Abstract
Background
Yarrowia lipolytica, one of the most charming chassis cells in synthetic biology, is unable to use xylose and cellodextrins.
Results
Herein, we present work to tackle for the first time the engineering of Y. lipolytica to produce lipids from cellodextrins and xylose by employing rational and combinatorial strategies. This includes constructing a cellodextrin-phosphorolytic Y. lipolytica by overexpressing Neurospora crassa cellodextrin transporter, Clostridium thermocellum cellobiose/cellodextrin phosphorylase and Saccharomyces cerevisiae phosphoglucomutase. The effect of glucose repression on xylose consumption was relieved by installing a xylose uptake facilitator combined with enhanced PPP pathway and increased cytoplasmic NADPH supply. Further enhancing lipid production and interrupting its consumption conferred the obese phenotype to the engineered yeast. The strain is able to co-ferment glucose, xylose and cellodextrins efficiently, achieving a similar μmax of 0.19 h−1, a qs of 0.34 g-s/g-DCW/h and a YX/S of 0.54 DCW-g/g-s on these substrates, and an accumulation of up to 40% of lipids on the sugar mixture and on wheat straw hydrolysate.
Conclusions
Therefore, engineering Y. lipolytica capable of assimilating xylose and cellodextrins is a vital step towards a simultaneous saccharification and fermentation (SSF) process of LC biomass, allowing improved substrate conversion rate and reduced production cost due to low demand of external glucosidase.
Similar content being viewed by others
Background
The use of renewable resource such as lignocellulosic biomass (or LC biomass) as feedstock for industrial activities will play an essential role for establishing a more sustainable society [1]. While hemicellulose degradation mainly releases xylose, the enzymatic hydrolysis of cellulose only generates hexose. Many of the commercially interest fungal cellulases display low β-glucosidase activity, rendering the hydrolysis of cellodextrins especially cellobiose a rate-limiting step during enzymatic hydrolysis of LC biomass [2]. To achieve efficient LC biomass conversion, different strategies have been employed in recent years. These include metabolic engineering of the production microorganisms [3, 4], engineering of the cellulases [5, 6], strain evolution [7], and the design and construction of artificial microbial consortia [8], etc.
The unconventional yeast Yarrowia lipolytica is an attractive workhorse for a variety of applications in detergent, food and pharmaceutical industries [9]. Advantageously, its extraordinary ability to accumulate high cellular content of lipids (more than 30% of its dry cell weight) [10] and its “Generally Recognized as Safe” (GRAS) status, have made this yeast an outstanding host for the production of commercially-useful lipids [11,12,13]. Nevertheless, despite these advantages, native strain of Y. lipolytica is unable to use xylose and cellodextrins as carbon sources. Therefore, engineering Y. lipolytica capable of assimilating xylose and cellodextrins is a vital step towards a simultaneous saccharification and fermentation (SSF) process of LC biomass, allowing improved substrate conversion rate and reduced production cost due to low demand of external glucosidase.
In this respect, examples of recent work performed on Y. lipolytica are noteworthy. A xylose-fermenting Y. lipolytica was constructed by introducing Scheffersomyces stipitis xylose reductase (SsXR) and xylitol dehydrogenase (SsXDH), plus overexpressing the endogenous xylulokinase (YlXK). The resulting strain was able to produce citric acid and lipid from xylose [14]. We also made the first attempt to develop an engineered strain of Y. lipolytica to co-ferment cellobiose and xylose [15]. However, the xylose-fermenting Y. lipolytica demonstrated a preferred glucose utilization over xylose. Also, the reported consumption rates of cellobiose and xylose, and the lipid production yield of the engineered strain were inferior to those obtained in glucose fermentation.
Herein, we present work to tackle for the first time the engineering of Y. lipolytica to produce lipids from cellodextrins and xylose by employing rational and combinatorial strategies. First of all, the construction of a cellodextrins-phosphorolytic Y. lipolytica was achieved by expressing the Neurospora crassa cellodextrin transporter (NcCdt1) [16], Clostridium thermocellum cellobiose/cellodextrin phosphorylase (CtCbp/CtCdp) [17], and the S. cerevisiae phosphoglucomutase (ScPgm2p) [15] (Fig. 1). This strain is capable of cellodextrin-phosphorolysis and glucose-1-phosphate (Glc-1P) production, and is expected to display an energetic advantage over the β-glucosidase-producing strains, since less ATP is consumed in glucose phosphorylation in glycolysis [18]. Then, xylose-fermenting ability was introduced into the above strain by expressing the three key genes in xylose assimilation pathway (SsXR, SsXDH and YlXK), and the gene CiGXF1 for xylose uptake. Finally, the obese phenotype was conferred to the engineered Y. lipolytica by overexpressing the genes GPD1 and DGA2. Gpd1p is involved in the production of precursor for TAG [19], while Dga2p catalyses the synthesis of TAG [20]. In addition, the genes involved in lipid degradation were interrupted. Both xylose assimilation and lipids production were enhanced by increasing the cytoplasmic NADPH supply via the overexpression of the genes ZWF1 and GND1, encoding glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase, respectively, in the pentose phosphate pathwapy (PPP). This work paved way for the development of the engineered strains of Y. lipolytica to produce valuable chemicals from lignocellulosic hydrolysates at low cost for the advanced generation biorefinery.
