Abstract
Background
Generalized anxiety disorder (GAD) is common among perimenopausal women. Acupuncture may be an effective treatment for GAD, but evidence is limited. The pathogenesis of GAD is not yet clear, but it is related to the hypothalamic-pituitary-adrenal axis and its excretion, cortisol (CORT), and adrenocorticotropic hormone (ACTH). The object of this study is to evaluate the efficacy of manual acupuncture (MA) versus placebo acupuncture (PA) for perimenopausal women with GAD.
Methods
This study is a single-center, randomized, single-blind clinical trial that will be conducted in the First Affiliated Hospital of Guangzhou University of Chinese Medicine. A total of 112 eligible GAD patients will be randomly assigned (1:1) to receive MA (n=56) or PA (n=56) three times per week for 4 weeks. The primary outcome measure will be the HAMA score. The secondary outcome measures will be the GAD-7 and PSQI scores and the levels of CORT and ACTH. The evaluation will be executed at baseline, 2 weeks, the end of the treatment, and a follow-up 3-month period. All main analyses will be carried out based on the intention-to-treat (ITT) principle.
Discussion
This study intends to compare the efficacy between MA and PA in the treatment of perimenopausal women with GAD and to further study the mechanisms underlying the effect.
Trial registration
Chinese Clinical Trial Registry ChiCTR2100046604. Registered on 22 May 2021.
Similar content being viewed by others
Background
Generalized anxiety disorder (GAD) is a kind of chronic mental disorder that includes both mental and physical symptoms [1]. GAD patients have general apprehensiveness or worry that is not restricted to any particular stimulus as the primary clinical feature [2]. According to epidemic research in Europe, the incidence rate of GAD is 6.2% throughout the lifetime [3]. Moreover, females have 1.5 to 2 times more exposure to GAD than males [4]. To our knowledge, the prevalence of females aged between 45 years old and 54 years old is the highest [5]. This phenomenon indicates that perimenopausal women are more likely to suffer from GAD. As one of the first-line pharmacotherapies for GAD [6], serotonin reuptake inhibitors (SSRIs) inhibit the reuptake of serotonin to increase the concentration of serotonin in the synaptic cleft to improve the level of serotonin in the brain which is believed to attenuate anxious symptoms [7]. However, patients’ conditions might be aggravated in the first week when first taking SSRIs [8]. Therefore, some patients refuse to take drugs because of adverse events. They seek for the help of psychiatrists and some of them probably receive cognitive behavioral therapy (CBT). Studies [9, 10] have shown that CBT is an effective treatment for GAD. However, due to the shortage of professionals and high cost, CBT is not beneficial for most patients in China [11]. Therefore, patients turn to find help from alternative therapies such as acupuncture.
Although the pathogenesis of GAD is not yet clear, current studies believe that it is related to abnormal secretory function of the hypothalamic-pituitary-adrenal axis (HPA axis) [12]. Cortisol (CORT) synthesized by the adrenal cortex is one of the final products of the HPA axis. CORT and adrenocorticotropic hormone (ACTH) levels in GAD patients are higher than those in normal people [13, 14]. The elevation of CORT and ACTH is detected as negative feedback by the hypothalamus which downregulates the stress response [15, 16]. Therefore, the decline in CORT and ACTH levels could predict the degree of improvement that a treatment produces [17].
Therefore, we designed a randomized controlled trial that focus on the efficacy of manual acupuncture (MA) versus placebo acupuncture (PA) on GAD in perimenopausal women. To evaluate the therapeutic efficacy of GAD, the primary outcome measure used in our protocol is the change in scores on the Hamilton Anxiety Scale (HAMA). The secondary outcome measures are the changes in scores on the Generalized Anxiety Disorder Scale (GAD-7) and Pittsburgh Sleep Quality Index (PQSI), and laboratory indicators related to the current possible pathogenesis of GAD, mainly including CORT and ACTH. This protocol aims to explore the efficacy of MA in GAD patients who would not prefer drug or CBT therapies. Meanwhile, we hope to explore the possible mechanism of acupuncture on GAD by measuring the levels of CORT and ACTH in serum.
