Abstract
Parasites of the genus Leishmania are unusual unicellular microorganisms in that they are characterized by the capability to subvert in their favor the immune response of mammalian phagocytes, including dendritic cells. Thus, in overt leishmaniasis, dendritic cells and macrophages are converted into a niche for Leishmania spp. in which the parasite, rather than being inactivated and disassembled, survives and replicates. In addition, Leishmania parasites hitchhike onto phagocytic cells, exploiting them as a mode of transport to lymphoid tissues where other phagocytic cells are potentially amenable to parasite colonization. This propensity of Leishmania spp. to target dendritic cells has led some researchers to consider the possibility that the non-pathogenic, reptile-associated Leishmania tarentolae could be exploited as a vaccine platform and vehicle for the production of antigens from different viruses and for the delivery of the antigens to dendritic cells and lymph nodes. In addition, as L. tarentolae can also be regarded as a surrogate of pathogenic Leishmania parasites, this parasite of reptiles could possibly be developed into a vaccine against human and canine leishmaniases, exploiting its immunological cross-reactivity with other Leishmania species, or, after its engineering, for the expression of antigens from pathogenic species. In this article we review published studies on the use of L. tarentolae as a vaccine platform and vehicle, mainly in the areas of leishmaniases and viral infections. In addition, a short summary of available knowledge on the biology of L. tarentolae is presented, together with information on the use of this microorganism as a micro-factory to produce antigens suitable for the serodiagnosis of viral and parasitic infections.
Graphical Abstract

Similar content being viewed by others
Background
Dendritic cells, antigen delivery and Leishmania tarentolae
Upon their invasion of vertebrate tissues, infectious microorganisms are typically exposed to the action of phagocytic cells, including dendritic cells (DCs). DCs subsequently transport the engulfed microbes to lymph nodes for processing of the microbial proteins into peptides and for the presentation of peptides to CD4+ T cells. DCs thus act as professional antigen presenting cells (APCs), playing a key role in the initiation of the adaptive immune response [1]. Compared to most pathogens, Leishmania parasites are rather unusual in that they exploit APCs of the myeloid cell line as a favourable habitat for their survival and reproduction [2]. In other words, Leishmania parasites are not only passively exposed to the phagocytic activity of DCs and macrophages but they specifically target phagocytic cells thanks to specific surface receptors. Once engulfed by DCs and macrophages, Leishmania parasites subvert the activation of these cells, inhibiting their oxidative and lytic antimicrobial responses, and use these cells as a mode of transport to the lymph nodes [3]. In summary, Leishmania parasites display one of the desired requirements for a vaccine vehicle: a specific targeting of DCs and lymph nodes (Fig. 1).
Schematic representation of the cutis of a mammal and a lymph node. A Leishmania-infected Phlebotominae sand fly is also represented (1). A dendritic cell (DC) is represented during the process of phagocytosis of a Leishmania promastigote (2), the transformation of the promastigote into the amastigote stage (3) and then the migration of the infected DC towards the lymph node (4). Inside the lymph node, DCs present the antigens to CD4+ T cells. A neutrophil is also shown as these cells play a role in the first phases of mammalian infection by Leishmania spp (5)
Despite this feature, however, candidate vaccines against leishmaniases based on the use of whole Leishmania cells, either inactivated or attenuated, have not yet been developed into licensed and available vaccines for human or canine use [4,5,6]. The major drawbacks of inactivated anti-Leishmania vaccines can be summarized as follows: (i) the limited immunogenicity of inactivated vaccines, as compared to attenuated ones; (ii) an improper modulation of the immune response, associated with the Th2-biasing properties of pathogenic Leishmania species; and (iii) the limits in the scalability to mass production of inactivated cells from pathogenic Leishmania species, and the logistical difficulties in the wide-scale distribution of the derived vaccine preparations [7]. These drawbacks, all of which are typical of inactivated vaccines—in particular the property of limited immunogenicity—have been overcome in other preparations using attenuated living microbes as vaccines. However, this latter approach entails possible safety issues [8] and even greater logistical difficulties for large-scale vaccine campaigns. In this context, the generation of gene-deleted clones to be used in vaccination programs can minimize the risk of reversal to virulence by attenuated pathogenic Leishmania strains [6].
The limits of inactivated and attenuated vaccines have been circumvented using surrogate pathogens as vaccines. For example, the Bacillus Calmette-Guerin (BCG) vaccine is derived from a cattle strain of the bacterium Mycobacterium bovis and developed as a vaccine against human tuberculosis (caused by Mycobacterium tuberculosis) after serial passages in vitro to achieve its attenuation [9]. An attractive feature of BCG vaccine is that this bacterium is suitable for genetic manipulation and therefore for the production of heterologous proteins. This led to the generation of BCG strains expressing antigens from other microorganisms, that have been assayed as candidate vaccines against a variety of pathogens unrelated with the mycobacteria [10]. Leishmania tarentolae, a parasite infecting reptiles, shares several features with BCG in terms of its potential to be exploited as a surrogate pathogen for use in vaccinations against leishmaniasis [11]. In addition, L. tarentolae has the potential to be developed as a vaccine platform of a wider interest, owing to the suitability of this parasite for genetic manipulation and, therefore, the production of heterologous protein antigens [11, 12].
In this article we review current knowledge on this parasite in relation with its potential use in the development of novel types of vaccines, not only against pathogenic Leishmania species, but also against a variety of viruses. The use of L. tarentolae to produce antigens for serological diagnosis will also be discussed. A schematic representation of the life-cycle of L. tarentolae is presented in Fig. 2.
