 4.8, P− 0.047). However, though deltamethrin mortality was the highest in the 1–3 allele category (0.78 vs. 0.57 for both of the other categories), the difference was not significant, indicating that resistance was not dependent on the number of kdr alleles.
Synergist assays
Piperonyl butoxide (PBO) increased the susceptibility of Ae. aegypti to pyrethroids across the sites and insecticides (χ2 = 26.100, df = 3, P < 0.001; GLM interaction terms involving site and insecticide with PBO each non-significant). Mosquitoes from Tema had an increase in mortality rates to deltamethrin (from 20 to 50%) and permethrin (from 70 to 85%) after PBO exposure (Fig. 4a). Pre-exposure of Ae. aegypti from Accra increased the mortality rates to deltamethrin (from 80 to 90%) and permethrin (70% to 80%) (Fig. 4b). For Ada Foah, synergist-insecticide combinations reversed permethrin resistance in Ae. aegypti from 75 to 100% while partial susceptibility restoration was observed with deltamethrin 80% to 95% (Fig. 4c). Similarly, pre-exposure of Ae. aegypti mosquitoes from Navrongo to PBO showed full recovery of susceptibility to permethrin (from 60 to 100%) and deltamethrin (from 75 to 100%) (Fig. 4d). PBO has a significant effect on mortality of Ae. aegypti to pyrethroids deltamethrin and permethrin. Overall, PBO increased the mortality from 0.68 to 0.89 (OR = 4.1; P < 0.001).
Discussion
This study provides evidence of the resistance of Ae. aegypti populations in Ghana to public health insecticides. Females were resistant to DDT and pyrethroids, deltamethrin and permethrin in all the study sites. Knockdown resistance mutations F1534C and VI016I were at high frequencies, whilst the V410L kdr mutation was present at lower frequencies in Tema, Ada and Navrongo. Increased mortality to both pyrethroids was observed in Ae. aegypti in all sites after pre-exposure to PBO.
All mosquitoes that were randomly sampled for morphological identification were found to be Ae. aegypti. These findings are similar to that of another study in Ghana, where the most predominant species in urban and suburban sites was Ae. aegypti [25] and generally more likely to be found in urban and suburban areas [1, 49]. Aedes aegypti was the most common species across six regions in Ghana based on surveillance data obtained from 2015 to 2016 by Amoa-Bosompem et al. [50]. Also, Ae. aegypti was the predominant species (75.5%) in an urban site, Accra, according to a study by Suzuki et al. [23]. Therefore, multiple studies enable the conclusion that Ae. aegypti is the dominant vector in urban and suburban areas in Ghana.
Overall, the resistance profile of Ae. aegypti mosquitoes to major insecticides used for public health varied across study sites. Pyrethroids and DDT resistance in Ae. aegypti populations were widespread across all the sites. Evidence of pyrethroid resistance in Ae. aegypti was also established in other previous studies from Ghana [23,24,25] and other African countries [20, 21, 28]. However, what is driving insecticide resistance in these populations is uncertain. This is because current vector control measures in Ghana involve the use of IRS and LLINs, which are mainly targeting indoor resting mosquitoes. Previous studies in West Africa have shown that Ae. aegypti mosquitoes tend to rest outdoors so are not likely to have as many IRS and LLINs encounters [25, 42]. The extent of the involvement of these measures on resistance in Ghanaian Ae. aegypti population is largely unknown. Thus, calls are being made for more studies on the mediators of insecticide resistance in Ae. aegypti populations to be better equipped for arboviral vector control in Ghana.
