Abstract
Background
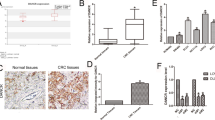
Triple-negative breast cancer (TNBC) is a subtype of breast cancer with higher aggressiveness and poorer outcomes. Recently, long non-coding RNAs (lncRNAs) have become the crucial gene regulators in the progression of human cancers. However, the function and underlying mechanisms of lncRNAs in TNBC remains unclear.
Methods
Based on public databases and bioinformatics analyses, the low expression of lncRNA MIDEAS-AS1 in breast cancer tissues was detected and further validated in a cohort of TNBC tissues. The effects of MIDEAS-AS1 on proliferation, migration, invasion were determined by in vitro and in vivo experiments. RNA pull-down assay and RNA immunoprecipitation (RIP) assay were carried out to reveal the interaction between MIDEAS-AS1 and MATR3. Luciferase reporter assay, Chromatin immunoprecipitation (ChIP) and qRT-PCR were used to evaluate the regulatory effect of MIDEAS-AS1/MATR3 complex on NCALD.
Results
LncRNA MIDEAS-AS1 was significantly downregulated in TNBC, which was correlated with poor overall survival (OS) and progression-free survival (PFS) in TNBC patients. MIDEAS-AS1 overexpression remarkably inhibited tumor growth and metastasis in vitro and in vivo. Mechanistically, MIDEAS-AS1 mainly located in the nucleus and interacted with the nuclear protein MATR3. Meanwhile, NCALD was selected as the downstream target, which was transcriptionally regulated by MIDEAS-AS1/MATR3 complex and further inactivated NF-κB signaling pathway. Furthermore, rescue experiment showed that the suppression of cell malignant phenotype caused by MIDEAS-AS1 overexpression could be reversed by inhibition of NCALD.
Conclusions
Collectively, our results demonstrate that MIDEAS-AS1 serves as a tumor-suppressor in TNBC through modulating MATR3/NCALD axis, and MIDEAS-AS1 may function as a prognostic biomarker for TNBC.
Similar content being viewed by others
Background
Breast cancer is one of the most common malignant tumors that has serious effects on the health of women worldwide [3,4,5]. TNBC is characterized by higher rates of relapse, greater metastatic potential, and shorter overall survival compared with other major breast cancer subtypes [4, 5]. Therapeutic methods for TNBC patients usually include surgery, chemotherapy, radiotherapy, and immunotherapy [6,7,8,9]. Clinically, although significant progress in the treatment of TNBC over the last decade, recurrence and metastasis remain the principal causes of mortality in patients with this disease [Full size image
MIDEAS-AS1 reduced the proliferation, migration and invasion of TNBC cells in vitro
To explore the potential roles of MIDEAS-AS1, we transfected the MIDEAS-AS1 overexpression plasmids into MDA-MB-231 and MDA-MB-468 cells, and the overexpression efficiency was determined by qRT-PCR (Additional file 1: Fig. S2A, S2B). Results from MTT assay indicated that the proliferation ability of MDA-MB-231 and MDA-MB-468 cells with MIDEAS-AS1 overexpression was reduced compared to control group (Fig. 2A). Meanwhile, overexpression of MIDEAS-AS1 also inhibited the proliferation of breast cancer organoids (Fig. 2B). Moreover, overexpression of MIDEAS-AS1 repressed cell colony-forming activity (Fig. 2C-D). Additionally, flow cytometry was used to detect the apoptosis rate and cell cycle after different treatments. Our results indicated that MIDEAS-AS1 overexpression led to remarkably increased apoptosis rate (Fig. 2E) and cell number in G1 phase (Additional file 1: Fig. S2C). On the other hand, MIDEAS-AS1 knockdown promoted the proliferation and colony-formation abilities of TNBC cells (Fig. 2A, D). Furthermore, the apoptosis rate was obviously decreased and the number of cells in G1 phase was less in MIDEAS-AS1 knockdown group compared to that in control group (Fig. 2E, Additional file 1: Fig. S2C). We then investigated the effect of MIDEAS-AS1 on TNBC cell migration and invasion using transwell assays and wound healing assay in MDA-MB-231 and MDA-MB-468 cells. The results of transwell assays showed a significant reduction in the migratory and invasive abilities of MDA-MB-231 and MDA-MB-468 cells after MIDEAS-AS1 overexpression (Fig. 2F). Furthermore, wound healing assay indicated that the wound closure area of MDA-MB-231 and MDA-MB-468 cells was dramatic decreased following MIDEAS-AS1 overexpression (Additional file 1: Fig. S2D). Consistently, MIDEAS-AS1 knockdown led to increased migratory and invasive abilities of TNBC cells (Fig. 2G). Given that epithelial-mesenchymal transition (EMT) is one of the major mechanisms for cancer metastasis, we further examined the effect of MIDEAS-AS1 on the expression of EMT-related marker proteins by Western blot. The results showed that N-cadherin, vimentin, fibronectin and MMP9 proteins were downregulated, while the E-cadherin protein was upregulated in MDA-MB-231 and MDA-MB-468 cells after overexpression of MIDEAS-AS1 (Fig. 2H). On the contrary, N-cadherin, vimentin, fibronectin and MMP9 proteins were upregulated, and the E-cadherin protein was downregulated in TNBC cells after MIDEAS-AS1 knockdown (Fig. 2I). Therefore, MIDEAS-AS1 could regulate the EMT process to modulate TNBC metastasis. Collectively, these results indicated that MIDEAS-AS1 played critical roles in suppressing cell proliferation and mobility of TNBC cells.