The contrustion of cellodextrin-phosphorololytic Y. lipolytica strains and their evluation. a the schematic diagram of the strategies used in strain engineering. Heterologous genes overexpressed included those encoding: CDT cellodextrin transporter, cbp cellobiose phosphorylase, cdp cellodextrin phosphorylase, PGM phosphoglucomutase, Comparison of the growth of (b) ylBPT on glucose, (c) ylBPT on cellobiose and cellotriose, and (d) yl4BPT on cellobiose and cellotriose. Shown are biomass, glucose, cellobiose, and cellotriose concentration versus time. Each data point represents the mean of three independent experiments and the error bar indicates the standard deviation
Results
Expression of cellodextrin transporter and cellodextrin phosphorylase in Y. lipolytica
To confer cellodextrin-phosphorolytic capacity to Y. lipolytica, the genes Ctcdp and NcCDT1 were introduced into Y. lipolytica Po1f (Fig. 1a). The Ura+ transformants were selected for their ability to grow on cellobiose, cellotriose and cellotetrose. The results revealed that yeast ylPT co-expressing NcCDT1 and Ctcdp can grow on cellobiose, cellotriose, but not on cellotetrose. Therefore, cellobiose and cellotriose were chosen as carbon sources for the following studies. In addition, the mono-transformant ylP (containing Ctcdp only) cannot grow in these conditions, despite the fact that phosphorylase activity was detectable (0.56 ± 0.04 U/mg-total protein on cellobiose and 0.19 ± 0.02 U/mg-total protein on cellotriose). Most likely, Y. lipolytica is unable to transport cellodextrins into the cell, or at least the rate of transport (and thus the feed rate to the phosphorylases) was too low to support the growth on these substrates.
The recombinant strains were further characterized in shake flask cultures. The results showed that the growth of ylPT (harboring the cellodextrin phosphorolysis pathway) on cellobiose was extremely poor, and only 4.0 g/L cellobiose was consumed over 36 h at a specific consumption rate of 0.17 g/g-DCW/h (Fig. 1, Table 1). Although it grew better on cellotriose, a lag phase of 12 h was detected. As a result, 7.5 g/L of cellotriose was consumed over 36 h and thereafter, the substrate remained unchanged despite prolonged incubation. The μmax and biomass yield of ylPT on cellotriose was calculated as 0.11 h−1 and 0.42 g-DCW/g-cellotriose, respectively, which was 58% and 78% of those obtained on glucose (a μmax of 0.19 h−1 and a biomass yield of 0.54 g-DCW/g-glucose) (Fig. 1, Table 1).
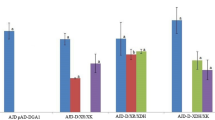
A previous work demonstrated that the low conversation of Glc-1P to Glc-6P, which resulted in Glc-1P accumulation and thus a greater flux towards glycogen synthesis, impeded the cellobiose phosphorolysis of an engineered yeast [15]. To investigate the limitations that characterize the phosphorolytic assimilation of cellodextrin in present study, the cellular concentrations of Glc-1P and the reserve carbon source of ylDPT grown on cellotriose were measured. The results showed that ylPT contained 30% more cellular Glc-1P and 5 times more glycogen than when it was grown on glucose (Fig. 2). Strikingly, this analysis also revealed the accumulation of intracellular cellobiose up to 20.0 mg/g-DCW in ylPT. Therefore, poor growth of ylPT on cellobiose is likely due to the low cellobiose phosphorolysis efficiency of Ctcdp. Moreover, cellobiose is also the primary product of cellotriose phosphorolysis whose accumulation may inhibit the following phosphorolysis reaction. Thus, the limiting factors of cellodextrin assimilation are the low conversion rate from Glc-1P to Glc-6P, and the low efficiency in the use of the released cellobiose (Fig. 1).