Life-cycle of Leishmania tarentolae in sand flies and vertebrate hosts. In reptiles, amastigote-like forms are known to develop in blood cells, but the details of the life-cycle in reptilian hosts are still unknown. Sand flies ingest infected blood cells, and parasites subsequently differentiate into promastigotes, with a hypopylarian or peripylarian type of development. The transmission to vertebrate hosts is likely to occur, in most cases, through the blood meal or through oral ingestion of the fly. Other modes of transmission have also been suggested, including contact with the mucosae of urine droplets containing the parasites. Urine droplets are indeed produced and released by sand fly females during the blood meal through the prediuresis process. Information on transmission and development in mammals is limited
Leishmania tarentolae: natural history and evidence of infectivity in mammalian hosts
Although L. tarentolae, the type species of the subgenus Sauroleishmania [13], was described more than one century ago in the gecko Tarentola mauritanica [14], information on its natural history remains limited [11] with respect to other species within the subgenera Leishmania and Viannia. Indeed, the 21 species within the subgenus Sauroleishmania have historically been overlooked in leishmaniasis research, being associated with reptiles (from the families Agamidae, Gekkonidae, Lacertidae, Scincidae and Varanidae) and generally regarded to be non-infectious to mammals. In addition, Sauroleishmania species are transmitted by sand flies of the genus Sergentomyia, which have a primarily herpetophilic feeding behavior [15]. The detection of promastigotes of a protist parasite (Paleoleishmania proterus) mixed with reptilian nucleated blood cells in the midgut lumen of a Palaemyia burmitis female sand fly in mid-Cretaceous amber (approx. 100 MYA) suggested that Sauroleishmania evolved in reptiles [16, 17]. However, phylogenetic analyses placing Sauroleishmania as the sister group of the subgenus Leishmania, after the divergence of Viannia, supported the hypothesis that the ancestor of Sauroleishmania evolved in mammals and then switched to reptiles [12,13,14,15,16,17,18]. The distribution of L. tarentolae is limited to the Old World [19] in association with geckoes in Europe, North Africa and the Middle East [20,21,22]. The tight association between Sauroleishmania spp. and reptile-biting Sergentomyia sand flies [23,24,25,26] was challenged by the detection of mammalian blood in these sand fly species [27, 28]. These results also raised questions about the potential role of Sergentomyia sand flies as vectors of Leishmania infantum. Meanwhile, individuals of different species of Phlebotomus (e.g. Phlebotomus perfiliewi, P. perniciosus [29,30,31]) have been found positive for L. tarentolae, most likely as an effect of their opportunistic feeding behavior, which may be linked with several ecological factors and host availability [32]. These observations are further supported by the experimental infection of Phlebotomus papatasi [33, 34], Ph. perniciosus and Phlebotomus sergenti [34] with Sauroleishmania spp. On these premises, we can conclude that Sergentomyia is not the sole genus of sand flies in which Sauroleishmania can develop and that these Leishmania protozoan parasites could also circulate in mammals. Moreover, laboratory experiments have shown that New World sand flies, such as Lutzomyia longipalpis, are competent as vectors for L. tarentolae [35], thus suggesting the potential circulation of this parasite also in the Americas.
In the sand fly vector, Sauroleishmania has traditionally been classified as an hypopylarian microorganism since it develops mainly in the hindgut [36]. However, the development of some Sauroleishmania species in the midgut [33, 34, 37] of Ph. papatasi and Ph. perniciosus (i.e. peripylarian or suprapylarian), but not in Ph. sergenti, suggests that Sauroleishmania development is influenced by the insect species [34]. In particular, the presence of L. tarentolae promastigotes in the stomodeal valve of some Phlebotomus species [33, 34] supports the possibility of transmission through the blood meal, whereas the localization in the Malpighian tubules suggests transmission through urine droplets (i.e. the product of prediuresis), bite wounds or contact with the mucosae of the hosts. In addition to the many aspects of the Sauroleishmania—sand fly interactions, the potential infectivity of L. tarentolae for mammals has recently been supported after the molecular detection of DNA from this parasite in a 300-year-old Brazilian mummy [38]. This discovery also raised some questions about the host range and geographical distribution of this species and advocated for specific investigations aimed at defining their ability to infect mammalian hosts. It is interesting to note that another species belonging to the subgenus Sauroleishmania, Leishmania adleri, while primarily associated with ectothermic hosts, may infect humans causing transient skin lesions, similar to those observed in cutaneous leishmaniasis [39], and has also been detected in asymptomatic hamsters and mice [40]. Additionally, under laboratory conditions, L. tarentolae promastigotes differentiate into an amastigote-like form after uptake by mammalian DCs and macrophages [41,42,43,44], hinting at the possibility of natural infection in mammals. In Mediterranean countries, human and canine leishmaniases are endemic, and L. tarentolae has been isolated from different species of reptiles [12, 45, 46] and sand flies [28], and its DNA has also been detected by molecular methods in mammals [29, 38, 46, 47]. A recent molecular screening revealed the presence of L. tarentolae DNA in humans and sand flies from central Italy and Linosa island [29, 47]. Importantly, infection by L. tarentolae has also been hypothesized as being associated with a reduction in anti-L. infantum antibody titers in dogs seropositive to canine leishmaniasis, but clinically healthy [48]. Therefore, in regions where L. tarentolae is highly prevalent in reptile and sand fly populations, the presence of this parasite may represent a factor capable of inhibiting the development of clinical forms of canine leishmaniasis, which is associated with antibody-mediated inflammatory reactions [11]. This possibility is further supported by the finding of Sergentomya minuta as the most abundant sand fly species in canine leishmaniasis endemic areas [31].
In summary, available data confirm the sympatric circulation of L. infantum and L. tarentolae in central and southern Italy, also suggesting that geckoes may be infected with L. infantum. Furthermore, the interactions of these two species of Leishmania in these areas are evidenced in their overlap** circulation in “non-natural” hosts and vectors. Therefore, hosts and vectors that have historically been considered to be non-permissive to the one or the other of the two species could actually play a role in the epidemiological cycle of L. infantum and L. tarentolae.
Leishmania tarentolae as a biotech tool: use in the production protein antigens for the serodiagnosis of infectious diseases
The classical prokaryotic systems for protein expression and production (e.g. the well-known Escherichia coli system) lack essential components for proper protein folding and eukaryotic-type post-translational modifications. Eukaryotic expression systems have thus been proposed in recent decades, although these are characterized by several limitations, such as slow generation time and high costs of culture maintenance [49]. Leishmania tarentolae has been proposed as an alternative system for protein production that overcomes these limitations since it combines the advantages of both eukaryotic and prokaryotic systems: the maintenance and growth of L. tarentolae is accomplished at a low cost and is easily scalable to industrial production using bioreactors. In addition, L. tarentolae boasts a range of post-translational modifications, including mammalian-type N-glycosylation [50]. A particular focus of research is a strain of L. tarentolae that was originally isolated from lizards and subsequently cultivated in axenic culture for decades. This strain is commercially available as a eukaryotic protein expression platform (In Vivo LEXSY translation system, Jena Bioscience GmbH) and can be used to express both intracellular or secreted proteins under the activity of constitutive or inducible promoters (https://www.jenabioscience.com/). This system has successfully been exploited to produce both antigens for sero-diagnostic applications and vaccine development (see following section), as well as a variety of proteins to be used in crystallography.
A first obvious application of L. tarentolae is the production of protein antigens from other trypanosomatids that can be used in serological diagnosis. In 2015 Rooney and co-workers published the first study in which L. tarentolae was used to produce antigens from Trypanosoma brucei, with the aim to develop a rapid diagnostic test for African trypanosomiases [51]. Another experimental work exploited L. tarentolae for the expression of the rK39 antigen of L. infantum for serodiagnosis of visceral leishmaniasis (VL); the results showed satisfactory diagnostic accuracy when the antigen was tested on sera from patients with VL [52].
Leishmania tarentolae has also been explored as a means to produce protein antigens for the serodiagnosis of viral infections. The gold standard for serological diagnosis of viral infections implies the use of antigens produced in mammalian cells [53], with all the potential limitations, particularly in terms of fast production and rapid application in the case of emerging epidemics from novel pathogens. Two studies have so far been published on the use of protein antigens produced in L. tarentolae for the diagnosis of viral infections. In the first study, an antigen from the hepatitis E virus capsid protein, produced in L. tarentolae, was successfully used in an enzyme-linked immunosorbent assay (ELISA) for the detection of antiviral antibodies in porcine sera [54]. In a more recent study, antigens from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were thoroughly tested on sera from humans affected by coronavirus disease 2019 (COVID-19) and found to display diagnostic performances comparable to those achieved by antigens produced in human cells [55].