Also, resistance to bendiocarb was observed in Tema while suspected resistance to bendiocarb was also observed in the other sites. An earlier study on Aedes mosquitoes in Ghana showed suspected resistance and susceptibility to bendiocarb in Ghanaian Aedes populations [25]. This provides evidence that bendiocarb resistance is increasing in Aedes populations. Other studies from Burkina Faso, Cameroon and Cote d’Ivoire also reported bendiocarb resistance in Ae. aegypti populations [33, 41, 51]. Our findings also showed resistance and suspected resistance to pirimiphos-methyl in all sites except in Accra, where it was susceptible. Other studies in Ghana and West Africa have reported susceptibility of Ae. aegypti populations to organophosphate insecticides [25, 41, 51]. However, our findings and those of other studies from Cote d’Ivoire and Senegal with recent evidence show that organophosphate resistance is also increasing [22, 33]. This calls for more surveillance of organophosphate and carbamate resistance in Ghanaian Ae. aegypti populations.
In this study, high frequencies of the F1534C and V1016I mutations were detected in both resistant and susceptible Ae. aegypti mosquitoes genotyped. Previous studies in Ghanaian Ae. aegypti in 2016 also detected high frequencies on the F1534C mutation and one heterozygote mutation of the V1016I mutation [28]. It is alarming to observe an increase in the frequency of V1016I to the point of nearing fixation in some of the study sites as well as the detection of the V410L mutation in Ghanaian Ae. aegypti populations. Similarly, the F1534C mutation has been found to be nearly fixed in Ae. aegypti mosquitoes from Cameroon (90%) and Burkina Faso (97%) [35, 52]. Relatively low allelic frequencies of the V410L mutation were observed across the study sites in both the resistant and susceptible groups of Ae. aegypti mosquitoes genotyped. This is the first report to our knowledge of this mutation in the northern part of Ghana, Navrongo. It was first reported in the southern part of Ghana, Accra, in only forest populations in 2022 [53]. This mutation was first detected in a Brazilian Ae. aegypti strain in 2017 [32]. It was detected in high frequencies in Angola (0.83), and low frequencies in Portugal (0.17) and Cote d’Ivoire (0.28) [32, 33]. These kdr mutations were found to be significantly associated with permethrin resistance. However, no significant association was observed between the kdr mutations and deltamethrin resistance. This is contrary to in vitro work by Haddi et al. [32] where both permethrin and deltamethrin resistance was significantly associated with the presence of the 410L allele. This finding of a limited impact of the 410L mutation on deltamethrin resistance was also evident in the analysis of the relationship between the number of kdr alleles and survival; the contrast was extremely strong for permethrin, especially for the 5–6 kdr allele category, all of which harboured 410L mutants.
Findings from this study revealed an increase in the mortality of Ae. aegypti mosquitoes to pyrethroids, deltamethrin and permethrin after pre-exposure to PBO. There was a significant increase in the mortality rates of Ae. aegypti mosquitoes after pre-exposure to PBO across all the sites. In sites Ada Foah and Navrongo, total restoration of susceptibility was observed after pre-exposure to PBO. Similar findings have been observed in Cameroon [21] and in Nigeria [37], where the mortality rate to pyrethroids was increased after pre-exposure to PBO synergist. Results obtained for PBO assays are useful for arboviral vector control, especially in endemic areas with high resistance among the vector populations. PBO can be incorporated in insecticide combinations to help increase the mortality of resistant Ae. aegypti mosquitoes to pyrethroids. The increase in mortality and restoration of susceptibility observed after PBO exposure confirms the role of monooxygenases in pyrethroid resistance that was observed. Therefore, we recommend that further studies should be done to identify the specific monooxygenases such as cytochrome P450s involved in pyrethroid resistance in Ae. aegypti populations in Ghana.
Conclusion
This study shows moderate to high phenotypic resistance among Ae. aegypti populations across the study sites. Knockdown resistance mutations F1534C and V1016I were found in high frequencies in Ae. aegypti populations across the study sites while V410L mutation was also detected in low frequencies. Pre-exposure of Ae. aegypti mosquitoes to PBO increased their mortalities to the pyrethroid insecticides tested. It is important to determine the intensity of resistance in Ae. aegypti populations in Ghana and also look into the possibility of adapting an integrated approach using newer classes of insecticides, larval source management, mass trap** and biological control toward the control of Aedes mosquitoes in Ghana [54].