Comparsion of cellular concentration of glycogen, Glc-1P and cellobiose in phosphorolytic Y. lipolytica ylPT (pTEF-cdp1, pTEF-CDT1) compared with Y. lipolytica ylBPT (pTEF-NcCDT1, pTEF-Ctcdp, pTEF-Ctcdp, pTEF-ScPGM2) in areobic growth on 20 g/L glucose, or cellobiose or cellobiose. Samples were taken from the cells grown at the exponential phase
Optimization of the cellodextrin-phosphorolytic pathway in Y. lipolytica
To tackle the issue of low growth rate of ylPT on cellobiose and -triose due to the Glc-1P accumulation, the gene PGM2 encoding phosphoglucomutase (PGM) in S. cerevisiae, which showed the preference in catalyzing the reaction from Glc-1P to Glc-6P [15], was introduced into ylPT. In addition, the cellobiose phosphorylase of C. thermocellum was expressed in ylPT to enhance cellobiose assimilation. The performance of the newly engineered strain ylBPT was evaluated in cellobiose and cellotriose fermentations. As illustrated in Fig. 1, ylBPT demonstrated a 12 h lag phase and a μmax of 0.14 h−1 when it was grown on cellobiose. As a result, it consumed all of the cellobiose (20 g/L) over 30 h at a specific consumption rate of 0.29 g/g-DCW/h. Similar results were obtained when the same strain grown on cellotriose. This was accompanied by the decreased cellular content of glycogen and cellobiose resulted from the increased conversion rate of Glc-1P to Glc-6P, which would provide thermodynamic ‘pull’ for cellodextrin phosphorolysis (Fig. 2).
Our last effort was to combine the genes Ctcbp, Ctcdp and NcCDT1, and overexpress them under 4UASTef promoter, aiming to construct a recombinant strain that is able to ferment a mixture of cellobiose and cellotriose efficiently. The resulting strain yl4BPT demonstrated a shortened lag phase of 6 h, a μmax of 0.18 h−1 and a biomass yield of 0.54 g-DCW/ g-cellobiose or g-cellotriose, and thus a shorter fermentation time (24 h), all of which were similar to those values of the same strain grown on glucose (Fig. 1, Table 1).
Engineering Y. lipolytica capable of fermenting xylose efficiently in the presence of glucose
In this work, we first enhanced the expression of the three key genes SsXR, SsXDH and YlXK for xylose assimilation using 4UASTef promoter. In addition, CiGXF1 was installed to facilitate xylose uptake and to bypass the glucose repression [21]. Moreover, the genes ZWF1 and GND1 of the pentose phosphate pathway, which may lead to increased cytoplasmic NADPH supply and enhanced xylose fermentation by intermediates feeding, were also overexpressed (Fig. 3) [22, 23]. Unlike S. cerevisiae for which the growth on xylose was always less efficient than glucose despites great endeavors made on pathway optimization [24, 25], our engineered strain yl4XRHK exhibited similar growth rate (0.18 h−1) and biomass yield (0.51 g-DCW/g-xylose) on xylose to that of obtained on glucose (Fig. 3, Table 1). Compared to the parental strain ylXHK, yl4XRHK exhibited a 6 h shorter fermentation time and a 30% higher specific xylose consumption rate. We also applied a high xylose and glucose ratio of 3:1 to investigate the impact of high glucose concentration on xylose consumption. Expressing CiGXF1 greatly relieved the glucose repression on xylose fermentation, as simultaneous co-consumption of xylose and glucose was observed for the strain yl4XRHK [21]. For all the cultures, xylitol production was negligible even at high xylose/glucose ratio, for which 24 g/L of xylose remained to be consumed after glucose depletion (Fig. 4d).
A schematic illustration of the strategies used in the current study to construct recombinant Y. lipolytica strains able to ferment xylose efficiently. The heterologous genes introduced are indicated in cyan box and the endogenous genes overexpressed are shown in purple box. XR xylose reductase, XDH xylitol dehydrogenase, GXF xylose facilitator, XK xylulokinase, ZWF phosphoglucose dehydrogenase, GND1 6-phosphoglucose dehydrogenase
Comparsion of the growth of recombinant Y. lipolytica ylHXK (pTEF-SsXR, pTEF-SsXDH, pTEF-XK) and yl4XRHK (p4UTef-SsXR, p4UTef-SsXDH, p4UTef-XK, pTEF-GiGXF1, pTEF-ZWF1, pTEF-GND1) in areobic growth on (a) xylose; (b), (c) and (d) mixed glucose and xylose. Shown are biomass, xylose and glucose concentration versus time. Each data point represents the mean of three independent experiments and the error bar indicates the standard deviation
Development of recombinant strains of Y. lipolytica to co-ferment cellodextrins and xylose efficiently
Encouraged by the success of the above work, we then pursued a more ambitious goal to render Y. lipolytica with both xylose utilization and cellodextrins catabolism ability (Fig. 5). To achieve this, the genes for xylose utilization (SsXR, SsXDH, YlXKS1 and CiGXF1) were introduced into the strain yl4BPT harboring cellodextrin-phosphorolysis pathway (Ctcbp1, Ctcdp1, NcCDT1 and ScPGM2), resulting the strain yl4BX. Please note that the key genes SsXR, SsXDH, ylXKS1, Ctcbp1, Ctcdp1 and NcCDT1 were expressed under 4UASTef promoter, while the rest genes were controlled by the normal TEF promoter.