Recombinant protein yield by L. tarentolae can be improved in bioreactors and altered culture growth conditions. Pion et al. [56], for example, engineered Leishmania for the production of recombinant influenza hemagglutinins in biofermenter culture; changes in the culture volume (2 l) and agitation of the culture led to a tenfold increase in the yield. Taking safety issues into account, the production of recombinant proteins to be used as vaccines or in therapeutic applications should ideally avoid the use of culture media containing components of animal origin [57]. However, promastigotes of L. tarentolae are normally cultivated in liquid media with the addition of blood or animal serum, or other components of animal origin [58]. A commonly used medium is brain heart infusion (BHI) growth medium supplemented with animal serum, but there are alternatives for culturing L. tarentolae. For example, Fritsche et al. [59] tested yeast extract medium, with hemin as the sole component of animal origin. Schneider's Drosophila medium was also successfully tested for cultivation of Leishmania spp., but always in combination with animal serum. Finally, a classic study proposed catalase or high concentrations of peroxidase as substitute for hemin in BHI medium [60]. The above efforts to set up optimal media and culture conditions for L. tarentolae cultivation will likely increase its suitability as a tool for protein production at the industrial level, a prerequisite for translational application of this microorganism to produce antigens, vaccines or therapeutics [61].
Leishmania tarentolae as a surrogate of pathogenic leishmaniae in terms of vaccination, and the issue of immune modulation in anti-Leishmania vaccines
The origin of vaccines is traced back to 1796, when Edward Jenner proposed the inoculation of material from cowpox lesions as a treatment to protect humans from smallpox. In modern terminology, the first vaccine comprised a surrogate pathogen (the cowpox virus) that causes only mild infections in humans but nonetheless elicits cross-protective immunity against the human pathogen, namely the smallpox virus [62]. During the first half of the twentieth century, BCG was developed as a surrogate of the human pathogen M. tuberculosis and is still widely used in vaccination campaigns against tuberculosis (see section Background). The use of microorganisms that infect animal species other than humans as surrogates of human pathogens for vaccination targets is thus part of the history of vaccinology and should not be dismissed as an obsolete or useless strategy. Several lines of evidence suggest that L. tarentolae could represent a surrogate pathogen and that it is suitable to be assayed as a vaccine against human and canine leishmaniases. First, cross-protective immunity is well documented in Leishmania infections (for example see [63] and citations therein). The existence of cross-protective immunity in Leishmania species has also been documented at the molecular level [64] or using sub-components of Leishmania cells, as in the LBSap vaccine which, while being composed of antigens from Leishmania braziliensis, is protective against Leishmania infantum [65, 66]. Second, comparative genomics show that L. tarentolae shares approximately 90% of its genes with pathogenic species, including L. infantum and Leishmania major. However, several genes possibly associated with virulence are lacking in L. tarentolae, compared to pathogenic Leishmania species [67], which is reassuring in relation to safety issues. Third, current evidence indicates that L. tarentolae is not pathogenic to mammals, while still being capable of infecting macrophages and DCs, reaching the amastigote state [41, 44, 68]. Finally, the evidence for a circulation of L. tarentolae in dogs, and the absence of any evidence for its association with pathological outcomes, further emphasizes the potential utility of this parasite in anti-Leishmania vaccination (see [11] and section on L. tarentolae natural history and the evidence of infectivity in mammalian hosts).
A major issue that should be addressed to determine the potential of L. tarentolae as a vaccine against pathogenic leishmaniae is the type of immune modulation that this reptile parasite determines in mammals. There is a general consensus that hosts displaying classical macrophage activation (M1 activation), with specific polarization of CD4+ T cells towards the Th1 phenotype, are protected against infection and overt disease with most forms of leishmaniases, in both dogs and humans, while hosts displaying alternative macrophage activation (M2 activation) and a Th2-biased response are more exposed to the risk of develo** active Leishmania infection and overt disease [69, 70]. For this reason, a central focus in leishmaniasis vaccine research is the identification of adjuvants capable of determining a proper polarization of the immune response towards the M1/Th1 profile [71]. Initial studies, using the murine model, showed that L. tarentolae determines Th1-type cytokine production in vivo [41]. However, most of the subsequent studies suggested that elicitation of a Th1 response requires co-administration of the appropriate adjuvants, or the concomitant expression of immune-modulating molecules [72, 73]. However, the expression of M2 markers in human macrophages, after stimulation with L. tarentolae, was also observed [74]. A recent in vitro study on human DCs reported moderate production of Th1-type cytokines after stimulation by living promastigotes of L. tarentolae and also confirmed the penetration of L. tarentolae into DCs and their maturation, with expression of surface markers of activation, including MHC class II and co-stimulatory molecules [44]. The capacity of DCs to engulf L. tarentolae, the maturation of these cells after exposure to the parasite and evidence for the production, even though moderate, of Th1-associated cytokines are all coherent with the possibility that L. tarentolae possesses some of the characteristics required by an anti-Leishmania vaccine.
Despite the above theoretical arguments in favor of the potential utility of L. tarentolae as a vaccine against pathogenic Leishmania species, only a few studies have investigated the issue experimentally, always in rodent models. In a first seminal study, intraperitoneal immunization with L. tarentolae was found to achieve a protective immune response against Leishmania donovani in BALB/c mice, associated with a strong Th1 response in the absence of adjuvant [41]; this result was, however, not confirmed by other studies. In that study, the promastigotes of L. tarentolae were not engineered for the expression of any antigen from pathogenic Leishmania species, nor for any proteins capable of stimulating the immune response, but only for the production of the green fluorescent protein. Live, non-engineered, promastigotes of L. tarentolae have also been shown to provide cross-protective immunity against L. major [75, 76]. The possibility of exploiting wild-type L. tarentolae as a vaccine against dog or human pathogenic Leishmania species is thus worthy of further investigations (see section Concluding remarks).
Engineered Sauroleishmania strains as vaccines against pathogenic Leishmania species
One possible strategy to increase the immunogenicity of L. tarentolae as a vaccine against human and dog leishmaniases is to engineer this microorganism for the expression of antigens from pathogenic Leishmania species or of immune-modulating proteins associated with M1/Th1 immune activation. Briefly, in anti-leishmania vaccine studies, L. tarentolae has been genetically modified (GM) for the production of the following categories of proteins: (i) antigens from pathogenic Leishmania species; (ii) immune-modulating proteins of human origin; and (iii) antigens or immune-modulating proteins from the sand fly saliva. Strains of L. tarentoale have also been engineered for the production of both immune-modulating proteins and antigens from pathogenic Leishmania species. Studies on GM L. tarentolae in anti-Leishmania vaccination tests are summarized in Table 1. A brief account, focused on protein categories, is also provided in the following paragraphs.