Availability of data and materials
All datasets generated and/or analyzed during this study are included in the manuscript.
Abbreviations
- WHO:
-
World Health Organization
- PBO:
-
Piperonyl butoxide
- VGSC:
-
Voltage-gated sodium channel
- kdr :
-
Knockdown resistance
- V:
-
Valine
- G:
-
Glycine
- I:
-
Isoleucine
- C:
-
Cysteine
- F:
-
Phenylalanine
- L:
-
Leucine
- DDT:
-
Dichlorodiphenyltrichloroethane
- GST's:
-
Glutathione S-transferase
- CYPs:
-
Cytochrome P450s
- Ae. :
-
Aedes
- UV:
-
Ultraviolet
- IRS:
-
Indoor residual spraying
- LLINs:
-
Long-lasting insecticide-treated nets
References
Weetman D, Kamgang B, Badolo A, Moyes CL, Shearer FM, Coulibaly M, et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: current and future threats. Int J Environ Res Public Health. 2018;15:220.
Mordecai EA, Ryan SJ, Caldwell JM, Shah MM, LaBeaud AD. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet Heal. 2020;4:e416–23.
Ahmed QA, Memish ZA. Yellow fever from Angola and Congo: a storm gathers. Trop Doct. 2017;47:92–6. https://doi.org/10.1177/0049475517699726.
Gainor EM, Harris E, LaBeaud AD. Uncovering the Burden of Dengue in Africa: considerations on magnitude, misdiagnosis, and ancestry. Viruses. 2022;14:233.
Im J, Balasubramanian R, Ouedraogo M, Wandji Nana LR, Mogeni OD, Jeon HJ, et al. The epidemiology of dengue outbreaks in 2016 and 2017 in Ouagadougou, Burkina Faso. Heliyon. 2020;6:e04389.
World Health Organization. Vector-borne Diseases. WHO Press; 2020. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Accessed 10 Mar 2022.
World Health Organization. Yellow Fever—West and Central Africa. 2021. https://www.who.int/emergencies/disease-outbreak-news/item/yellow-fever---west-and-central-africa. Accessed 9 Mar 2022.
Appawu M, Dadzie S, Abdul H, Asmah R, Boakye D, Wilson M, et al. Surveillance of viral haemorrhagic fevers in Ghana: Entomological assessment of the risk transmission in the northern regions, Ghana. Med J. 2007;40:137–41.
World Health Organization. Yellow Fever- Ghana. WHO Press; 2021. https://www.who.int/emergencies/disease-outbreak-news/item/yellow-fever---ghana. Accessed 9 Mar 2022.
Amoako N, Duodu S, Dennis FE, Bonney JHK, Asante KP, Ameh J, et al. Detection of Dengue virus among children with suspected malaria, Accra, Ghana. Emerg Infect Dis. 2018;24:1544–7.
Bonney JHK, Hayashi T, Dadzie S, Agbosu E, Pratt D, Nyarko S, et al. Molecular detection of dengue virus in patients suspected of Ebola virus disease in Ghana. PLoS ONE. 2018;13:e0208907.
Stoler J, Delimini RK, Bonney JHK, Oduro AR, Owusu-Agyei S, Fobil JN, et al. Evidence of recent dengue exposure among malaria parasite-positive children in three urban centers in Ghana. Am J Trop Med Hyg. 2015;92:497–500.
Adusei JA, Narkwa PW, Owusu M, Domfeh SA, Alhassan M, Appau E, et al. Evidence of chikungunya virus infections among febrile patients at three secondary health facilities in the Ashanti and the Bono Regions of Ghana. PLoS Negl Trop Dis. 2021;15:e0009735.
Manu SK, Bonney JHK, Pratt D, Abdulai FN, Agbosu EE, Frimpong PO, et al. Arbovirus circulation among febrile patients at the greater Accra Regional Hospital, Ghana. BMC Res Notes. 2019;12:332. https://doi.org/10.1186/s13104-019-4378-x.