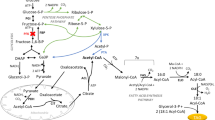
A schematic illustration of the strategies used in the current study to construct a cellodextrin- and xylose-fermenting Y. lipolytica for lipid overproduction. The pathway for cellodextrin phosphorolysis is marked with light yellow box, while xylose assimilation pathway is marked with pink box. Lipid pathway engineering is indicated in cyan box. PPP pathway overexpression to enhance NADPH supply is marked with gray box. The heterologous genes are written in red and the native genes are given in blue. The interrupted genes are marked with a red deletion line. GPD1 glycerol-3-phosphate dehydrogenase, DGA2 acyl-CoA:diacylglycerol acyl transferase, MFE1 multifunctional beta-oxidation enzyme hydratase-dehydrogenase-epimerase, PEX10 peroxisome biogenesis, TGL TAG lipase
Thereafter, the strain yl4BX was characterized in YNB media containing different carbon source. The results showed that yl4BX required 26 h, 30 h, 32 h and 32 h to consume the single carbon source, glucose (20 g/L), xylose (20 g/L), cellobiose (19 g/L) and cellotriose (18.6 g/L), respectively (Table 2). Generally speaking, the lag phase for the cultures containing glucose was slightly shorter than those were deficient in glucose, and simultaneous co-consumption of these carbon sources was achieved. These results confirmed the positive effect of so-called ‘helper substrate’ to promote the use of less favourable substrate by nourishing the cells to achieve good fitness [14, 26]. As a result, the time needed for the complete depletion of carbon source including xylose (10 g/L)/cellobiose (9.5 g/L), xylose (10 g/L)/cellotriose (9.3 g/L) and xylose (10 g/L)/cellobiose (4.8 g/L)/cellotriose (4.7 g/L) was similar (32 h), but slightly longer than that of the fermentation on the mixture of xylose (10 g/L)/glucose (10 g/L), xylose (10 g/L)/glucose (5 g/L)/cellobiose (4.8 g/L), xylose (10 g/L)/glucose (5 g/L)/cellotriose (4.7 g/L). Strikingly, similar biomass yields (≈ 0.52 g/g-glucose equivalent in average) and growth rates (≈ 0.18 h−1 in average) were achieved for the above fermentations (Table 2). The production of xylitol was less than 1.0 g/L for all the xylose containing cultures.
Engineering Y. lipolytica to accumulate high cellular content of lipids on LC biomass derived sugars
After the construction of a recombinant Y. lipolytica that is able to ferment xylose and cellodextrins in the presence of glucose efficiently, we then explored how his strain can be applied for lipids production on these carbon sources. The strategy we used to increase lipid accumulation is to overexpress GPD1 and DGA2, which are involved in TAG formation, and to interrupt MFE1, PEX10 and TGL4 to prevent ß-oxidation, peroxisome biogenesis [27] and the release of fatty acids from the lipid body [28] (Fig. 5). The contribution of each single overexpression and gene deletion was verified in lipid production on glucose (Additional file 1: Fig. S1). The strain yl4BXP which exhibited the highest lipid accumulation yield on glucose (45% of the biomass) was inoculated into the media containing a mixture of xylose, cellobiose and cellotriose for further investigation. Lipid production was conducted in a 3L-bioreactor in the presence of 25 g/L of each sugar. A second addition of 25 g/L of each substrate was carried out when the total amount of carbon source dropped below 20 g/L. A C/N ratio of 60 was used according to the previous literature, which was reported as the best C/N ratio for lipid accumulation from different carbon sources [29].
As illustrated in Fig. 6, co-consumption of the three sugars by yl4BXP was observed, with xylose being the fastest fermented sugar followed by cellobiose and cellotriose. In the end, yl4BXP consumed a total amount of 150 g/L mixed sugars in less than 5 days, and produced 59.6 g/L of biomass, a yield of 0.39 g-DCW/g-sugar. Lipid accumulation reached 23.8 g/L as estimated from the sum of the extracted fatty acids, a production of 0.16 g-lipid/g-sugar, corresponding to 40.0% of biomass (in DCW) and a productivity yield of 0.22 g/L/h (Fig. 6). Analysis of the fatty acid profile of yl4BXP illustrated a twofold increase in oleic acid (C18:1, 67%) and 73% decrease in linoleic acid (C18:2, 9.7%) in the engineered strain compared with the parental strain po1f-control. While linoleic acid (36%) and oleic acid (34%) was the first and second most abundant fatty acid in the parental strain, palmitoleic acid (C16:1), represents only 13% of total fatty acids, was the second most abundant fatty acid in yl4BXP (Fig. 7).
