Abstract
Background
Laparoscopic one anastomosis gastric bypass (LOAGB) is a simple variation of gastric bypass and has gained worldwide popularity with clinical outcomes similar to laparoscopic Roux-en-Y gastric bypass (LRYGB) for weight loss and management of comorbidities. However, biliary reflux to the esophagus negates the benefits of LOAGB. In addition, weight gain after LOAGB and after LRYGB is a major problem in bariatric surgery.
The aim of this article is to describe the design and protocol of a randomized controlled trial comparing the outcomes of two methods of LOAGB: experimental method with wrap** versus standard method nonwrap** fundus of the excluded part of the stomach to prevent weight regain and biliary reflux after LOAGB.
Methods
The study was designed as a single-center prospective, interventional, randomized controlled trial. Masking: None (open label). Allocation: randomized. Enrollment: 100 obese patients. The relevant ethics committee approved the trial protocol.
The endpoints (body mass index, bile reflux in the esophagus, other reflux symptoms) will be assessed presurgery and postsurgery (12, 24, and 36 months postoperatively).
Discussion
With its 3-year follow-up time, this RCT will provide important data on the impact of wrap** the fundus of the excluded part of the stomach to prevent weight regain and biliary reflux after LOAGB.
Trial registration
ClinicalTrials.govNCT04834635. Registered on 8 April 2021.
Similar content being viewed by others
Administrative information
Data of FundoRingOAGB trial.
Title {1} | A laparoscopic one anastomosis gastric bypass with wrap** versus nonwrap** fundus of the excluded part of the stomach to treat obese patients (FundoRingOAGB trial): study protocol for a randomized controlled trial |
Trial registration {2a and 2b}. | ClinicalTrials.gov, NCT04834635 |
Protocol version {3} | Registered on 8 April 2021. Version 8 April 2021. |
Funding {4} | The work will be supported by The Society of Bariatric and Metabolic Surgeons of Kazakhstan, Nur-Sultan, Kazakhstan, grant number 2021/1. |
Author details {5a} | Oral Ospanov1 Galymzhan Yeleuov1, Alexandr Fursov1, Bakhtiyar Yelembayev1, Roman Fursov1, Zhenis Sergazin1, Adil Mustafin1 1. Department of Surgical Disease and Bariatric Surgery, Astana Medical University, Nur-Sultan, Kazakhstan |
Name and contact information for the trial sponsor {5b} | The Society of Bariatric and Metabolic Surgeons of Kazakhstan, Nur-Sultan, Kazakhstan |
Role of sponsor {5c} | The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript |
Introduction
Background and rationale {6a}
Typical early weight loss following bariatric surgery ranges from 47 to 80% of excess weight [1]. However, typical weight regain is 15–25% of that lost weight [2]. Weight regain after surgery is a major problem in bariatric practice. Therefore, adjustable bands and rings are used, such as “FobiRing” for banded gastric bypass [3]. However, foreign material can cause complications, such as erosion of the stomach wall [4]. Therefore, surgeons avoid the use of mechanical devices for banded gastric bypass.
Currently, laparoscopic one anastomosis gastric bypass (LOAGB) or minigastric bypass (MGB) is a common bariatric procedure for treating obesity [5]. The greatest criticism of the LOAGB is for the likelihood of biliary reflux [6]. Biliary reflux resistant to medical treatment has an incidence of 0.6–10% after one anastomosis gastric bypass and may be a reason for revisional surgery [7].
In addition, along with obesity, gastroesophageal reflux disease (GERD) is steadily increasing worldwide, and antireflux surgery must be performed simultaneously with bariatric surgery in obese patients [8]. In these cases, fundoplication may be performed using the fundus of the excluded part of the stomach [9]. Colpaert J et al. reported good short-term results with modified Nissen fundoplication for patients with reflux after RYGB [10]. They opted to perform the same procedure to slow down the passage of food through the gastric pouch. This procedure can be performed as a revision procedure after Roux-en-Y gastric bypass for the treatment of postoperative dum** syndrome [11].