Methods
Study design
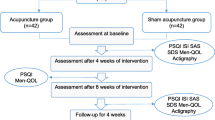
This is a randomized controlled and patient-and-assessor-blind trial. A total of 112 participants will be enrolled from the outpatient Acupuncture and Moxibustion Department of the First Affiliated Hospital of Guangzhou University of Chinese Medicine. The flow chart is shown in Fig. 1. The protocol is composed based on the SPIRIT checklist [18] and the Declaration of Helsinki [19]. The trial was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (K[2021]014) and has been registered at the Chinese Clinical Registry (ChiCTR2100046604).
Sample size
According to preliminary trials [20, 21], we chose the HAMA score as the outcome to calculate the sample size. After treatment, the HAMA score of MA was 14.0±3.2, and the HAMA score of PA was 19.4±5.3. PASS version 15.0 (NCSS, LLC. Kaysville, UT, USA) was used to estimate the sample size with a power level of 90% and a two-sided significance level of 5%. After calculation, we set the sample size of 44 patients in each group to observe the significant difference between the two groups. With a 20% withdrawal rate, we plan to enroll a total of 112 patients with 56 patients in each group.
Randomization and allocation concealment
An independent researcher sets a random number as a seed and used IBM SPSS Statistics version 26 (IBM SPSS Inc., Chicago, USA) to generate a random allocation sequence. According to the size of the random number sequence, patients who received the first 56 random numbers will be allocated into the MA group, and patients who received the last 56 random numbers will be allocated into the PA group in a 1:1 ratio. The researcher will put the cards with written random numbers and group assignments into sealed envelopes. The intervenors will allocate enrolled patients into the MA group or PA group on the basis of the card in the envelope.
Blinding
Due to the nature of acupuncture, it is difficult to blind patients and intervenors at the same time. We designed a new placebo acupuncture needle pedestal to blind patients, and the pedestal was applied for a patent in China (202121352221.7). The new placebo needle can be used at different positions with the acupoints and at different needling angles (Fig. 2). The PA group used a special needle (φ0.30 × 40 mm, produced by Huanqiu Co. Ltd., China) in which the tip is blunt so that the needle could not be pricked into the skin. In addition, the diameter of the blunt needle is 0.3 mm. It is thin enough to make patients feel like they are pricked. The pedestal is opaque so patients would not know whether the needle is pricked. Therefore, patients are unaware of whether they receive MA or PA. In addition, there is adhesive tape below the base to ensure that the whole pedestal can adhere to the head and body (Fig. 3). To test the participant-blinding effects, the first two patients will be randomly selected from the study to guess whether they have received MA or PA within 5 minutes after one of the treatment sessions in weeks 2 and 4. In addition, researchers who analyze the data are unaware of group assignments either to minimize the potential source of bias.
Patient recruitment
A total of 112 GAD patients will be recruited through, but not limited to, posters pasted in the hospital lobby and social media (WeChat), which can be seen by potential patients. Meanwhile, we will cooperate with the gynecology and psychology departments of the hospital for potential patients to ensure the target sample size is reached. Eligible patients who meet the inclusion criteria and exclusion criteria will be enrolled in the outpatient department of the First Affiliated Hospital of Guangzhou University of Chinese Medicine.
Inclusion criteria
Patients who meet all of the following criteria will be allowed to enrollment:
-
1.
Aged 45 to 55 years old (female only).
-
2.
Meet the diagnostic criteria of DSM-5 [1] for GAD.
-
3.
Excessive anxiety and worry (apprehensive expectation), occurring more days than not for at least 6 months.
-
4.
The individual finds it difficult to control the worry.
-
5.
The anxiety and worry are associated with three (or more) of the following six symptoms (with at least some symptoms having been present for more days than not for the past 6 months):
-
a.
Restlessness or feeling keyed up or on edge.
-
b.
Being easily fatigued.
-
c.
Difficulty concentrating or mind going blank.
-
d.
Irritability.
-
e.
Muscle tension.
-
f.
Sleep disturbance (difficulty falling or staying asleep, or restless, unsatisfying sleep).
-
(4)
The anxiety, worry, or physical symptoms cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.
-
(5)
The disturbance is not attributable to the physiological effects of a substance (e.g., a drug of abuse, a medication) or another medical condition (e.g., hyperthyroidism).