Lipid prodution of recombinant Y. lipolytica yl4BXP (p4UTef-SsXR, p4UTef-SsXDH, p4UTef- XK, pTEF-GiGXF1, pTEF-ZWF1, pTEF-GND1, pTEF-GPD1, pTEF-DGA2) from mixed carbon source including xylose, cellobiose and cellotriose in YNB meida. Shown are xylose, cellobiose, cellotriose, biomass, xylitol, citrate and lipids concentration versus time
Lipid production using recombinant Y. lipolytica in fed-batch SSF of wheat straw in bioreactors
To investigate whether the recombinant Y. lipolytica could be useful in the real scenario of LC biomass conversion, fed-batch SSF of steam-pretreated wheat straw was carried out in a 3L bioreactor using the strain yl4BXP. A sequential co-fermentation scheme was employed comprising an initial phase of batch culture on hydrolysate liquor followed by three additions of the cellulase and unwashed solids of the wheat straw slurry. It has been suggested that such a fermentation strategy is advantageous due to the detoxification of the inhibitory compounds by yeast during the batch phase and the improved tolerance of cells through progressive adaptation [30].
During the initial 48 h of batch cultivation, 8.6 g/L glucose was depleted and 26 g/L of xylose representing 79 wt% of the total available xylose in hydrolysate was consumed, and 2.76 g/L of lipids were produced (Fig. 8). To evaluate the feasibility of the process, Cellic CTec2 was added at a dosage to avoid excessive hydrolysis of glucan into glucose (10.0 FPU/g cellulose), and also at a higher dosage of 15.0 FPU/g cellulose for comparison. During the subsequent fed-batch SSF at lower enzyme dosage, xylose was co-consumed with the released sugars from enzymatic hydrolysis of glucan. No accumulation of glucose and cellodextrins was observed, and this observation was correlated with lipid production (detected in the form of FAMEs), reflecting a continuous biomass formation. After 144 h of cultivation, the fed-batch SSF resulted in a lipid concentration of 8.5 g/L, which corresponded to an overall production yield of 0.1 g-lipid/g-sugar (Fig. 8). Glucose was depleted, and 2.3 g/L of xylose sustained in the end of fermentation. Xylose utilization reached 96 wt% of the total xylose loading. The co-consumption of glucose, xylose and cellodextrins were sustained throughout the 144 h of co-fermentation. Prolonging the fermentation did not yield further gain in lipid titer.
Lipid prodution of recombinant Y. lipolytica yl4BXP (p4UTef-SsXR, p4UTef-SsXDH, p4UTef- XK, pTEF-GiGXF1, pTEF-ZWF1, pTEF-GND1, pTEF-GPD1, pTEF-DGA2) by fed-batch SSF of steam-pretreated wheat straw in bioreactor. Shown are glucose, xylose, biomass, xylitol, citrate and lipid concentration versus time
Discussions
It is interesting to note that phosphorolytic capability was recently conferred to S. cerevisiae (expressing NcCdt1 and a Cbp from Saccharomyces degradans) [18]. In the previous work, the energetic benefits of phosphorolysis were evidenced because S. cerevisiae endowed with the cellobiose phosphorolysis pathway produced more biomass and ethanol than a cellobiose-hydrolyzing strain. Consistent with these observations, the newly developed strain in this work showed better performance, e.g., shorter lag phase, faster rates of growth and substrate consumption, than the previously engineered yeast relied on cellobio-hydrolytic activity [31]. However, our results imply that the presence of the phosphorolysis pathway in Y. lipolytica did not procure an obvious energetic benefit, as the biomass yield of yl4BPT was similar to the previous reported Y. lipolytica possessing cellobiose hydrolytic activity [31]. It is noteworthy that further overexpressing either Ctcbp, Ctcdp or CDT1 using 8UASTef promoter did not improve the growth rate and biomass yield further. Nevertheless, yl4BPT is still the most efficient cellodextrin-fermenting recombinant yeast reported so far.
Several recent studies have shared the same interest in develo** a xylose-fermenting Y. lipolytica by employing different strategies. As such, although Y. lipolytica PO1g overexpressing SsXR and SsXDH was unable to grow on xylose, the following adaptation has enabled the growth of the strain on xylose with a doubling time of 25 h [26]. In a second work, Y. lipolytica YlSR001 was directly adapted in xylose culture which allowed the isolation of a mutant strain capable of slightly growing on xylose [32]. The author disclosed that the insufficient XDH activity was the limiting factor for xylose assimilation. The overexpression of XDH enhanced the growth of the strain on xylose, but unfortunately, still at an extremely slow rate [32]. Although our previous strategy to construct a xylose-fermenting Y. lipolytica by overexpressing SsXR, SsXDH and YlXK was successful, xylose assimilation in the presence of glucose of the engineered strain was not optimal as sequential consumption of xylose after glucose was still the case due to glucose repression [14]. In addition, the accumulation of xylitol on xylose occurred after glucose depletion when a high initial concentration of xylose was applied, which indicated Y. lipolytica suffered from the cofactor imbalance issue since ssXR consumes NADPH and XDH generates NADH [26]. In this respect, our current strategy was obviously more successful. CiGxf1p is a non-glucose preference transporter which shows highest efficacy in xylose uptake in the presence of glucose [21]. Its Vmax value is one order of magnitude higher than the xylose-H+ symporter CiGxs1p [33], and the overexpression of CiGxf1p in Kluyveromyces marxianus has greatly improved xylitol production due to the enhanced xylose uptake [Calculations and statistics Specific rate of substrate consumption (g-s/g-DCW/h) was estimated according to the following equation:\({\mathrm{q}}_{\text{sub}}=\frac{{\mu }_{\mathrm{max}}}{{Y}_{\text{X/s}}}\), where \({Y}_{\text{X/s}}=\frac{\text{dX}}{{\text{ds}}}\) represents the biomass yield coefficient. The biomass and product yields were calculated as the ratio of the amount of biomass or products formed divided by the amount of carbon source consumed. The maximum specific growth rate μmax (h−1) was calculated from the plot of biomass concentration versus time for exponentially growing cells. The duration of lag phase was estimated using an online tool as described previously [50]. All the experiments were performed at least in triplicate, and the mean values ± standard deviation were shown.