MIDEAS-AS1 inhibits TNBC cell proliferation and migration in vitro. A–D The effects of MIDEAS-AS1 overexpression and knockdown on the proliferation were examined by MTT assay (A-B) and colony formation assays (C-D). E Flow cytometry was performed to measure the effect of MIDEAS-AS1 on apoptosis. F-G Transwell and invasion assays were used to evaluate the motility of MDA-MB-231 and MDA-MB-468 cells transfected with MIDEAS-AS1-overexpressing vector or control vector (F) and si-NC or si-MIDEAS-AS1 (G). H Western blot analysis of EMT-related proteins in MDA-MB-231 and MDA-MB-468 cells after overexpression of MIDEAS-AS1. I Western blot analysis of EMT-related proteins in MDA-MB-231 and MDA-MB-468 cells after knockdown of MIDEAS-AS1. Data were shown as mean ± SD. (*p < 0.05, ** p < 0.01, *** p < 0.001)
MIDEAS-AS1 directly interacts with MATR3 to carry out its function
It had been suggested that the regulatory mechanisms of lncRNAs were closed associated with their subcellular localization [25]. Therefore, we firstly detected the subcellular localization of MIDEAS-AS1 in TNBC cells. Following isolation of the nuclear and cytoplasmic RNA in MDA-MB-231 and MDA-MB-468 cells, qRT-PCR analysis demonstrated that MIDEAS-AS1 was mainly located in the nucleus (Fig. 3A). Furthermore, FISH assay also obtained the similar results (Fig. 3B), indicating the potential role of MIDEAS-AS1 in transcriptional regulation through acting as a scaffold for TFs [25, 27]. To identify proteins interacted with MIDEAS-AS1, RNA pull-down assay was performed. We incubated MDA-MB-231 cell lysates with biotinylated MIDEAS-AS1 or its antisense RNA transcribed in vitro, and the silver staining and mass spectrometry (MS) identified MATR3 as one of the major proteins in the MIDEAS-AS1 pull-down precipitations (Fig. 3C). Moreover, mass spectrometry analysis found that MIDEAS-AS1 interacted with MATR3 at “DLSAAGIGLLAAATQSLSMPASLGR” and “YQLLQLVEPFGVISNHLILNK” peptide sequences (Fig. 3D). The specific binding between MATR3 protein and MIDEAS-AS1 was further confirmed by RNA pull down following Western blot (Fig. 3E). Then, we performed RNA immunoprotein (RIP) assay using flag antibodies and revealed that MIDEAS-AS1 was significantly enriched in MATR3 immunoprecipitations (Fig. 3F). Meanwhile, RNA FISH technology combined with immunofluorescence analysis demonstrated the co-localization of MIDEAS-AS1 and MATR3 protein in MDA-MB-231 cells (Fig. 3G). To identify the specific binding regions between MIDEAS-AS1 and MATR3, we constructed vectors containing full-length MIDEAS-AS1 or three truncated sequences (Fig. 3H). The RNA pull down assay indicated that deleting the sequence of 217–439 bp (Δ2 vector) could abolish the MIDEAS-AS1-MATR3 interaction, which was consist with the prediction results obtained from catRAPID database (Additional file 1: Fig. S3A). Furthermore, we wonder whether MIDEAS-AS1 could regulate the expression of MATR3. Significantly, overexpression or knockdown of MIDEAS-AS1 shows no effect on the RNA and protein expression levels of MATR3 (Additional file 1: Fig. S3B), indicating that MIDEAS-AS1 was not involved in the post-transcriptional regulation of MATR3. Collectively, these results revealed that MIDEAS-AS1 specifically interacted with MATR3 in TNBC cells.