Different protein antigens from pathogenic Leishmania species have been investigated in anti-Leishmania vaccine research, with L. tarentolae used as a vehicle for their production and delivery. For example, administration of L. tarentolae engineered to express the A2 antigen from L. donovani provided protection against L. infantum in a murine model of the infection [77]. Other studies using L. tarentolae as a vaccine vehicle exploited the LACK and KMP11 antigens (for the full name of these antigens, see Table 1), that are expressed both in the promastigote and the amastigote stage and are involved in the infection of mammalian hosts [78, 79]. Immunization with L. tarentolae expressing these proteins, co-adjuvated with CpG motifs, achieved a reduction of parasite load by Leishmania major in a murine model [73]. As a third example, live L. tarentolae expressing LPG3 from L. infantum, a protein chaperon involved in the biosynthesis of lipophosphoglycans, induced the expression of high level of interferon gamma (IFN-γ) in the murine model, and partial protection against L. infantum infection, without co-administration of any adjuvant [80].
As already emphasized, L. tarentolae has been exploited not only to express and deliver protein antigens from pathogenic Leishmania parasites, but also for the production and delivery of immune-modulating molecules. Recent findings point to CXCL-10 (C-X-C motif chemokine 10) as a potential tool for immunotherapy of cutaneous leishmaniasis, since this molecule favors a shift towards a Th1 pro-inflammatory response [81]. Indeed, inoculation of L. tarentolae expressing CXCL-10 in mice inhibited arginase activity and Th2 cytokine expression towards IFN-γ and nitric oxide production [82]. Another example of an immune-modulating molecule investigated in vaccine research is the human neutrophil peptide (HNP1), which is a member of the large antimicrobial peptides (AMPs) group [83]. In a study published in 2017, a strain of L. tarentolae engineered for the expression of HPN1 from L. major was tested in the murine model; the results showed containment of the parasite load after challenge with L. major itself [84].
The contribution of a vector’s saliva in the immunology of leishmaniases has been extensively been studied in recent decades, with identification of highly immunogenic molecules, such as the PpSP15 protein from Phlebotomus papatasi [85]. Regarding the use of engineered Sauroleishmania in vaccination, the administration of GM L. tarentolae expressing the cysteine proteinases A and B (CPA/CPB) genes, in co-administration with a DNA plasmid coding for the sand fly salivary antigen PpSP15, was shown to be very effective in murine models as a vaccine against L. major [86]. Leishmania tarentolae expressing PpSP15 was also effective in terms of immunization against L. major infection when administered in combination with CpG oligodeoxynucleotides [87]. More recently, the combination of two salivary proteins from the sand fly, PpSP15 and PpSP9, co-expressed and delivered by L. tarentolae, proved to be effective in immunization against two different Leishmania species, L. major and L. tropica [88].
Microbial vaccine vehicles: Leishmania tarentolae as an anti-viral vaccine
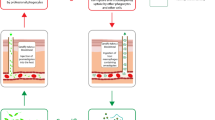
At the time of writing this article, four major vaccine technologies or platforms are being used extensively for the production of antiviral vaccines: (i) RNA vaccines delivered through lipid nanoparticles; (ii) adenovirus-based viral vectors; (iii) subunit vaccines, using recombinant protein antigens, produced in eukaryotic expression systems; and (iv) inactivated or attenuated viruses [89]. In addition to these types of platforms, a fifth approach is based on the administration of whole bacterial cells that are engineered for the expression of viral antigens (Fig. 3) [90,91,92].
Use of microbial vectors in vaccination against viruses and other pathogens. A gene coding for an antigen is selected from a pathogen and cloned for its expression into a microbial vehicle (1). Different types of expression could be exploited (2). The selected microbial vehicle is then used for the immunization of the host (3). GM, Genetically modified, WT, wild type
The core idea of this strategy is that GM bacteria can be exploited not only as micro-factories to produce the desired antigen, but also as vehicles for the delivery of the antigens themselves, ideally targeting DCs and lymph nodes. These GM bacteria are referred to as bacterial vaccine vehicles, or as living bacterial vehicles, when used without inactivation. In the context of antiviral vaccination, a major limitation with the use of bacteria as antigen vehicles, or even for the simple production of the antigens, is that protein expressed in bacteria are not subjected to post-translational modifications, which are instead characteristic of proteins produced in mammalian cells [93, 94]. Therefore, viral antigens expressed in bacteria are expected to present structural differences in comparison with antigens produced by mammalian cells during viral infections. In addition to bacteria, yeasts have also been proposed as vaccine vehicles for the production and delivery of viral antigens (see, for example, [95]). Indeed, production of protein antigens in yeasts ensures post-translational modification of the antigen, but the glycosylation pattern in yeast-produced proteins is rather different from that of mammalian proteins [96].
Considering the glycosylation issue, L. tarentolae emerges as an alternative solution to produce viral antigens: as outlined above, the glycosylation pattern in this microorganism resembles that of mammals. In addition, both the easy culturing and genetic engineering of L. tarentolae, as well as its propensity to target DCs, make this protozoon an ideal candidate for its use as a microbial antigen vehicle for anti-viral vaccination. We emphasize that the use of GM L. tarentolae as candidate anti-Leishmania vaccines (see above sections) also exploits this microorganism as a vehicle for the antigen. Published studies on the use of L. tarentolae as an antigen vehicle in anti-viral vaccination have so far targeted eight different viruses (Table 1). A first study in this area exploited L. tarentolae for the expression and delivery of the Gag protein from human immunodeficiency virus type 1 (HIV-1). The assays in the murine model led to an effective immune response, with the production of neutralizing antibodies [42]. Another virus that has been targeted in preclinical studies using GM L. tarentolae is human papillomavirus (HPV), in a murine model of the tumor associated with this virus [97]; the results of that study revealed a contained growth of the tumor in vaccinated mice. Finally, GM L. tarentolae expressing the spike protein of SARS-CoV-2 has recently been assayed in mice, and the production of neutralizing antibodies was observed [98]. In the latter study, rectal administration of the engineered strain of L. tarentolae was also shown to be effective in the induction of the neutralizing response, paving the way toward the use of L. tarentolae as an antigen vehicle for mucosal vaccination.
In addition to the exploitation of L. tarentolae as an antigen vehicle, this protozoon has also been used to produce viral protein antigens that have been assayed as purified proteins, alone or in combination with GM L. tarentolae strains [e.g. 98], or virus-like particles, that have been assayed in preclinical studies against four different viruses (Table 1). Finally, L. tarentolae has also been investigated for the production of a candidate vaccine against the apicomplexan protozoon Toxoplasma gondii [99]; in that study, a multi-epitope vector-based vaccine, based on immunodominant epitopes of five Toxoplasma antigens, conferred protective immunity against acute toxoplasmosis in BALB/c mice [99].