In the literature, we did not find data on the use of a similar method for primary procedures and examples of use for patients without GERD to prevent biliary reflux in one anastomosis gastric bypass and de novo postoperative reflux esophagitis.
Objectives {7}
We hypothesize that total fundoplication can not only treat GERD but also significantly prevent the return of weight, such as after banded gastric bypass, prevent postoperative bile reflux in the esophagus, and decrease de novo GERD.
The aim of this article is to describe the design and protocol of a randomized controlled trial comparing the outcomes of two methods of LOAGB: the experimental method with wrap** versus the standard method nonwrap** fundus of the excluded part of the stomach.
The main aim of the study are as follows:
-
1.
Comparing the primary outcome measure: change in body mass index in both groups and weight regain.
-
2.
Comparing the secondary outcome measures: postoperative bile reflux in the esophagus; assessment of GERD if preoperative GERD symptoms (GERD-HRQL) were present or if GERD symptoms occurred postoperatively (de Novo GERD).
Trial design {8}
The study was designed as a single-center prospective, interventional, randomized controlled trial. Masking: None (Open Label) Allocation: randomized. Enrollment: 100 obese patients.
Methods: participants, interventions, and outcomes
Study setting {9}
Participant data will be collected from “Green Clinic,” Nur-Sultan, Kazakhstan. All operations are performed by one surgeon.
Eligibility criteria {10}
Inclusion criteria:
-
Obese patients had a body mass index (BMI) from 30 to 50 kg/m2.
-
The patient is generally fit for anesthesia (ASA grading 1–2) and surgery.
-
The patient commits to the need for long-term follow-up.
Exclusion criteria:
-
Patients with superobesity—BMI more than 50 kg/m2.
-
Prosthetic (mesh) Hiatal herniorrhaphy or large hiatal hernia
-
Esophageal shortening
-
Los Angeles Classification of Esophagitis (LA grade) C or D reflux esophagitis
-
History of surgery on the stomach or esophagus
-
Less than 18 or more than 60 years of age
-
Not fit for bariatric surgery
-
Psychiatric illness
-
Patients unwilling or unable to provide informed consent
The drop out criteria:
-
1.
Refusal to participate
-
2.
Patient not available for long-term follow-up
Who will take informed consent? {26a}
Written informed consent will be obtained from all participants or their authorized representatives.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
Not applicable.
Interventions
Explanation for the choice of comparators {6b}
The choice of a comparator—the method of laparoscopic one-anastomosis (mini) gastric bypass by M. Carbajo-based long-term follow-up results in 1200 patients [12]
Intervention description {11a}
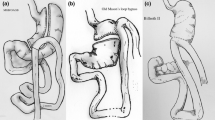
Experimental surgical bariatric procedure in the first (A) group: patients (n=50) underwent laparoscopic anastomosis (mini) gastric bypass with total wrap** of the fundus of the gastric excluded part and suture cruroplasty if hiatal hernia was present (FundoRingMGB group) (Fig. 1) [13]; active comparator surgical bariatric procedure in the second (B) group: patients (n=50) underwent laparoscopic anastomosis (mini) gastric bypass by Carbajo MA (Fig. 2) [14]. Only suture cruroplasty was performed if hiatal hernia was present (LOAGB group).
Criteria for discontinuing or modifying allocated interventions {11b}
Not applicable
Strategies to improve adherence to interventions {11c}
Not applicable
Relevant concomitant care permitted or prohibited during the trial {11d}
Relevant concomitant care and interventions that are permitted during the trial: using drainage in the abdominal cavity for the first 4 days after surgery.
Relevant concomitant care and interventions are prohibited during the trial: balloon dilatation in the fundoplication area after surgery.
Provisions for posttrial care {30}
Compensation for those who suffered from participation in the trial is not provided. Elimination of treatment gaps is carried out on a general basis in the national health system.
Outcomes {12}
Primary outcome measure
-
1.