Availability of data and materials
All data generated or analyzed in the present study are included in this published article and a supporting material “Additional file 1”.
References
Raj T, Chandrasekhar K, Naresh Kumar A, Rajesh Banu J, Yoon JJ, Kant Bhatia S, Yang YH, Varjani S, Kim SH. Recent advances in commercial biorefineries for lignocellulosic ethanol production: Current status, challenges and future perspectives. Bioresour Technol. 2022;344(Pt B): 126292.
Stockton BC, Mitchell DJ, Grohmann K, Himmel ME. Optimumβ-D-glucosidase supplementation of cellulase for efficient conversion of cellulose to glucose. Biotech Lett. 1991;13(1):57–62.
Cunha JT, Soares PO, Romaní A, Thevelein JM, Domingues L. Xylose fermentation efficiency of industrial Saccharomyces cerevisiae yeast with separate or combined xylose reductase/xylitol dehydrogenase and xylose isomerase pathways. Biotechnol Biofuels. 2019;12:20.
Peña-Castro JM, Muñoz-Páez KM, Robledo-Narvaez PN, Vázquez-Núñez E. Engineering the metabolic landscape of microorganisms for lignocellulosic conversion. Microorganisms. 2023. https://doi.org/10.3390/microorganisms11092197.
Kao MR, Yu SM, Ho TUD. Improvements of the productivity and saccharification efficiency of the cellulolytic β-glucosidase D2-BGL in Pichia pastoris via directed evolution. Biotechnol Biofuels. 2021;14(1):126.
You S, Li J, Zhang F, Bai Z-Y, Shittu S, Herman R-A, Zhang W-X, Wang J. Loop engineering of a thermostable GH10 xylanase to improve low-temperature catalytic performance for better synergistic biomass-degrading abilities. Biores Technol. 2021;342: 125962.
Moreno AD, Carbone A, Pavone R, Olsson L, Geijer C. Evolutionary engineered Candida intermedia exhibits improved xylose utilization and robustness to lignocellulose-derived inhibitors and ethanol. Appl Microbiol Biotechnol. 2019;103(3):1405–16.
Vu VNH, Kohári-Farkas C, Filep R, Laszlovszky G, Ban MT, Bujna E, Gupta VK, Nguyen QD. Design and construction of artificial microbial consortia to enhance lignocellulosic biomass degradation. Biofuel Research Journal. 2023;10(3):1890–900.
Groenewald M, Boekhout T, Neuvéglise C, Gaillardin C, van Dijck PW, Wyss M. yarrowia lipolytica: safety assessment of an oleaginous yeast with a great industrial potential. Crit Rev Microbiol. 2014;40(3):187–206.
Beopoulos A, Chardot T, Nicaud JM. Yarrowia lipolytica: a model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie. 2009;91(6):692–6.
Guo Q, Peng QQ, Chen YY, Song P, Ji XJ, Huang H, Shi TQ. High-yield α-humulene production in Yarrowia lipolytica from waste cooking oil based on transcriptome analysis and metabolic engineering. Microb Cell Fact. 2022;21(1):271.
Gallego-García M, Moreno AD, González A, Negro MJ. Efficient use of discarded vegetal residues as cost-effective feedstocks for microbial oil production. Biotechnol Biofuels Bioprod. 2023;16(1):21.
Wang J, Yu X, Wang K, Lin L, Liu HH, Ledesma-Amaro R, Ji XJ. Reprogramming the fatty acid metabolism of Yarrowia lipolytica to produce the customized omega-6 polyunsaturated fatty acids. Bioresour Technol. 2023;383: 129231.
Ledesma-Amaro R, Lazar Z, Rakicka M, Guo Z, Fouchard F, Coq AC, Nicaud JM. Metabolic engineering of Yarrowia lipolytica to produce chemicals and fuels from xylose. Metab Eng. 2016;38:115–24.
Guo ZP, Borsenberger V, Croux C, Duquesne S, Truan G, Marty A, Bordes F. An artificial chromosome ylAC enables efficient assembly of multiple genes in Yarrowia lipolytica for biomanufacturing. Commun Biol. 2020;3(1):199.