Concluding remarks
Leishmania tarentolae has a great potential as a candidate vaccine, both as non-engineered, WT organism, possibly in combination with adjuvating molecules favoring Th1-biased responses, or after its engineering, for the expression of antigens and/or immune-modulating molecules. However, several research issues still need to be addressed for an effective translation into practice. The use of L. tarentolae as a wild-type whole organism for vaccination of dogs (and possibly humans) will require further studies in rodent models, with also the need to understand whether the parasite can actually replicate within the mammalian host, ensuring adequate stimulation of the immune response, without causing any pathological alteration. In this context, a very important issue is whether wild-type L. tarentolae administered as living cells would benefit from co-administration with an adjuvant. Indeed, co-administration with adjuvants could stimulate an excessive immune response against L. tarentolae, with early inactivation of the parasites, and thus reduced replication. Therefore, investigations on adjuvating strategies capable of favoring the immune response in the Th1 direction while allowing L. tarentolae to undergo a few replication cycles will be of great importance. Regarding the strains of L. tarentolae engineered for vaccination against pathogenic protozoa of the genus Leishmania, viruses or other infectious agents, the effective use of these strains will require addressing the problems related with their status as GM organisms, obtained by incorporating one or more genes for antibiotic resistance. The actual use of these strains could therefore require the removal of resistance genes or the development of metabolic complementation strategies for the selection of transformed strains as an alternative to antibiotic-based selection. More generally, the production of L. tarentolae cells for an effective use in vaccination campaigns will require production to be scaled up to the level of industrial bio-fermenters, with an optimization of the culture media, in the absence of antibiotic pressure (see above). In any case, the increased interest in L. tarentolae, both as a vaccine platform and more generally as a micro-factory to produce recombinant proteins for a variety of applications, will likely lead to an acceleration of research on this organism and to solutions to the above issues.
Availability of data and materials
Not applicable.
References
Worbs T, Hammerschmidt SI, Förster R. Dendritic cell migration in health and disease. Nat Rev Immunol. 2017;17:30–48.
Martínez-López M, Soto M, Iborra S, Sancho D. Leishmania hijacks myeloid cells for immune escape. Front Microbiol. 2018;9:883.
Liu D, Uzonna JE. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol. 2012;2:83.
Saljoughian N, Taheri T, Rafati S. Live vaccination tactics: possible approaches for controlling visceral leishmaniasis. Front Immunol. 2014;5:134.
Moafi M, Rezvan H, Sherkat R, Taleban R. Leishmania vaccines entered in clinical trials: a review of literature. Int J Prev Med. 2019;10:95.
Volpedo G, Bhattacharya P, Gannavaram S, Pacheco-Fernandez T, Oljuskin T, Dey R, et al. The history of live attenuated centrin gene-deleted Leishmania vaccine candidates. Pathogens. 2022;11:431.
Kedzierski L, Zhu Y, Handman E. Leishmania vaccines: progress and problems. Parasitology. 2006;133:87–112.
Detmer A, Glenting J. Live bacterial vaccines—a review and identification of potential hazards. Microb Cell Fact. 2006;5:23.
Lobo N, Brooks NA, Zlotta AR, Cirillo JD, Boorjian S, Black PC, et al. 100 years of Bacillus Calmette-Guérin immunotherapy: from cattle to COVID-19. Nat Rev Urol. 2021;18:611–22.
Mouhoub E, Domenech P, Ndao M, Reed MB. The diverse applications of recombinant BCG-based vaccines to target infectious diseases other than tuberculosis: an overview. Front Microbiol. 2021;12:757858.
Mendoza-Roldan JA, Zatelli A, Latrofa MS, Iatta R, Bezerra-Santos MA, Annoscia G, et al. Leishmania (Sauroleishmania) tarentolae isolation and sympatric occurrence with Leishmania (Leishmania) infantum in geckoes, dogs and sand flies. PLoS Negl Trop Dis. 2022;16:e0010650.
Klatt S, Simpson L, Maslov DA, Konthur Z. Leishmania tarentolae: Taxonomic classification and its application as a promising biotechnological expression host. PLoS Negl Trop Dis. 2019;13:e0007424.
Ranque P. Étude morphologique et biologique de quelques Trypanosomidés récoltés au Sénégal. PhD thesis. Marseille: Université d’Aix-Marseille II; 1973.
Wenyon CM. Observations on the intestinal protozoa of three Egyptian lizards, with a note on a cell-invading fungus. Parasitology. 1920;12:350–65.
Lewis DJ. The phlebotomine sandflies (Diptera: Psychodidae) of the Oriental Region. Syst Entomol. 1987;12:163–80.
Poinar G Jr, Poinar R. Evidence of vector-borne disease of early Cretaceous reptiles. Vector Borne Zoonotic Dis. 2004;4:281–4.
Poinar G Jr, Poinar R. Paleoleishmania proterus n. gen., n. sp., (Trypanosomatidae: Kinetoplastida) from Cretaceous Burmese amber. Protist. 2004;155:305–10.
Schönian G. Genetics and evolution of Leishmania parasites. Infect Genet Evol. 2017;50:93–4.
Akhoundi M, Kuhls K, Cannet A, Votýpka J, Marty P, Delaunay P, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10:e0004349.
Telford SRJ. A review of trypanosomes of gekkonid lizards, including the description of five new species. Syst Parasitol. 1995;31:37–52.
Sloboda M, Kamler M, Bulantová J, Votýpka J, Modrý D. A new species of Hepatozoon (Apicomplexa: Adeleorina) from Python regius (Serpentes: Pythonidae) and its experimental transmission by a mosquito vector. J Parasitol. 2007;93:1189–98.
Halla U, Korbel R, Mutschmann F, Rinder M. Blood parasites in reptiles imported to Germany. Parasitol Res. 2014;113:4587–99.
Killick-Kendrick R, Lainson R, Rioux JA, Saf’janova VM. The taxonomy of Leishmania-like parasites of reptiles. In: Rioux JA, editor. Leishmania: Taxonomie et phylogenèse. Application Éco-epidemiologiques (Colloque International du CNRS/INSERM, 1984). Montpellier: MEE; 1986. p. 143–8.
Maroli M, Gramiccia M, Gradoni L, Ready PD, Smith DF, Aquino C. Natural infections of Phlebotomine sandflies with Trypanosomatidae in central and south Italy. Trans R Soc Trop Med Hyg. 1988;82:227–8.
Rashti MS, Mohebali M. Natural promastigote infection of Sergentomyja sintoni its seasonal variation and reservoir host in Turkemen Sahapa Iran. Iran J Public Health. 1994;23:41–50.
Karimi A, Hanafi-Bojd AA, Yaghoobi-Ershadi MR, Akhavan AA, Ghezelbash Z. Spatial and temporal distributions of phlebotomine sand flies (Diptera: Psychodidae), vectors of leishmaniasis, in Iran. Acta Trop. 2014;132:131–9.
Abbate JM, Maia C, Pereira A, Arfuso F, Gaglio G, Rizzo M, et al. Identification of trypanosomatids and blood feeding preferences of phlebotomine sand fly species common in Sicily, Southern Italy. PLoS ONE. 2020;15:e0229536.