Change of body mass index: The measure assesses a change of body mass index. Weight (kg) and height (cm) will be combined with the body mass index (BMI) kg/m2. Time frame: Baseline at 12, 24, and 36 months after surgery. Ideal body weight was calculated as 25 × [patient’s height in meters]2. Effectiveness endpoint follow-up included body mass index (BMI, kg/m2), change in BMI (ΔBMI [initial BMI − postoperative BMI]), excess BMI loss (EBMIL, % [(ΔBMI/initial BMI − 25) × 100]), and total weight loss (TWL, % [initial weight − postoperative weight/initial weight × 100]).
Secondary outcome measure
-
2.
Postoperative bile reflux in the esophagus. The endoscopic finding of postoperative bile reflux in the esophagus. Mild bile reflux (BR) was assessed if the biliary reflux was asymptomatic and detected only by an endoscopic picture of gastritis near the gastroenteroanastomosis. A grade of average BR was assessed by the presence of pain or total gastritis of the pouch caused by the presence of bile. A grade of severe BR was assessed if BR symptoms of stomach pain or heartburn were intolerable or by endoscopic verification of esophagitis by the presence of bile in the esophagus [15]. Time frame: at 12, 24, and 36 months after surgery.
-
3.
Assessment of GERD if preoperative GERD symptoms (GERD-HRQL) were present or if GERD symptoms occurred postoperatively (de novo GERD).
Participant timeline {13}
The flow diagram of the study design is shown in Table 1.
Sample size {14}
Purpose sampling
-
Effect size (d) (postoperative change in BMI (%)) = 15%.
-
SD = 10.
Formula for calculating sample size is:
Level of significance = 5%, power = 80% (Sample size increases as power increases. Higher the power, lower the chance of missing a real effect), Zα = Z is constant set by convention according to accepted α error and Z (1-β) = Z is constant set by convention according to the power of study [16].
Sample size calculations in our study:
n = 2 (1.96 + 1.28) 2 × 102/152 = 9.33 individuals in each group should be recruited in the study. However, we plan to include more than five times the number of patients in the sample.
Recruitment {15}
We will sample a homogenous population group (obese patients with body mass index (BMI) from 30 to 50 kg/m2), thus reducing the standard deviation and hence the sample size. We will recruit 100 obese patients (50 patients in two groups).
Assignment of interventions: allocation
Sequence generation {16a}
Informed consent will be obtained from each participant before patient enrollment in the study. Patients who meet all of the inclusion criteria and none of the exclusion criteria will be consecutively included and randomized into one of the two study arms by the study statistician, who is not involved in the enrollment, assignment, or assessment of patients, according to a random allocation sequence generated by Stata 7.0. The randomization list is kept strictly confidential. Allocation concealment is ensured by using sequentially numbered, identical, opaque, sealed envelopes (n = 100). Patients will be randomized to one of two groups at a 1:1 allocation ratio.
Concealment mechanism {16b}
Sealed envelopes will be used during the visit before surgery.
Implementation {16c}
The intervention will be communicated to the patient by a nurse who has no involvement in the enrollment or assessment of patients and who will open the sealed envelope during the visit before surgery.
Assignment of interventions: blinding
Who will be blinded {17a}
We will use an open-label trial, or open trial, when a type of clinical trial in which information is not withheld from trial participants. Bath, our open-label trial, is still randomized.
Procedure for unblinding if needed {17b}
Not applicable.
Data collection and management
Plans for assessment and collection of outcomes {18a}
Treatment-related data were collected at V1 (baseline). According to the study protocol, follow-up data will be collected from V2 to 12 months, 24 months (V3), and 36 months after surgery (V4) (Table 1).
Plans to promote participant retention and complete follow-up {18b}
Data collection begins on the day a participant signs the informed consent form and continues until the termination of the trial or until the participant withdraws from the trial for any reason. If participants discontinue or deviate from the study protocols, the investigators will attempt to minimize the missing data. All original data are kept in chronological order for verification.
Data management {19}
Original data are transferred in a timely manner to a paper-based case report form (CRF) and an electronic database system located in a guarded facility at the trial site. Access to the study data is restricted. The PI will have access to the final dataset. An independent steering committee will monitor and examine adherence to the study protocol.
Confidentiality {27}
The relevant regulations of the data protection legislation will be maintained.