Galazka JM, Tian C, Beeson WT, Martinez B, Glass NL, Cate JH. Cellodextrin transport in yeast for improved biofuel production. Science. 2010;330(6000):84–6.
Nakai H, Hachem MA, Petersen BO, Westphal Y, Mannerstedt K, Baumann MJ, Dilokpimol A, Schols HA, Duus J, Svensson B. Efficient chemoenzymatic oligosaccharide synthesis by reverse phosphorolysis using cellobiose phosphorylase and cellodextrin phosphorylase from Clostridium thermocellum. Biochimie. 2010;92(12):1818–26.
Ha SJ, Galazka JM, Joong OhE, Kordić V, Kim H, ** YS, Cate JH. Energetic benefits and rapid cellobiose fermentation by Saccharomyces cerevisiae expressing cellobiose phosphorylase and mutant cellodextrin transporters. Metab Eng. 2013;15:134–43.
Dulermo T, Nicaud JM. Involvement of the G3P shuttle and beta-oxidation pathway in the control of TAG synthesis and lipid accumulation in Yarrowia lipolytica. Metab Eng. 2011;13(5):482–91.
Beopoulos A, Haddouche R, Kabran P, Dulermo T, Chardot T, Nicaud JM. Identification and characterization of DGA2, an acyltransferase of the DGAT1 acyl-CoA:diacylglycerol acyltransferase family in the oleaginous yeast Yarrowia lipolytica. New insights into the storage lipid metabolism of oleaginous yeasts. Appl Microbiol Biotechnol. 2012;93(4):1523–37.
Runquist D, Hahn-Hägerdal B, Rådström P. Comparison of heterologous xylose transporters in recombinant Saccharomyces cerevisiae. Biotechnol Biofuels. 2010;3(1):5.
Qiao K, Wasylenko TM, Zhou K, Xu P, Stephanopoulos G. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat Biotechnol. 2017;35(2):173–7.
Zhang P, Wei W, Shang Y, Ye BC. Metabolic engineering of Yarrowia lipolytica for high-level production of scutellarin. Bioresour Technol. 2023;385: 129421.
Subtil T, Boles E. Competition between pentoses and glucose during uptake and catabolism in recombinant Saccharomyces cerevisiae. Biotechnol Biofuels. 2012;5:14.
Kim SR, Park YC, ** YS, Seo JH. Strain engineering of Saccharomyces cerevisiae for enhanced xylose metabolism. Biotechnol Adv. 2013;31(6):851–61.
Stephanopoulos G, Tai M: Engineered microbes and methods for microbial oil overproduction from cellulosic materials. WO 2013/192520 A1 2013.
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, Otoupal P, Alper HS. Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun. 2014;5:3131.
Dulermo T, Treton B, Beopoulos A, Kabran Gnankon AP, Haddouche R, Nicaud JM. Characterization of the two intracellular lipases of Y lipolytica encoded by TGL3 and TGL4 genes: new insights into the role of intracellular lipases and lipid body organisation. Biochim Biophys Acta. 2013;1831(9):1486–95.
Lazar Z, Dulermo T, Neuveglise C, Crutz-Le Coq AM, Nicaud JM. Hexokinase-A limiting factor in lipid production from fructose in Yarrowia lipolytica. Metab Eng. 2014;26C:89–99.
Nielsen F, Zacchi G, Galbe M, Wallberg O. Prefermentation improves ethanol yield in separate hydrolysis and cofermentation of steam-pretreated wheat straw. Sustainable Chem Processes. 2016;4(1):10.
Guo Z, Duquesne S, Bozonnet S, Cioci G, Nicaud JM, Marty A, O’Donohue MJ. Development of cellobiose-degrading ability in Yarrowia lipolytica strain by overexpression of endogenous genes. Biotechnol Biofuels. 2015;8:109.
Ryu S, Hipp J, Trinh CT. Activating and elucidating complex sugar metabolism in yarrowia lipolytica. Appl Environ Microbiol. 2015. https://doi.org/10.1128/AEM.03582-15.
Leandro MJ, Gonçalves P, Spencer-Martins I. Two glucose/xylose transporter genes from the yeast Candida intermedia: first molecular characterization of a yeast xylose-H+ symporter. Biochem J. 2006;395(3):543–9.
Ren L, Liu Y, **a Y, Huang Y, Liu Y, Wang Y, Li P, Chang K, Xu D, Li F, et al. Improving glycerol utilization during high-temperature xylitol production with Kluyveromyces marxianus using a transient clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 system. Biores Technol. 2022;365: 128179.
Liu N, Qiao K, Stephanopoulos G. (13)C Metabolic flux analysis of acetate conversion to lipids by yarrowia lipolytica. Metab Eng. 2016;38:86–97.
Cheng H, Wang S, Bilal M, Ge X, Zhang C, Fickers P, Cheng H. Identification, characterization of two NADPH-dependent erythrose reductases in the yeast Yarrowia lipolytica and improvement of erythritol productivity using metabolic engineering. Microb Cell Fact. 2018;17(1):133.