Maia C, Depaquit J. Can Sergentomyia (Diptera, Psychodidae) play a role in the transmission of mammal-infecting Leishmania? Parasite. 2016;23:55.
Pombi M, Giacomi A, Barlozzari G, Mendoza-Roldan J, Macrì G, Otranto D, et al. Molecular detection of Leishmania (Sauroleishmania) tarentolae in human blood and Leishmania (Leishmania) infantum in Sergentomyia minuta: unexpected host-parasite contacts. Med Vet Entomol. 2020;34:470–5.
Latrofa MS, Mendoza-Roldan J, Manoj R, Dantas-Torres F, Otranto D. A duplex real-time PCR assay for the detection and differentiation of Leishmania infantum and Leishmania tarentolae in vectors and potential reservoir hosts. Entomol Gen. 2021;41:543–51.
Mendoza-Roldan JA, Latrofa MS, Iatta R, Manoj RRS, Panarese R, Annoscia G, et al. Detection of Leishmania tarentolae in lizards, sand flies and dogs in southern Italy, where Leishmania infantum is endemic: hindrances and opportunities. Parasit Vectors. 2021;14:1–12.
Quate LW. Phlebotomus sandflies of the Paloich area in the Sudan (Diptera, Psychodidae). J Med Entomol. 1964;1:213–68.
Adler S, Theodor O. Observations on Leishmania ceramodactyli n.sp. Trans R Soc Trop Med Hyg. 1929;22:343–55.
Ticha L, Kykalova B, Sadlova J, Gramiccia M, Gradoni L, Volf P. Development of various Leishmania (Sauroleishmania) tarentolae strains in three Phlebotomus species. Microorganisms. 2021;9:2256.
Diaz-Albiter HM, Regnault C, Alpizar-Sosa EA, McGuinness D, Barrett M, Dillon RJ. Non-invasive visualisation and identification of fluorescent Leishmania tarentolae in infected sand flies. Wellcome Open Res. 2018;3:160.
Lainson R, Shaw JJ. Evolution, classification and geographical distribution. In: Peters W, Killick-Kendrick R, editors. The Leishmaniases in biology and medicine, vol. 1. Biology and epidemiology. London: Academic; 1987. p. 1–120.
Adler S, Theodor O. Investigation on Mediterranean kala azar X—a note on Trypanosoma platydactyli and Leishmania tarentolae. Proc R Soc B Biol Sci. 1935;116:543–4.
Novo SP, Leles D, Bianucci R, Araujo A. Leishmania tarentolae molecular signatures in a 300 hundred-years-old human Brazilian mummy. Parasit Vectors. 2015;8:72.
Manson-Bahr PE, Heisch RB. Transient infection of man with a Leishmania (L. adleri) of lizards. Ann Trop Med Parasitol. 1961;55:381–2.
Adler S. The behavior of a lizard Leishmania in hamsters and baby mice. Rev Inst Med Trop Sao Paulo. 1962;4:61–4.
Breton M, Tremblay MJ, Ouellette M, Papadopoulou B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect Immun. 2005;73:6372–82.
Breton M, Zhao C, Ouellette M, Tremblay MJ, Papadopoulou B. A recombinant non-pathogenic Leishmania vaccine expressing human immunodeficiency virus 1 (HIV-1) Gag elicits cell-mediated immunity in mice and decreases HIV-1 replication in human tonsillar tissue following exposure to HIV-1 infection. J Gen Virol. 2007;88:217–25.
Taylor VM, Muñoz DL, Cedeño DL, Vélez ID, Jones MA, Robledo SM. Leishmania tarentolae: utility as an in vitro model for screening of antileishmanial agents. Exp Parasitol. 2010;126:471–5.
Varotto-Boccazzi I, Garziano M, Cattaneo GM, Bisaglia B, Gabrieli P, Biasin M, et al. Leishmania tarentolae as an antigen delivery platform: dendritic cell maturation after infection with a clone engineered to express the SARS-CoV-2 spike protein. Vaccines. 2022;10:803.
Pozio E, Gramiccia M, Gradoni L, Maroli M. Hemoflagellates in Cyrtodactylus kotschyi (Steindachner, 1870) (Reptilia, Gekkonidae) in Italy. Acta Trop. 1983;40:399–400.
Mendoza-Roldan JA, Latrofa MS, Tarallo VD, Manoj RR, Bezerra-Santos MA, Annoscia G, et al. Leishmania spp. in Squamata reptiles from the Mediterranean basin. Transbound Emerg Dis. 2021;69:2856–66.
Iatta R, Mendoza-Roldan JA, Latrofa MS, Cascio A, Brianti E, Pombi M, et al. Leishmania tarentolae and Leishmania infantum in humans, dogs and cats in the Pelagie archipelago, southern Italy. PLoS Negl Trop Dis. 2021;15:e0009817.
Cavalera MA, Iatta R, Panarese R, Mendoza-Roldan JA, Gernone F, Otranto D, et al. Seasonal variation in canine anti-Leishmania infantum antibody titres. Vet J. 2021;271:105638.
Gomes AR, Byregowda SM, Veeregowda BM, Balamurugan V. An overview of heterologous expression host systems for the production of recombinant proteins. Adv Anim Vet Sci. 2016;4:346–56.
Niimi T. Recombinant protein production in the eukaryotic protozoan parasite Leishmania tarentolae: a review. Methods Mol Biol. 2012;824:307–15.
Rooney B, Piening T, Büscher P, Rogé S, Smales CM. Expression of Trypanosoma brucei gambiense antigens in Leishmania tarentolae. Potential for use in rapid serodiagnostic tests (RDTs). PLoS Negl Trop Dis. 2015;9:e0004271.
Rezaei Z, Van Reet N, Pouladfar G, Kühne V, Ramezani A, Sarkari B. Expression of a rK39 homologue from an Iranian Leishmania infantum isolate in Leishmania tarentolae for serodiagnosis of visceral leishmaniasis. Parasit Vectors. 2019;12:593.
Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–3.
Baechlein C, Meemken D, Pezzoni G, Engemann C, Grummer B. Expression of a truncated hepatitis E virus capsid protein in the protozoan organism Leishmania tarentolae and its application in a serological assay. J Virol Methods. 2013;193:238–43.
Varotto-Boccazzi I, Manenti A, Dapporto F, Gourlay LJ, Bisaglia B, Gabrieli P, et al. Epidemic preparedness-Leishmania tarentolae as an easy-to-handle tool to produce antigens for viral diagnosis: application to COVID-19. Front Microbiol. 2021;12:736530.
Pion C, Courtois V, Husson S, Bernard MC, Nicolai MC, Talaga P, et al. Characterization and immunogenicity in mice of recombinant influenza haemagglutinins produced in Leishmania tarentolae. Vaccine. 2014;32:5570–6.
Sodoyer R. Expression systems for the production of recombinant pharmaceuticals. BioDrugs. 2004;18:51–62.