The participants’ confidentiality was maintained at all times. For confidentiality reasons, case report forms (CRFs) do not contain any personal data on study participants. Members of the ethics committees are obliged to respect confidentiality and to refrain from divulging the participants’ identities or any other personal information they might be aware of. Source data in the hospital’s electronic patient information systems are secured by personal passwords and medical secrecy is maintained.
Plans for collection, laboratory evaluation, and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
Not applicable.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Statistical analysis will be performed using Microsoft Excel for Mac (Microsoft Corp., Redmond, WA) and StatPlus: MacPro (AnalystSoft Inc., Walnut, CA).
The normal distribution of the variables will be tested using the Kolmogorov–Smirnov test. Subsequently, quantitative demographic and outcome variables will be reported as the means and standard deviations (SD); qualitative variables will be reported as counts and percentages. Between-group comparisons among quantitative variables will be performed using independent one-way ANOVA. Where ANOVA omnibus F test is significant, pairwise comparisons using Fisher's least significant difference (LSD) method will be performed. Between-group differences among qualitative variables will be assessed using the chi-square test or Fisher's exact probability test. Measures of within-group change in quantitative variables, from baseline out to 12, 24, and 36 months, will be analyzed using the dependent-samples t test. Correlation and linear regression analyses will be performed to assess the strength and direction of the relationship between changes in BMI and telomere length and to estimate the predictive value of BMI change relative to telomere length at the 12-, 24-, and 36-month follow-ups. Statistical significance was set at p < 0.05.
Interim analyses {21b}
The PI will have access to interim results and make the final decision to terminate the trial.
Methods for additional analyses (e.g., subgroup analyses) {20b}
Not applicable
Methods in analysis to handle protocol nonadherence and any statistical methods to handle missing data {20c}
Randomized clinical trials analyzed using the intent-to-treat (ITT) approach provide unbiased comparisons between treatment groups. In cases of nonadherence, intent-to-treat analysis will be performed to avoid overlap and exclusion effects that could disrupt the random assignment of treatment groups in the study. The ITT analysis will provide information on the potential effects of treatment policies rather than on the potential effects of a particular treatment.
Plans to give access to the full protocol, participant-level data, and statistical code {31c}
Not applicable.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
The principal investigator (PI) is responsible for the overall project and for organizing steering committee meetings. An independent steering committee will be responsible for ensuring the overall safety of participants, coordinating study meetings, supervising the study, monitoring data safety, and overseeing the quality control.
Composition of the data monitoring committee, its role, and reporting structure {21a}
The composition of the data monitoring committee (DMC) is not needed because the PI organizes an independent steering committee meeting.
Adverse event reporting and harms {22}
An adverse event (AE) refers to any untoward event that occurs during the clinical study but that does not necessarily have a causal relationship with the surgical treatment. Safety evaluations are performed from the point at which the signature on the informed consent form is obtained until the end of the study or until the patient withdraws from the trial, according to the management requirements. AEs and serious adverse events (SAEs) will be reported.
An SAE is an event that causes hospitalization, prolonged hospitalization, disability, incapacity, life-threatening illness or death, or congenital malformation during the clinical trial.
During the study, all AEs will be recorded. These records include the type of AE (using standard medical terminology), the date of the AE, the date of the disappearance/stabilization of the AE, the severity of the AE, the impact of the AE on the surgery, the relationship of the AE with the surgery, the treatment measures, and the outcomes. If an SAE occurs, researchers fill out an SAE report form. The report is signed and dated and reported to the Ethics Committee of The Society of Bariatric and Metabolic Surgeons of Kazakhstan (Nur-Sultan, Kazakhstan) within 24 h.
Frequency and plans for auditing trial conduct {23}
Frequency for auditing trial conduct: 12, 24, and 36 months after surgery.
The process will be independent from investigators and the sponsor.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
If the acceptance criteria, the results, and analyses are changed, this information will be communicated to all trial participants and to the ethics committee in writing (letters).
A sponsor will submit a protocol amendment in cases when there are changes in the existing protocol that significantly affect the safety of subjects, scope of the investigation, or scientific quality of the study.