-
(6)
The disturbance is not better explained by another mental disorder (e.g., anxiety or worry about having panic attacks in panic disorder).
-
(7)
The score of HAMA is between 14 and 28.
-
(8)
No anti-anxiety drugs or other psychotropic drugs in the last 2 weeks.
-
(9)
No participation in any other research in the last 1 month.
Exclusion criteria
Patients who meet any of the following criteria will be excluded:
-
1.
Diagnosed by other severe illnesses such as heart failure, tumors, renal failure, and so on.
-
2.
Diagnosed by psychotic illness such as schizophrenia, agoraphobia, and so on clearly.
-
3.
Addicted to alcohol or drugs in the past 3 months.
-
4.
Afraid of acupuncture.
-
5.
Pregnancy or breastfeeding.
Drop out criteria
Patients who meet any of the following criteria will be excluded from the study:
-
1.
Cannot complete all treatment sessions.
-
2.
Outbreaks of serious illness threatening life include disability, cancer, organ failure, and so on.
-
3.
Unwilling to comply with acupuncture treatment.
-
4.
Anti-anxiety drugs or other psychotropic drugs were administered when enrolled in the study.
-
5.
Pregnancy when enrolled in the study.
-
6.
Patient quits the study treatment by herself.
Inventions
All eligible patients will receive 12 sessions of MA or PA (4 weeks, three times a week). Each session will take 30 min. The acupuncture therapists have acupuncture education from Guangzhou University of Chinese Medicine (range 1–3 years of full-time academic studies) or have clinical experience in the First Affiliated Hospital of Guangzhou University of Chinese Medicine (range 2–5 years). In addition, all intervenors are trained to completely understand the standard of operation. The acupoints of the MA and PA groups are the same, including Sishenzhen (one of the main acupoints in **’s 3 Needle [22]), Shenting (DU24), Yintang (DU29), Shenmen (HT7), and Sanyinjiao (SP6). The acupoint locations are according to the “2006 People’s Republic of China National Standard” (GB/T1234-2006) with the exhibition in Fig. 4. The acupuncture operation will be performed according to the textbook of the 10th Five-Year Plan of the Ministry of Health. Enrolled patients will be treated one-on-one by a trained acupuncturist from beginning to the end. In addition, an independent assistant will follow the intervention procedures to ensure the consistency of treatment.
MA group
Patients in the MA group will be pricked by disposable, stainless steel needles (φ0.30 × 40 mm, produced by Huanqiu Co. Ltd., China) through our device. The needles will be inserted into the skin to ensure that patients have a Deqi sensation [23] which is considered the essential reason for the efficacy of acupuncture [24]. Depth of puncture is approximately 25–30 mm. The needles will be manipulated three times (at the start, middle, and end of every session) by twirling and lifting until the Deqi sensation occurs.
Placebo acupuncture (PA) group
Patients in the PA group will receive placebo acupuncture in which the needles will not be inserted into the skin and will not induce the Deqi sensation. The blunt-tipped placebo needle (φ0.30 × 40 mm, produced by Huanqiu Co. Ltd., China) can provide participant-blinding effects with a similar appearance to conventional needles but no skin penetration.
Outcome measure
The schedule of the whole procedure is shown in Fig. 5. An independent assessor who does not know the group assignment will collect outcome data for analysis.
Primary outcome
HAMA score
The primary outcome of this study is the HAMA [25] score, which measures the severity of anxiety. The HAMA is a 14-item scale rated on a 4-point scale with a total score ranging from 0 to 56. A higher score means a higher range of anxiety. According to studies, a score higher than 28 indicates severe anxiety, a score between 28 and 21 indicates moderate anxiety, a score between 20 and 14 indicates mild anxiety, and a score lower than 14 indicates no anxiety. HAMA will be assessed on the first day, the 14th day, and the 28th day of the trial.
Secondary outcome
GAD-7 score
The secondary outcome of this study is the GAD-7 [26] score, which measures the severity of GAD particularly. The GAD-7 is a 7-item scale rated on a 3-point scale with a total score ranging from 0 to 21. In addition, the GAD-7 score indicates the level of general anxiety. The higher the GAD-7 score is, the more anxious the patient is. If the patient is enrolled, GAD-7 will be assessed on the first day, the 14th day, and the 28th day of the trial.