Chang KP, Fish WR. Leishmania. In: Jensen JB, editor. In vitro cultivation of protozoan parasites. Boca Raton: CRC Press Inc; 1983. p. 111–53.
Fritsche C, Sitz M, Weiland N, Breitling R, Pohl HD. Characterization of the growth behavior of Leishmania tarentolae: a new expression system for recombinant proteins. J Basic Microbiol. 2007;47:384–93.
Gaughan PLZ, Krassner SM. Hemin deprivation in culture stages of the hemoflagellate, Leishmania tarentolae. Comp Biochem Physiol B. 1971;39:5–18.
Habibzadeh S, Doroud D, Taheri T, Seyed N, Rafati S. Leishmania parasite: the impact of new serum-free medium as an alternative for fetal bovine serum. Iran Biomed J. 2021;25:349–58.
Riedel S. Edward Jenner and the history of smallpox and vaccination. Proc (Bayl Univ Med Cent). 2005;18:21–5.
Aguilar-Be I, da Silva Zardo R, Paraguai de Souza E, Borja-Cabrera GP, Rosado-Vallado M, Mut-Martin M, et al. Cross-protective efficacy of a prophylactic Leishmania donovani DNA vaccine against visceral and cutaneous murine leishmaniasis. Infect Immun. 2005;73:812–9.
Nico D, Gomes DC, Alves-Silva MV, Freitas EO, Morrot A, Bahia D, et al. Cross-protective immunity to Leishmania amazonensis is mediated by CD4+ and CD8+ epitopes of Leishmania donovani nucleoside hydrolase terminal domains. Front Immunol. 2014;5:189.
Resende LA, Roatt BM, Aguiar-Soares RD, Viana KF, Mendonça LZ, Lanna MF, et al. Cytokine and nitric oxide patterns in dogs immunized with LBSap vaccine, before and after experimental challenge with Leishmania chagasi plus saliva of Lutzomyia longipalpis. Vet Parasitol. 2013;198:371–81.
de Mendonça LZ, Resende LA, Lanna MF, Aguiar-Soares RD, Roatt BM, Castro RA, et al. Multicomponent LBSap vaccine displays immunological and parasitological profiles similar to those of Leish-Tec® and Leishmune® vaccines against visceral leishmaniasis. Parasit Vectors. 2016;9:472.
Raymond F, Boisvert S, Roy G, Ritt JF, Legare D, Isnard A, et al. Genome sequencing of the lizard parasite Leishmania tarentolae reveals loss of genes associated to the intracellular stage of human pathogenic species. Nucleic Acids Res. 2012;40:1131–47.
Geroldinger G, Rezk M, Idris R, Gruber V, Tonner M, Moldzio R, et al. Techniques to study phagocytosis and uptake of Leishmania tarentolae by J774 macrophages. Exp Parasitol. 2019;197:57–64.
Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392:951–70.
Rossi M, Fasel N. How to master the host immune system? Leishmania parasites have the solutions! Int Immunol. 2018;30:103–11.
Varotto-Boccazzi I, Epis S, Arnoldi I, Corbett Y, Gabrieli P, Paroni M, et al. Boosting immunity to treat parasitic infections: Asaia bacteria expressing a protein from Wolbachia determine M1 macrophage activation and killing of Leishmania protozoans. Pharmacol Res. 2020;161:105288.
Ansari N, Rafati S, Taheri T, Roohvand F, Farahmand M, Hajikhezri Z, et al. Non-pathogenic Leishmania tarentolae vector based- HCV polytope DNA vaccine elicits potent and long lasting Th1 and CTL responses in BALB/c mice model. Mol Immunol. 2019;111:152–61.
Salari S, Sharifi I, Keyhani AR, GhasemiNejadAlmani P. Evaluation of a new live recombinant vaccine against cutaneous leishmaniasis in BALB/c mice. Parasit Vectors. 2020;13:415.
Badirzadeh A, Montakhab-Yeganeh H, Miandoabi T. Arginase/nitric oxide modifications using live non-pathogenic Leishmania tarentolae as an effective delivery system inside the mammalian macrophages. J Parasit Dis. 2021;45:65–71.
Keshavarzian N, Noroozbeygi M, Haji M, Hoseini M, Yeganeh F. Evaluation of leishmanization using Iranian lizard Leishmania mixed with CpG-ODN as a candidate vaccine against experimental murine leishmaniasis. Front Immunol. 2020;11:1725.
Haghdoust S, Noroozbeygi M, Hajimollahoseini M, Masooleh MM, Yeganeh F. A candidate vaccine composed of live nonpathogenic Iranian lizard Leishmania mixed with chitin microparticles protects mice against Leishmania major infection. Acta Trop. 2021;227:106298.
Mizbani A, Taheri T, Zahedifard F, Taslimi Y, Azizi H, Azadmanesh K, et al. Recombinant Leishmania tarentolae expressing the A2 virulence gene as a novel candidate vaccine against visceral leishmaniasis. Vaccine. 2009;28:53–62.
Kelly BL, Stetson DB, Locksley RM. Leishmania major LACK antigen is required for efficient vertebrate parasitization. J Exp Med. 2003;198:1689–98.
de Mendonça SC, Cysne-Finkelstein L, Matos DC. Kinetoplastid membrane protein-11 as a vaccine candidate and a virulence factor in Leishmania. Front Immunol. 2015;6:524.
Pirdel L, Farajnia S. A non-pathogenic recombinant Leishmania expressing Lipophosphoglycan 3 against experimental infection with Leishmania infantum. Scand J Immunol. 2017;86:15–22.
Vasquez RE, Soong L. CXCL10/gamma interferon-inducible protein 10-mediated protection against Leishmania amazonensis infection in mice. Infect Immun. 2006;74:6769–77.
Montakhab-Yeganeh H, Abdossamadi Z, Zahedifard F, Taslimi Y, Badirzadeh A, Saljoughian N, et al. Leishmania tarentolae expressing CXCL-10 as an efficient immunotherapy approach against Leishmania major-infected BALB/c mice. Parasite Immunol. 2017;39:e12461.
Dutta P, Das S. Mammalian antimicrobial peptides: promising therapeutic targets against infection and chronic inflammation. Curr Top Med Chem. 2016;16:99–129.
Abdossamadi Z, Taheri T, Seyed N, Montakhab-Yeganeh H, Zahedifard F, Taslimi Y, et al. Live Leishmania tarentolae secreting HNP1 as an immunotherapeutic tool against Leishmania infection in BALB/c mice. Immunotherapy. 2017;9:1089–102.
Lestinova T, Rohousova I, Sima M, de Oliveira CI, Volf P. Insights into the sand fly saliva: blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl Trop Dis. 2017;11:e0005600.
Zahedifard F, Gholami E, Taheri T, Taslimi Y, Doustdari F, Seyed N, et al. Enhanced protective efficacy of nonpathogenic recombinant Leishmania tarentolae expressing cysteine proteinases combined with a sand fly salivary antigen. PLoS Negl Trop Dis. 2014;8:e2751.