Dissemination plans {31a}
The trial results will be distributed in journal publications.
Discussion
Advances in bariatric and metabolic surgery make it possible to include this subspecialty in our national health systems as an independent specialization [17]. LOAGB is recognized as a simple and safe gastric bypass method [12], and LOAGB has been shown to match RYGB in weight loss and metabolic improvement after 2 years [18].
However, the high likelihood of intractable biliary reflux in LOAGB, 2.8% after revisional bariatric procedures (r-LOAGB) and 0.4% after primary LOAGB (p-LOAGB), is a serious complication. A. Soprani et al. [19] collected a series of 16 patients who underwent laparoscopic Nissen/MGB for large sliding hiatal hernia or paraesophageal hernia between 2013 and 2016. The surgery consisted of a standard MGB combined with crural repair and Nissen fundoplication using the remnant stomach as an anti-reflux valve. During this period, ten patients underwent Nissen/MGB after laparoscopic adjustable gastric band (LAGB) (seven two-stage and three one-stage procedures). None of these patients developed postoperative symptomatic bile reflux.
Our current study, for the first time, investigates the results of applying a similar method for all cases of morbid obesity according to the specified inclusion criteria. Thus, regardless of the presence or absence of insufficiency of the lower esophageal sphincter and hiatal hernia, in the experimental group, we will perform a bariatric surgical procedure, which we called “FundoRingOAGB.” The combination of gastric bypass and fundoplication (CGB&F) as described in the publication can be classified into three main types:
-
Type 1 CGB&F—One-stage: simultaneous gastric bypass and fundoplication.
-
Type 2 CGB&F—Two-stage: consequent gastric bypass after fundoplication with (a) complete takedown of the wrap; (b) partial takedown of the wrap; and (c) without takedown of the wrap.
-
Type 3 CGB&F—Two-stage: sequential fundoplication after a previous bariatric surgery.
-
Type 1 CGB&F includes primary bariatric procedures, and types 2–3 are secondary surgical procedures [13].
The return of weight after any gastric bypass method significantly reduces the preference for using bariatric and metabolic surgery for the treatment of morbid obesity; therefore, we first examined the likelihood of weight return after the experimental (FundoRingOAGB) and standard procedure of LOAGB. For the primary outcome measure, the change in body mass index will be assessed 12, 24, and 36 months after the surgical procedure.
Simultaneously, secondary outcome measures were the likelihood of biliary reflux within 12, 24, and 36 months after this procedure. Finally, GERD will be assessed if preoperative GERD symptoms are present or if GERD symptoms occur postoperatively (de novo GERD). For this aim, we will use a quality-of-life scale for gastroesophageal reflux disease (GERD-HRQL) [20].
In the present randomized controlled trial, we expect to show that one anastomosis gastric bypass with fundoplication ring for obese patients will better prevent the return of weight and bile reflux than the standard method. The idea to investigate in a randomized study the combination of two procedures as a primary procedure for one-anastomotic gastric bypass was expressed by Oral Ospanov [13, 21].
Trial status
At the time of the initial manuscript submission, recruitment had started on March 29, 2021, and the last patient is expected to be included in the study in December 2021. The study will be completed on April 5, 2024.
Abbreviations
- AE:
-
Adverse event
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- BR:
-
Bile reflux
- CRF:
-
Case report form
- DMC:
-
Data monitoring committee
- EBMIL:
-
Excess body mass index loss
- GERD:
-
Gastroesophageal reflux disease
- ITT:
-
Intent to treat
- LMGB:
-
Laparoscopic minigastric bypass gastric bypass
- LOAGB:
-
Laparoscopic one anastomosis gastric bypass
- LRYGB:
-
Laparoscopic Roux-en-Y gastric bypass
- LSD:
-
Least significant difference
- PI:
-
Principal investigator
- REC/IRB:
-
Research ethics committee/institutional review board
- SAEs:
-
Serious adverse events
- SBMSK:
-
Society of Bariatric and Metabolic Surgeons of Kazakhstan
- SD:
-
Standard deviation
- TWL:
-
Total weight loss
References
Golzarand M, Toolabi K, Farid R. The bariatric surgery and weight losing: a meta-analysis in the long- and very long-term effects of laparoscopic adjustable gastric banding, laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy on weight loss in adults. Surg Endosc. 2017;31(11):4331–45. https://doi.org/10.1007/s00464-017-5505-1 Epub 2017 Apr 4.