PSQI score
The secondary outcome of this study is the PSQI [27] score, which measures the quality of sleep in the last month. The PSQI is a 19-item questionnaire, divided into 7 parts: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of slee** medication, and daytime dysfunction. The PSQI score is inversely proportional to the quality of sleep. PSQI will be assessed on the first day, the 14th day, and the 28th day of the trial.
CORT and ACTH
It has been suggested that anxious subjects demonstrate greater CORT and ACTH concentrations [28]. In this study, we will draw serum samples and investigate CORT and ACTH before and after acupuncture. An enzyme-linked immunosorbent assay (ELISA) will be used to measure CORT and ACTH. These related tests will be carried out by the clinical laboratory of the First Affiliated Hospital of Guangzhou University of Chinese Medicine.
Safety outcome
Researchers will record body temperature, respiratory rate, pulse rate, and blood pressure on the first day, the 14th day, and the 28th day of the treatment. Adverse events (AEs) caused by acupuncture should be truthfully recorded in case report forms (CRFs). In addition, intervenors should treat patients with AEs as soon as possible to comfort patients and eliminate AEs. In the meanwhile, we will inform AEs to the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine. After treatment of AEs, researchers will ask patients whether they would like to continue the trial or not and respect their will.
Ancillary and post-trial care
After the whole treatment, patients who received PA treatment will receive 12 sessions of MA treatment to compensate. If patients who receive MA treatment want to continue treatment, they can appoint and be treated in the outpatient department.
Follow-up
After the completion of 12 sessions of acupuncture, we will follow patients via telephone or WeChat to score the HAMA, GAD-7, and PSQI scores in the first month and the third month to determine whether the efficacy can be maintained.
Data monitoring and analysis
Data will be saved on paper as recorded by the assessors. All original data will be stored in the medical record room of the First Affiliated Hospital of Guangzhou University of Chinese Medicine for 3 years. An independent assistant will monitor the process of collecting data and the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine will carry out audits at regular intervals.
Two independent study assistants will input the data, check by themselves whether the input data have discrepancies, and correct them. IBM SPSS Statistics version 26 (IBM SPSS Inc., Chicago, USA) will be used to analyze the data. The HAMA, GAD-7, and PSQI scores assessed in each group will be subjected to normality tests. If the scores obey a normal distribution, a one-way analysis of variance will be performed. If not, the Kruskal-Wallis rank sum test will be performed. The repeatedly assessed HAMA, GAD-7, and PSQI scores in the MA or PA group will be subjected to a normality test. If the scores obey a normal distribution, repeated measurement analysis of variance will be performed. If not, the Friedman rank sum test will be performed. If the significance of the F-test of the univariate linear model is greater than 0.05, CORT and ACTH will be subjected to covariance analysis. If not, the Kruskal-Wallis rank sum test will be performed.
All statistical tests will be two-sided, and the significance level will be set at 0.05. If the patient drops out, we will follow the intention-to-treat (ITT) and use the last score or serological indices assessed as the final score or indices.
Quality control
Two independent study assistants will input the data to avoid misty**. Before the beginning of the trial, all intervenors will be trained, and another assistant will follow the intervention procedures and stay in the outpatient department to ensure consistency of acupuncture intervention among intervenors. In addition, quality inspectors will check the CRFs randomly to ensure that there are no recorded errors.
Discussion
According to recent meta-analyses or systematic reviews [ The trial is currently being carried out right now and the end date of enrollment is expected on May 1, 2022.Trial status
References
Pub AP. American Psychiatric Association: Diagnostic and statistical manual of mental disorders (DSM-5®); 2013.
Reed G, First M, Kogan C, et al. Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders. World Psychiatry. 2019;18(1):3–19.
Alonso J, Lépine J. Overview of key data from the European Study of the Epidemiology of Mental Disorders (ESEMeD). J Clin Psychiatry. 2007:3–9.
Thibaut F. Anxiety disorders: a review of current literature. Dialogues Clin Neurosci. 2017;19(2):87–8. https://doi.org/10.31887/DCNS.2017.19.2/fthibaut.