Buchwald AG, Hayden MH, Dadzie SK, Paull SH, Carlton EJ. Aedes-borne disease outbreaks in West Africa: a call for enhanced surveillance. Acta Trop. 2020;209:105468.
Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4:e646–e646.
Gaye A, Wang E, Vasilakis N, Guzman H, Diallo D, Talla C, et al. Potential for sylvatic and urban Aedes mosquitoes from Senegal to transmit the new emerging dengue serotypes 1, 3 and 4 in West Africa. PLoS Negl Trop Dis. 2019;13:e0007043.
Horstick O, Runge-Ranzinger S, Nathan MB, Kroeger A. Dengue vector-control services: how do they work? A systematic literature review and country case studies. Trans R Soc Trop Med Hyg. 2010;104:379–86. https://doi.org/10.1016/j.trstmh.2009.07.027.
Machani MG, Ochomo E, Zhong D, Zhou G, Wang X, Githeko AK, et al. Phenotypic, genotypic and biochemical changes during pyrethroid resistance selection in Anopheles gambiae mosquitoes. Sci Rep. 2020;10:19063. https://doi.org/10.1038/s41598-020-75865-1.
Badolo A, Sombié A, Pignatelli PM, Sanon A, Yaméogo F, Wangrawa DW, et al. Insecticide resistance levels and mechanisms in Aedes aegypti populations in and around Ouagadougou. Burkina Faso PLoS Negl Trop Dis. 2019;13:e0007439.
Kamgang B, Yougang AP, Tchoupo M, Riveron JM, Wondji C. Temporal distribution and insecticide resistance profile of two major arbovirus vectors Aedes aegypti and Aedes albopictus in Yaoundé, the capital city of Cameroon. Parasit Vectors. 2017;10:469. https://doi.org/10.1186/s13071-017-2408-x.
Sene NM, Mavridis K, Ndiaye EH, Diagne CT, Gaye A, Ngom EHM, et al. Insecticide resistance status and mechanisms in Aedes aegypti populations from Senegal. PLoS Negl Trop Dis. 2021;15:e0009393.
Suzuki T, Osei JH, Sasaki A, Adimazoya M, Appawu M, Boakye D, et al. Risk of transmission of viral haemorrhagic fevers and the insecticide susceptibilitystatus of Aedes aegypti (linnaeus) in some sites in Accra, Ghana. Ghana Med J. 2016;50:136–41.
Kudom AA. Entomological surveillance to assess potential outbreak of Aedes-borne arboviruses and insecticide resistance status of Aedes aegypti from Cape Coast, Ghana. Acta Trop. 2020;202:105257.
Owusu-Asenso CM, Mingle JAA, Weetman D, Afrane YA. Spatiotemporal distribution and insecticide resistance status of Aedes aegypti in Ghana. Parasit Vectors. 2022;15:61. https://doi.org/10.1186/s13071-022-05179-w.
Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017;11:e0005625.
Granada Y, Mejía-Jaramillo AM, Strode C, Triana-Chavez O. A point mutation V419L in the sodium channel gene from natural populations of Aedes aegypti is involved in resistance to λ-cyhalothrin in Colombia. Insects. 2018. https://doi.org/10.3390/insects9010023.
Kawada H, Higa Y, Futami K, Muranami Y, Kawashima E, Osei JHN, et al. Discovery of point mutations in the voltage-gated sodium channel from African Aedes aegypti populations: potential phylogenetic reasons for gene introgression. PLoS Negl Trop Dis. 2016;10:e0004780.
Marcombe S, Poupardin R, Darriet F, Reynaud S, Bonnet J, Strode C, et al. Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: a case study in Martinique Island (French West Indies). BMC Genomics. 2009;10:494. https://doi.org/10.1186/1471-2164-10-494.
Ayres CFJ, Seixas G, Borrego S, Marques C, Monteiro I, Marques CS, et al. The V410L knockdown resistance mutation occurs in island and continental populations of Aedes aegypti in West and Central Africa. PLoS Negl Trop Dis. 2020;14:e0008216. https://doi.org/10.1371/journal.pntd.0008216.