Faria SL, de Oliveira Kelly E, Lins RD, Faria OP. Nutritional Management of Weight Regain After Bariatric Surgery. Obes Surg. 2010;20(2):135–9. https://doi.org/10.1007/s11695-008-9610-z.
Fobi M, Malgorzata V, Stanczyk JN, Che-Senge K. The banded gastric bypass: principles of metabolic surgery III: Springer; 2012. p. 227–38. https://doi.org/10.1007/978-3-642-02411-5_20.
Spann MD, Aher C. V, English W.J., Williams D.B. Endoscopic management of erosion after banded bariatric procedures. Surg Obes Relat Dis. 2017;13(11):1875–9. https://doi.org/10.1016/j.soard.2017.07.025.
Deitel M. International MGB-OAGB Club affiliates with IJS. Int. J. Surg. 2019;61:76–7. https://doi.org/10.1016/j.ijsu.2018.09.008.
De Luca M, Piatto G, Merola G, et al. IFSO Update Position Statement on One Anastomosis Gastric Bypass (OAGB). Obes Surg. 2021;31(7):3251–78. https://doi.org/10.1007/s11695-021-05413-x.
Kassir R, Petrucciani N, Debs T, Juglard G, Martini F, Liagre A. Conversion of One Anastomosis Gastric Bypass (OAGB) to Roux-en-Y Gastric Bypass (RYGB) for Biliary Reflux Resistant to Medical Treatment: Lessons Learned from a Retrospective Series of 2780 Consecutive Patients Undergoing OAGB. Obes Surg. 2020;30(6):2093–8. https://doi.org/10.1007/s11695-020-04460-0.
Ospanov O, Maleckas A, Orekeshova A. Gastric greater curvature plication combined with Nissen fundoplication in the treatment of gastroesophageal reflux disease and obesity. Medicina. 2016;52(5):283–90. https://doi.org/10.1016/j.medici.2016.08.001.
Kawahara NT, Alster C, Maluf-Filho F, Polara W, Campos GM, Poli-de-Figueiredo LF. Modified Nissen fundoplication: laparoscopic antireflux surgery after Roux-en-Y gastric bypass for obesity. Clinics (Sao Paulo). 2012;67(5):531–3. https://doi.org/10.6061/clinics/2012(05)23.
Colpaert J, Horevoets J, Maes L, Uijtterhaegen G, Dillemans B. Surgical treatment of therapy-resistant reflux after Roux-en-Y gastric bypass. A case series of the modified Nissen fundoplication. Acta Chir Belg. 2020;120(4):291–6. https://doi.org/10.1080/00015458.2019.1696028.
Maurissen J, Yercovich N, Van Aelst P, et al. Modified Nissen Fundoplication for Late Dum** Syndrome After Roux-en-Y Gastric Bypass. Obes Surg. 2021;31(5):2353–5. https://doi.org/10.1007/s11695-021-05310-3.
Carbajo MA, Luque-de-León E, Jiménez JM, Ortiz-de-Solórzano J, Pérez-Miranda M, Castro-Alija MJ. Laparoscopic one-anastomosis gastric bypass: technique, results, and long-term follow-up in 1200 patients. Obes Surg. 2017;27(5):1153–67. https://doi.org/10.1007/s11695-016-2428-1.
Ospanov OB. The Gastric Bypass and Fundoplication in Bariatric Surgery: the Comments on Published Papers and Our Classification of Combination Procedures. Obes Surg. 2021;31(10):4643–4. https://doi.org/10.1007/s11695-021-05505-8.
Bhandari M, Fobi MAL, Buchwald JN, et al. Standardization of Bariatric Metabolic Procedures: World Consensus Meeting Statement. Obes Surg. 2019;29(S4):309–45. https://doi.org/10.1007/s11695-019-04032-x.