Ma X, **ang Y, Cai Z, et al. Generalized anxiety disorder in China: prevalence, sociodemographic correlates, comorbidity, and suicide attempts. Perspect Psychiatric Care. 2009;45(2):119–27. https://doi.org/10.1111/j.1744-6163.2009.00212.x.
Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19(2):93–107. https://doi.org/10.31887/DCNS.2017.19.2/bbandelow.
Arias H, Targowska-Duda K, García-Colunga J, et al. Is the antidepressant activity of selective serotonin reuptake inhibitors mediated by nicotinic acetylcholine receptors? Molecules. 2021;26(8):2149.
Warden D, Trivedi M, Wisniewski S, et al. Early adverse events and attrition in selective serotonin reuptake inhibitor treatment: a suicide assessment methodology study report. J Clin Psychopharmacol. 2010;30(3):259–66. https://doi.org/10.1097/JCP.0b013e3181dbfd04.
Hall J, Kellett S, Berrios R, et al. Efficacy of cognitive behavioral therapy for generalized anxiety disorder in older adults: systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry. 2016;24(11):1063–73.
Linden M, Zubraegel D, Baer T, Franke U, Schlattmann P. Efficacy of cognitive behaviour therapy in generalized anxiety disorders. Results of a controlled clinical trial (Berlin CBT-GAD Study). Psychother Psychosom. 2005;74(1):36–42. https://doi.org/10.1159/000082025.
Edinger J, Sampson W. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26(2):177–82. https://doi.org/10.1093/sleep/26.2.177.
Fiksdal A, Hanlin L, Kuras Y, Gianferante D, Chen X, Thoma MV, et al. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology. 2019;102:44–52. https://doi.org/10.1016/j.psyneuen.2018.11.035.
Shan Q, Ke Y, Wang Y. Value of plasma and salivary cortisol tests in generalized anxiety disorder. Chin Gen Pract. 2020;23(11):1372–5.
Vreeburg S, Zitman F, Van Pelt J, et al. Salivary cortisol levels in persons with and without different anxiety disorders. Psychosom Med. 2010;72(4):340–7. https://doi.org/10.1097/PSY.0b013e3181d2f0c8.
De Weerth C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci Biobehav Rev. 2017;83:458–71. https://doi.org/10.1016/j.neubiorev.2017.09.016.
Kinlein S, Phillips D, Keller C, et al. Role of corticosterone in altered neurobehavioral responses to acute stress in a model of compromised hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2019;102:248–55. https://doi.org/10.1016/j.psyneuen.2018.12.010.
Fischer S, Cleare A. Cortisol as a predictor of psychological therapy response in anxiety disorders-Systematic review and meta-analysis. J Anxiety Disord. 2017;47:60–8. https://doi.org/10.1016/j.janxdis.2017.02.007.
Chan A, Tetzlaff J, Altman D, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7. https://doi.org/10.7326/0003-4819-158-3-201302050-00583.
Association WM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. https://doi.org/10.1001/jama.2013.281053.
Carvalho F, Weires K, Ebling M, et al. Effects of acupuncture on the symptoms of anxiety and depression caused by premenstrual dysphoric disorder. Acupunct Med. 2013;31(4):358–63.
Dai J, Qin Y, Hao M. Effect of head acupuncture combined with renmai acupoint acupuncture on symptoms and sleep quality of elderly patients with anxiety disorder. J Sichuan Tradit Chin Med. 2020;38(11):184–7.
Yuan Q, Li J, Liu B, Wu ZF, ** R. Effect of **-3-needling therapy on plasma corticosteroid, adrenocorticotrophic hormone and platelet 5-HT levels in patients with generalized anxiety disorder. Chin J Integr Med. 2007;13(4):264–8. https://doi.org/10.1007/s11655-007-0264-9.
Park H, Park J, Lee H, Lee H. Does Deqi (needle sensation) exist? Am J Chinese Med. 2002;30(1):45–50. https://doi.org/10.1142/S0192415X02000053.
Chen S, Guo S, Marmori F, Wang Y, Zhao Q, Wang B, et al. Appraisal of the Deqi Concept among Contemporary Chinese Acupuncturists. eCAM. 2013;2013:538476–7. https://doi.org/10.1155/2013/538476.