Dusfour I, Vontas J, David J-P, Weetman D, Fonseca DM, Corbel V, et al. Management of insecticide resistance in the major Aedes vectors of arboviruses: Advances and challenges. PLoS Negl Trop Dis. 2019;13:e0007615.
Haddi K, Tomé HVV, Du Y, Valbon WR, Nomura Y, Martins GF, et al. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: a potential challenge for mosquito control. Sci Rep. 2017;7:46549.
Konan LY, Oumbouke WA, Silué UG, Coulibaly IZ, Ziogba JCT, N’Guessan RK, et al. Insecticide resistance patterns and mechanisms in Aedes aegypti (Diptera: Culicidae) populations across Abidjan, Côte d’Ivoire reveal emergent pyrethroid resistance. J Med Entomol. 2021;58:1808–16. https://doi.org/10.1093/jme/tjab045.
Toé HK, Zongo S, Guelbeogo MW, Kamgang B, Viana M, Tapsoba M, et al. Multiple insecticide resistance and first evidence of V410L kdr mutation in Aedes (Stegomyia) aegypti (Linnaeus) from Burkina Faso. Med Vet Entomol. 2022;36:309–19. https://doi.org/10.1111/mve.12602.
Sombié A, Saiki E, Yaméogo F, Sakurai T, Shirozu T, Fukumoto S, et al. High frequencies of F1534C and V1016I kdr mutations and association with pyrethroid resistance in Aedes aegypti from Somgandé (Ouagadougou), Burkina Faso. Trop Med Health. 2019;47:2.
Fagbohun IK, Idowu ET, Olakiigbe AK, Oyeniyi AT, Otubanjo OA, Awolola TS. Metabolic resistance mechanism in Aedes aegypti from Lagos State, Nigeria. J Basic Appl Zool. 2020;81:59. https://doi.org/10.1186/s41936-020-00194-8.
Olusegun-Joseph T, Oboh M, Awoniyi A, Adebowale A, Agbaso M, Fagbohun I. Efficacy of piperonyl butoxide (PBO) synergist on pyrethroid and dichlorodiphenyl trichloroethane (DDT) resistant mosquitoes in Lekki, Lagos State, Nigeria. Anim Res Int. 2020;17:3821–8.
Vijayan V, Kumar B, Ganesh K, Jagadeshwaran U, Fakoorziba MR, Makkapati A. Effects of piperonyl butoxide (PBO) as a synergist with deltamethrin on five species of mosquitoes. J Commun Dis. 2007;39:159–63.
Gleave K, Lissenden N, Richardson M, Choi L, Ranson H. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. Cochrane database Syst Rev. 2018;11:CD012776.
Kamgang B, Wilson-Bahun TA, Yougang AP, Lenga A, Wondji CS. Contrasting resistance patterns to type I and II pyrethroids in two major arbovirus vectors Aedes aegypti and Aedes albopictus in the Republic of the Congo, Central Africa. Infect Dis poverty. 2020;9:23.
Yougang AP, Kamgang B, Bahun TAW, Tedjou AN, Nguiffo-Nguete D, Njiokou F, et al. First detection of F1534C knockdown resistance mutation in Aedes aegypti (Diptera: Culicidae) from Cameroon. Infect Dis poverty. 2020;9:152.
Badolo A, Sombié A, Yaméogo F, Wangrawa DW, Sanon A, Pignatelli PM, et al. First comprehensive analysis of Aedes aegypti bionomics during an arbovirus outbreak in West Africa: dengue in Ouagadougou, Burkina Faso, 016–2017. PLoS Negl Trop Dis. 2022;16:e0010059.
WHO. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. WHO. WHO Press; 2018. https://apps.who.int/iris/bitstream/handle/10665/250677/9789241511575-eng.pdf
WHO. Monitoring and managing insecticide resistance in Aedes mosquito populations, interim guidance for entomologists. Geneva, World Health Organization. 2016. https://www.who.int/csr/resources/publications/zika/insecticide-resistance/en/. Accessed 20 Jul 2022.