Singh A, Mishra P. Microbial pentose utilization: current applications in biotechnology. Amsterdam: Elsevier Science; 1995.
Jafarihaghighi F, Ardjmand M, Salar Hassani M, Mirzajanzadeh M, Bahrami H. Effect of fatty acid profiles and molecular structures of nine new source of biodiesel on combustion and emission. ACS Omega. 2020;5(26):16053–63.
Nielsen F, Zacchi G, Galbe M, Wallberg O. Sequential targeting of xylose and glucose conversion in fed-batch simultaneous saccharification and co-fermentation of steam-pretreated wheat straw for improved xylose conversion to ethanol. BioEnergy Research. 2017;10(3):800–10.
Madzak C, Tréton B, Blanchin-Roland S. Strong hybrid promoters and integrative expression/secretion vectors for quasi-constitutive expression of heterologous proteins in the yeast Yarrowia lipolytica. J Mol Microbiol Biotechnol. 2000;2(2):207–16.
Guo Z-p, Duquesne S, Bozonnet S, Nicaud J-M, Marty A, O’Donohue MJ. Expressing accessory proteins in cellulolytic Yarrowia lipolytica to improve the conversion yield of recalcitrant cellulose. Biotechnol Biofuels. 2017;10(1):298.
Xu P, Qiao K, Ahn WS, Stephanopoulos G. Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc Natl Acad Sci USA. 2016;113(39):10848–53.
Guo ZP, Duquesne S, Bozonnet S, Cioci G, Nicaud JM, Marty A, O’Donohue MJ. Conferring cellulose-degrading ability to Yarrowia lipolytica to facilitate a consolidated bioprocessing approach. Biotechnol Biofuels. 2017;10:132.
Blazeck J, Liu L, Knight R, Alper HS. Heterologous production of pentane in the oleaginous yeast Yarrowia lipolytica. J Biotechnol. 2013;165(3):184–94.
Xue Z, Sharpe PL, Hong S-P, Yadav NS, **e D, Short DR, Damude HG, Rupert RA, Seip JE, Wang J, et al. Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat Biotechnol. 2013;31(8):734–40.
Dulermo T, Tréton B, Beopoulos A, Kabran Gnankon AP, Haddouche R, Nicaud J-M. Characterization of the two intracellular lipases of Y. lipolytica encoded by TGL3 and TGL4 genes: new insights into the role of intracellular lipases and lipid body organisation. Biochimica et Biophysica Acta BBA Mol Cell Biol Lipids. 2013;1831(9):1486–95.
Borsenberger V, Onesime D, Lestrade D, Rigouin C, Neuveglise C, Daboussi F, Bordes F. Multiple parameters drive the efficiency of CRISPR/Cas9-Induced gene modifications in yarrowia lipolytica. J Mol Biol. 2018;430(21):4293–306.
Reichenbecher M, Lottspeich F, Bronnenmeier K. Purification and properties of a cellobiose phosphorylase (CepA) and a cellodextrin phosphorylase (CepB) from the cellulolytic thermophile Clostridium stercorarium. Eur J Biochem. 1997;247(1):262–7.
Guo ZP, Olsson L. Physiological responses to acid stress by Saccharomyces cerevisiae when applying high initial cell density. FEMS Yeast Res. 2016. https://doi.org/10.1093/femsyr/fow072.
Rolfe MD, Rice CJ, Lucchini S, Pin C, Thompson A, Cameron AD, Alston M, Stringer MF, Betts RP, Baranyi J, et al. Lag phase is a distinct growth phase that prepares bacteria for exponential growth and involves transient metal accumulation. J Bacteriol. 2012;194(3):686–701.
Acknowledgements
We thank the analytic platform of the State Key laboratory of Food Science and Resources for providing access to its analytical facilities.
Funding
This work was funded by the National Key Research and Developmental Program of China (2021YFC2100300) and the Fundamental Research Funds for the Central Universities (JUSRP123006 and JUSRP123008).
Author information
Authors and Affiliations
Contributions
YRZ, LZ, ZPG conceived of the study and YX, ZG, MYL, RZ, ZHG participated in its design. YRZ, LZ, ZPG designed the constructs, and YRZ carried out all the experiments and drafted the manuscript. YRZ, YX, ZG, MYL, RZ, ZHG, LZ, ZPG revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
All authors consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1 Plasmids used or created in the present study. Table S2 The sequences of the oligonucleotide primers used in this study. Table S3 sgRNA for gene deletion. Table S4 The composition of steam-pretreated wheat straw.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, Y., Li, M., Zhu, R. et al. Installing xylose assimilation and cellodextrin phosphorolysis pathways in obese Yarrowia lipolytica facilitates cost-effective lipid production from lignocellulosic hydrolysates. Biotechnol Biofuels 16, 186 (2023). https://doi.org/10.1186/s13068-023-02434-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-023-02434-9