Hamilton M. The assessment of anxiety states by rating [J]. Br J Med Psychol. 1959;32(1):50–5. https://doi.org/10.1111/j.2044-8341.1959.tb00467.x.
Spitzer R, Kroenke K, Williams J, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092–7. https://doi.org/10.1001/archinte.166.10.1092.
Buysse D, Reynolds C, Monk T, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4.
Bandelow B, Baldwin D, Abelli M, Bolea-Alamanac B, Bourin M, Chamberlain SR, et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162–214. https://doi.org/10.1080/15622975.2016.1190867.
Li M, **ng X, Yao L, Li X, He W, Wang M, et al. Acupuncture for treatment of anxiety, an overview of systematic reviews. Complement Ther Med. 2019;43:247–52. https://doi.org/10.1016/j.ctim.2019.02.013.
Yang X, Yang N, Huang F, Ren S, Li ZJ. Effectiveness of acupuncture on anxiety disorder: a systematic review and meta-analysis of randomised controlled trials. Ann General Psychiatry. 2021;20(1):9. https://doi.org/10.1186/s12991-021-00327-5.
Kim J, Kim S, Lee H, et al. Comparing verum and sham acupuncture in fibromyalgia syndrome: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:8757685.
Lenoir D, De Pauw R, Van Oosterwijck S, et al. Acupuncture versus sham acupuncture: a meta-analysis on evidence for longer-term effects of acupuncture in musculoskeletal disorders. Clin J Pain. 2020;36(7):533–49. https://doi.org/10.1097/AJP.0000000000000812.
Zhang J, He Y, Huang X, Liu Y, Yu H. The effects of acupuncture versus sham/placebo acupuncture for insomnia: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2020;41:101253. https://doi.org/10.1016/j.ctcp.2020.101253.
Zhong Y, Luo X, Chen Y, et al. Acupuncture versus sham acupuncture for simple obesity: a systematic review and meta-analysis. Postgrad Med J. 2020;96(1134):221–7. https://doi.org/10.1136/postgradmedj-2019-137221.
**ang Y, He J, Li R. Appropriateness of sham or placebo acupuncture for randomized controlled trials of acupuncture for nonspecific low back pain: a systematic review and meta-analysis. J Pain Res. 2018;11:83–94. https://doi.org/10.2147/JPR.S152743.
**ng J, Wu X, Liu H, et al. Effects of electroacupuncture therapy and cognitive behavioral therapy in chronic insomnia: a randomized controlled study [J]. eCAM. 2020;2020:5630130.
Ohayon M. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. https://doi.org/10.1053/smrv.2002.0186.
Alvaro P, Roberts R, Harris J. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36(7):1059–68. https://doi.org/10.5665/sleep.2810.
Funding
This work was supported by the surface of the State Natural Science Fund projects (8217150722), the National Administration of Traditional Chinese Medicine (GZY-KJS-2020-072), the Science and Technology Department of Guangdong Province (2018-5), National Administration of Traditional Chinese Medicine ([2019]62) and the First Affiliated Hospital of Guangzhou University of Chinese Medicine (2019ZWB07). The funding body had no role in the study design, data collection, statistical analysis, or interpretation or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Lixing Zhuang conceptualized and designed the study. **n Liu and **aoyan **e drafted the manuscript. **aoyan **e evaluates the GAD-7 and the HAMA. Yingjia Li and Meichen Li helped to perform the GAD data collection. Yuting Wang designed the new placebo acupuncture needle pedestal and performed the Clinical Trial Registration. Nanbu Wang was in charge of data analysis. Muxi Liao will help analyze the data. Lixing Zhuang provided methodological recommendations and will manage the research. All authors have agreed on the journal to which the article has been submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethic approval and consent to participate
The protocol has been amended and the trial has been approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine (K[2021]014). Before inclusion in the study, all patients have to provide written informed consent.
Consent for publication
All authors have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, X., **e, X., Li, Y. et al. Efficacy of manual acupuncture versus placebo acupuncture for generalized anxiety disorder (GAD) in perimenopause women: study protocol for a randomized controlled trial. Trials 22, 833 (2021). https://doi.org/10.1186/s13063-021-05756-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-021-05756-x