Huang YM. The subgenus stegomyia of Aedes in the Afrotropical Region with keys to the species (Diptera: Culicidae). Zootaxa. 2004;700:1–120.
Linss JGB, Brito LP, Garcia GA, Araki AS, Bruno RV, Lima JBP, et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasit Vectors. 2014;7:25. https://doi.org/10.1186/1756-3305-7-25.
Villanueva-Segura OK, Ontiveros-Zapata KA, Lopez-Monroy B, Ponce-Garcia G, Gutierrez-Rodriguez SM, Davila-Barboza JA, et al. Distribution and frequency of the kdr mutation V410L in natural populations of Aedes aegypti (L.) (Diptera: Culicidae) from Eastern and Southern Mexico. J Med Entomol. 2020;57:218–23. https://doi.org/10.1093/jme/tjz148.
World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2018. http://www.who.int/malaria/publications/atoz/9789241511575/en/%22. Accessed 8 Feb 2023.
Overgaard HJ, Olano VA, Jaramillo JF, Matiz MI, Sarmiento D, Stenström TA, et al. A cross-sectional survey of Aedes aegypti immature abundance in urban and rural household containers in central Colombia. Parasit Vectors. 2017;10:356. https://doi.org/10.1186/s13071-017-2295-1.
Amoa-Bosompem M, Kobayashi D, Murota K, Faizah AN, Itokawa K, Fujita R, et al. Entomological assessment of the status and risk of mosquito-borne arboviral transmission in Ghana. Viruses. 2020;12:147. https://doi.org/10.3390/v12020147.
Namountougou M, Soma DD, Balboné M, Kaboré DA, Kientega M, Hien A, et al. Monitoring insecticide susceptibility in Aedes aegypti populations from the two biggest cities, Ouagadougou and Bobo-Dioulasso, in Burkina Faso: implication of metabolic resistance. Trop Med Infect Dis. 2020;5:84.
Djiappi-Tchamen B, Nana-Ndjangwo MS, Mavridis K, Talipouo A, Nchoutpouen E, Makoudjou I, et al. Analyses of insecticide resistance genes in Aedes aegypti and Aedes albopictus mosquito populations from Cameroon. Genes. 2021;12:828. https://doi.org/10.3390/genes12060828.
Kwame Amlalo G, Akorli J, EtornamAkyea-Bobi N, Sowa Akporh S, Aqua-Baidoo D, Opoku M, et al. Evidence of high frequencies of insecticide resistance mutations in Aedes aegypti (Culicidae) mosquitoes in urban Accra, Ghana: implications for insecticide-based vector control of Aedes-borne arboviral diseases. J Med Entomol. 2022. https://doi.org/10.1093/jme/tjac120.
Roiz D, Wilson AL, Scott TW, Fonseca DM, Jourdain F, Müller P, et al. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl Trop Dis. 2018;12:e0006845. https://doi.org/10.1371/journal.pntd.0006845.
Acknowledgements
We thank the residents of the study sites for their support during our study. Our sincere gratitude goes to Mr Sylvester Coleman and all staff of the President Malaria Initiative, Tamale, Mr Sebastian Mensah and all staff of the animal house unit of the Department of Medical Microbiology, University of Ghana for their field and laboratory assistance.
Funding
This study was supported by grants from the National Institute of Health (D43 TW 011513).
Author information
Authors and Affiliations
Contributions
AA, AOF, SKA, DW and YAA were responsible for the study design, supervised the data collection, and contributed to the writing of the manuscript. AA, CMO-A, GA-B, ARM and IS performed the data collection, laboratory work and analysis. AA and CMO-A drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Abdulai, A., Owusu-Asenso, C.M., Akosah-Brempong, G. et al. Insecticide resistance status of Aedes aegypti in southern and northern Ghana. Parasites Vectors 16, 135 (2023). https://doi.org/10.1186/s13071-023-05752-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05752-x