Ospanov O, Buchwald JN, Yeleuov G, Bekmurzinova F. Laparoscopic One-Anastomosis Gastric Bypass with Band-Separated Gastric Pouch (OAGB-BSGP): a Randomized Controlled Trial. Obes Surg. 2019;29(12):4131–7. https://doi.org/10.1007/s11695-019-04236-1.
Brasher PM, Brant RF. Sample size calculations in randomized trials: common pitfalls. Can J Anaesth. 2007;54(2):103–6. https://doi.org/10.1007/BF03022005.
Ospanov O. Thank IFSO for the Support that Allowed to Include the Specialization “Bariatric and Metabolic Surgery” in the Healthcare System of Kazakhstan. Obes Surg. 2021;31(6):2774–5. https://doi.org/10.1007/s11695-021-05232-0.
Robert M, Espalieu P, Pelascini E, Caiazzo R, Sterkers A, Khamphommala L, Poghosyan T, Chevallier JM, Malherbe V, Chouillard E, Reche F, Torcivia A, Maucort-Boulch D, Bin-Dorel S, Langlois-Jacques C, Delaunay D, Pattou F, Disse E. Efficacy and safety of one anastomosis gastric bypass versus Roux-en-Y gastric bypass for obesity (YOMEGA): a multicentre, randomised, open-label, non-inferiority trial. Lancet. 2019;393(10178):1298. doi: https://doi.org/10.1016/S0140-6736(19)30475-1. Epub 2019 Mar 6. Erratum in: Lancet. 2019;393(10178):1298.
Soprani A, Carandina S, El Kareh I, Genser L, Cady J. Revision of Lap-Band to MGB. In: Deitel M, editor. Essentials of Mini – One Anastomosis Gastric Bypass. Cham: Springer; 2018. https://doi.org/10.1007/978-3-319-76177-0_22.
Velanovich V. The development of the GERD-HRQL symptom severity instrument. Dis Esophagus. 2007;20(2):130–4. https://doi.org/10.1111/j.1442-2050.2007.00658.x.
Ospanov O. Biography: Oral Ospanov, M.D., PhD, DMedSc, Professor. Obes Surg. 2021;31(7):2849–50. https://doi.org/10.1007/s11695-021-05501-y.
Acknowledgements
The authors would like to thank all support personnel of the Department of Surgical Disease and Bariatric Surgery, Astana Medical University, and Hospital “Green Clinic” for their hard work and dedication.
Authors’ contributions {31b}
OO is the principal investigator; he conceived the study and led the proposal and protocol development. BY and AF contributed to the study design and to the development of the proposal. GY, RF, and ZS are the lead trial methodologists. AM will be involved in the recruitment of patients and the acquisition of data. The authors read and approved the final manuscript.
Funding {4}
The work will be supported by The Society of Bariatric and Metabolic Surgeons of Kazakhstan, Nur-Sultan, Kazakhstan, grant number 2021/1. The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Availability of data and materials {29}
The datasets generated or analyzed during the current study are available from the corresponding author upon reasonable request. Requests for data should be directed to the Primary Investigator, Oral Ospanov, bariatric.kz@gmail.com.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate {24}
The study protocol, patients’ information sheets, and informed consent forms were approved by the local ethics committee (Research ethics committee/institutional review board (REC/IRB)): Ethics committee of SBMSK approved trial 29.03.2021, Approval Number: #1. The study will be conducted in accordance with the ethical standards of the 1964 Helsinki Declaration and its later amendments and with the approval of the regional ethics committee of Nur-Sultan, Kazakhstan. Written informed consent will be obtained and documented for all study participants.
Consent for publication {32}
Not applicable.
Competing interests {28}
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ospanov, O., Yeleuov, G., Fursov, A. et al. A laparoscopic one anastomosis gastric bypass with wrap** versus nonwrap** fundus of the excluded part of the stomach to treat obese patients (FundoRingOAGB trial): study protocol for a randomized controlled trial. Trials 23, 264 (2022). https://doi.org/10.1186/s13063-022-06252-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-022-06252-6