Katebi A, Gholami E, Taheri T, Zahedifard F, Habibzadeh S, Taslimi Y, et al. Leishmania tarentolae secreting the sand fly salivary antigen PpSP15 confers protection against Leishmania major infection in a susceptible BALB/c mice model. Mol Immunol. 2015;67:501–11.
Lajevardi MS, Gholami E, Taheri T, Sarvnaz H, Habibzadeh S, Seyed N, et al. Leishmania tarentolae as potential live vaccine co-expressing distinct salivary gland proteins against experimental cutaneous leishmaniasis in BALB/c mice model. Front Immunol. 2022;13:895234.
Hahn WO, Wiley Z. COVID-19 vaccines. Infect Dis Clin North Am. 2022;36:481–94.
Lee MH, Kim BJ. COVID-19 vaccine development based on recombinant viral and bacterial vector systems: combinatorial effect of adaptive and trained immunity. J Microbiol. 2022;60:321–34.
Soto JA, Díaz FE, Retamal-Díaz A, Gálvez NMS, Melo-González F, Piña-Iturbe A, et al. BCG-Based vaccines elicit antigen-specific adaptive and trained immunity against SARS-CoV-2 and Andes orthohantavirus. Vaccines. 2022;10:721.
Yoon W, Park Y, Kim S, Bang IS. Development of an oral Salmonella-based vaccine platform against SARS-CoV-2. Vaccines. 2022;10:67.
Walsh G, Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol. 2006;24:1241–52.
McElwain L, Phair K, Kealey C, Brady D. Current trends in biopharmaceuticals production in Escherichia coli. Biotechnol Lett. 2022;44:917–31.
Lei H, **e B, Gao T, Cen Q, Ren Y. Yeast display platform technology to prepare oral vaccine against lethal H7N9 virus challenge in mice. Microb Cell Fact. 2020;19:53.
Brooks SA. Protein glycosylation in diverse cell systems: implications for modification and analysis of recombinant proteins. Expert Rev Proteomics. 2006;3:345–59.
Salehi M, Taheri T, Mohit E, Zahedifard F, Seyed N, Taslimi Y, et al. Recombinant Leishmania tarentolae encoding the HPV type 16 E7 gene in tumor mice model. Immunotherapy. 2012;4:1107–20.
Epis S, Varotto Boccazzi I, Manenti A, Rubolini D, Gabrieli P, Cattaneo G, et al. Efficacy of mucosal vaccination using a protozoan parasite as a vehicle for antigen delivery: IgG and neutralizing response after rectal administration of LeCoVax-2, a candidate vaccine against COVID-19. Pharmacol Res. 2022;186:106546.
Majidiani H, Dalimi A, Ghaffarifar F, Pirestani M. Multi-epitope vaccine expressed in Leishmania tarentolae confers protective immunity to Toxoplasma gondii in BALB/c mice. Microb Pathog. 2021;155:104925.
Grzyb K, Czarnota A, Brzozowska A, Cieślik A, Rąbalski Ł, Tyborowska J, et al. Immunogenicity and functional characterization of Leishmania-derived hepatitis C virus envelope glycoprotein complex. Sci Rep. 2016;6:30627.
Hosseinzadeh S, Bolhassani A, Rafati S, Taheri T, Zahedifard F, Daemi A, et al. A non-pathogenic live vector as an efficient delivery system in vaccine design for the prevention of HPV16 E7-overexpressing cancers. Drug Deliv. 2013;20:190–8.
Bolhassani A, Shirbaghaee Z, Agi E, Davoudi N. VLP production in Leishmania tarentolae: a novel expression system for purification and assembly of HPV16 L1. Protein Expr Purif. 2015;116:7–11.
Czarnota A, Tyborowska J, Peszyńska-Sularz G, Gromadzka B, Bieńkowska-Szewczyk K, Grzyb K. Immunogenicity of Leishmania-derived hepatitis B small surface antigen particles exposing highly conserved E2 epitope of hepatitis C virus. Microb Cell Fact. 2016;15:62.
Czarnota A, Offersgaard A, Pihl AF, Prentoe J, Bukh J, Gottwein JM, et al. Specific antibodies induced by immunization with hepatitis B virus-like particles carrying hepatitis C virus envelope glycoprotein 2 epitopes show differential neutralization efficiency. Vaccines. 2020;8:294.
Panasiuk M, Zimmer K, Czarnota A, Grzyb K, Narajczyk M, Peszyńska-Sularz G, et al. Immunization with Leishmania tarentolae-derived norovirus virus-like particles elicits high humoral response and stimulates the production of neutralizing antibodies. Microb Cell Fact. 2021;20:186.
Saljoughian N, Taheri T, Zahedifard F, Taslimi Y, Doustdari F, Bolhassani A, et al. Development of novel prime-boost strategies based on a tri-gene fusion recombinant L. tarentolae vaccine against experimental murine visceral leishmaniasis. PLoS Negl Trop Dis. 2013;7:e2174.
Taslimi Y, Zahedifard F, Taheri T, Doroud D, Latif Dizaji S, Saljoughian N, et al. Comparison of protective potency of DNA and live vaccines expressing A2-CPA-CPB-CTE antigens against visceral leishmaniasis in syrian hamster as preliminary study. Iran J Parasitol. 2020;15:383–92.
Nasiri V, Dalimi A, Ghaffarifar F, Bolhassani A. Immunogenicity and efficacy of live L. tarentolae expressing KMP11-NTGP96-GFP fusion as a vaccine candidate against experimental visceral Leishmaniasis caused by L. infantum. Iran J Parasitol. 2016;11:144–58.
Shirbaghaee Z, Bolhassani A, Mirshafiey A, Motevalli F, Zohrei N. A live vector expressing HPV16 L1 generates an adjuvant-induced antibody response in-vivo. Iran J Cancer Prev. 2015;8:e3991.
Acknowledgements
Figures were created with BioRender.com. CB and SE thank the GSA-IDEA project. CB, SE and DO thank the PNRR project PE-13, INF-ACT One Health Basic and Translational Research Actions addressing Unmet Needs on Emerging Infectious Diseases.
Funding
CB and SE received funding from “Fondazione Romeo ed Enrica Invernizzi” (Grant Agreements No. LIB_VT21SEPIS and LIB_VT22CBAND).
Author information
Authors and Affiliations
Contributions
CB, DO, SE: conceived and designed the structure and overall content of this article. MP, MB, AA, IVB, JAMR, DO, VNLF, AM, GVZ, EM: conducted the literature search, screened articles, extracted data and conceived the figures. CB, SE, JAMR, DO: wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Bandi, C., Mendoza-Roldan, J.A., Otranto, D. et al. Leishmania tarentolae: a vaccine platform to target dendritic cells and a surrogate pathogen for next generation vaccine research in leishmaniases and viral infections. Parasites Vectors 16, 35 (2023). https://doi.org/10.1186/s13071-023-05651-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05651-1