MIDEAS-AS1 interacts with MATR3. A The expression level of MIDEAS-AS1 in the subcellular fractions of MDA-MB-231 and MDA-MB-468 cells was detected by qRT-PCR, with U6 and GAPDH as nuclear and cytoplasmic markers, respectively. B FISH analysis of the location of MIDEAS-AS1 (red) in the cytoplasm and nuclear fractions of MDA-MB-231 and MDA-MB-468 cells. Scale bars, 20 μm. C Biotin-labeled MIDEAS-AS1 was incubated with MDA-MB-231 cell lysates for pull-down, followed by SDS-PAGE separation, silver staining. D Peptide sequences analysis interacted with MIDEAS-AS1 was performed by mass spectrometry. E Western blot analysis following RNA pull-down assay indicated that MIDEAS-AS1 interacted with MATR3. F RIP assay using Flag antibody showed that MIDEAS-AS1 interacted with MATR3. G Colocalization of MIDEAS-AS1 and MATR3 by immunofluorescence following transfected with MIDEAS-AS1-overexpressing vector or control vector. Scale bars, 20 μm. H The interaction between the truncated MIDEAS-AS1 and MATR3 was confirmed by RNA pull-down and western blot. Data were shown as mean ± SD. (*p < 0.05, ** p < 0.01, *** p < 0.001)
MIDEAS-AS1 affects the progression and metastasis of TNBC by promoting NCALD expression
Given the critical role of nuclear matrix-associated protein MATR3 in transcriptional regulation, we wonder whether MIDEAS-AS1 could regulate the expression of downstream genes through interacting with MATR3 to further affect the progression and metastasis of TNBC. We performed an RNA-sequencing analysis on MIDEAS-AS1-overexpressing and control cells, and 471 differentially expressed genes were identified (Fig. 4A-B), including 252 up-regulated and 219 down-regulated genes. Meanwhile, the top 20 up-regulated genes are shown in Fig. 4C. Due to the tumor-suppressive role of MIDEAS-AS1 in TNBC, we integrated the down-regulated genes in breast cancer tissues from TCGA database and the up-regulated genes after MIDEAS-AS1 overexpression in TNBC cells, and then, 9 candidate genes were selected as potential downstream targets of MIDEAS-AS1 (Fig. 4D). It has been reported in the literature that antisense lncRNA may affect the expression of sense gene [28], so we also examined the influence of MIDEAS-AS1 on its sense gene (MIDEAS) by qRT-PCR. However, the results showed that MIDEAS-AS1 did not affect the expression of MIDEAS (Additional file 1: Fig. S4B). Meanwhile, the qRT-PCR results revealed that the expression of AF131215.5, CXCL1, and NCALD was remarkably increased after MIDEAS-AS1 overexpression and reduced after MIDEAS-AS1 knockdown (Additional file 1: Fig. S4A). CXCL1 (C-X-C motif chemokine ligand 1) was the most abundant chemokine secreted by TAMs, and CXCL1 could promote migration, invasion ability, and EMT in breast cancer [29, 30]. AF131215.5 was a lncRNA whose function remained unclear in cancer [31, 32]. Interestingly, NCALD (neurocalcin delta) expression was lower in lung adenocarcinoma tissues [33], and patients with higher NCALD levels exhibited a higher survival rate [34, 35]. Among these genes, we hypothesized that NCALD, the tumor suppressor gene, might be a potential target of MIDEAS-AS1 in breast cancer. Significantly, NCALD expression was markedly downregulated in breast cancer tissues compared with normal tissues (Additional file 1: Fig. S4C-D). Moreover, immunohistochemistry (IHC) showed higher expression of NCALD in normal tissues than breast cancer tissues according to Human Protein Atlas database (Additional file 1: Fig. S4E). These results indicated that NCALD might play a significant tumor suppressor role in breast cancer. Therefore, NCALD was selected as the functional downstream mediator for MIDEAS-AS1-mediated cell migration and metastasis in TNBC. Significantly, the qRT-PCR and Western blot results revealed that the expression of NCALD was remarkably increased after MIDEAS-AS1 or MATR3 overexpression and reduced after MIDEAS-AS1 or MATR3 knockdown in MDA-MB-231 and MDA-MB-468 cells (Fig. 4E-F, Additional file 1: Fig. S4E), indicating the regulatory effect of MIDEAS-AS1 and MATR3 on the expression of NCALD. Furthermore, rescue experiments were performed to demonstrate the causal link between MIDEAS-AS1, MATR3 and NCALD. The qRT-PCR analysis indicated that MIDEAS-AS1 overexpression promoted the expression of NCALD, while MATR3 knockdown could weaken the increased tendency of NCALD expression caused by overexpression of MIDEAS-AS1 in MDA-MB-231 and MDA-MB-468 cells (Fig. 4G). Furthermore, we found that the increasing trend of NCALD protein level caused by MIDEAS-AS1 overexpression could be attenuated by MATR3 knockdown in MDA-MB-231 and MDA-MB-468 cells (Fig. 4H). Then, we further investigated the transcriptional regulation effect of MIDEAS-AS1 and MATR3 on the expression of NCALD. The dual luciferase reporter assay showed that overexpression of MIDEAS-AS1 or MATR3 substantially increased the luciferase activity of NCALD vectors, while knockdown of MIDEAS-AS1 or MATR3 significantly inhibited the luciferase activity of NCALD vectors in HK293T cells (Fig. 4I). Furthermore, rescue experiments revealed that the promoter activity of NCALD was activated by MIDEAS-AS1 overexpression, which was inhibited after co-transfection with si-MATR3 (Fig. 4J). These results indicated that MIDEAS-AS1 could regulate the expression of NCALD through modulating the function of MATR3. To identify the specific binding regions of MIDEAS-AS1 and MATR3 on the NCALD promoter, we ectopically expressed full-length NCALD promoter (−2000 to + 100 bp) as well as five mutants: the truncated NCALD-1 (−2000 to −1564 bp) mutant, the truncated NCALD-2 (−1563 to −1094 bp) mutant, the truncated NCALD-3 (−1093 to −654 bp) mutant, the truncated NCALD-4 (−653 to −269 bp) mutant, and the truncated NCALD-5 (−268 to + 100 bp) mutant (Fig. 4K), and a luciferase assay was performed. We found that the luciferase activity was most significantly increased in −1563 bp to −1094 bp, indicating the presence of positive regulatory elements, which enhanced NCALD transcription in this region (Fig. 4L). In addition, the ChIP followed by qPCR assays was subsequently performed in Flag-MATR3 transfected breast cancer cells with or without MIDEAS-AS1 overexpression to determine the effect of MIDEAS-AS1 on MATR3 recruitment to the NCALD promoter. Consistent with the luciferase assay, MIDEAS-AS1-MATR3 complex was identified to significantly bound to −1563 bp to −1094 bp sites of the NCALD promoter region in MDA-MB-231 and MDA-MB-468 cells (Fig. 4M). These findings suggested that MIDEAS-AS1-MATR3 complex could enhance NCALD transcription by directly binding to its promoter.
MIDEAS-AS1 activates NCALD expression via recruiting the MATR3 complex to the NCALD promoter. A Heat map of differentially expressed gene based on RNA-seq analysis between MIDEAS-AS1-overexpression cells and control cells. B There were 471genes differentially expressed between MIDEAS-AS1-overexpression and control (252 up and 219 down) cells (|log2(Fold change) |> 1). C Top 20 up-regulated genes were showed. D Venn-diagrams showing intersect between down-regulated genes in TCGA data (1648 genes) and up-regulated DEGs after MIDEAS-AS1-overexpressing data (252 genes). E–F qRT-PCR and Western blot analysis of NCALD expression in MDA-MB-231cells and MDA-MB-468 cells with knockdown or overexpression MIDEAS-AS1 or MATR3. G qRT-PCR analysis of the expression of NCALD in MDA-MB-231 and MDA-MB-468 cells co-transfected with MIDEAS-AS1 overexpression vector or empty vector together with si-MATR3 or si-NC. H Western blot analysis of the expression of NCALD in MDA-MB-231 and MDA-MB-468 cells co-transfected with MIDEAS-AS1 overexpression vector or empty vector together with si-MATR3 or si-NC. I Luciferase reporter assays validated the binding of MIDEAS-AS1 and MATR3 with NCALD. J Luciferase assays in HK293T cells was determined by co-transfected with MIDEAS-AS1-overexpressing vector and si-MATR3. K-L Relative luciferase activity of full-length promoter and the other five truncated promoter regions of NCALD in HK293T cells by transfected with MIDEAS-AS1-overexpressing vector. M ChIP-qPCR experiments on ten different NCLAD promoter primer using anti-Flag antibody in MDA-MB-231 and MDA-MB-468 cells transfected with MIDEAS-AS1 overexpression plasmid and N-terminal FLAG-tagged MATR3 plasmid. Data were shown as mean ± SD. (*p < 0.05, ** p < 0.01, *** p < 0.001)
NCALD inhibits TNBC cell proliferation, migration, and invasion in vitro
Previous studies had found that NCALD was not only involved in cell apoptosis, cell cycle progression and other biological processes in several cancers [36], but also associated with the prognosis of cancers [34, 35]. However, there is no report about the function of NCALD in breast cancer. Our above results revealed downregulated expression of NCALD in breast cancer tissues and breast cancer cells, indicating the tumor-suppressive role of NCALD in breast cancer. To confirm the function of NCALD, we transfected NCALD overexpressing vectors and si-NCALD into MDA-MB-231 and MDA-MB-468 cells, and the overexpression and knockdown efficiency were determined by qRT-PCR and Western blot assays (Fig. 5A-B). The MTT and colony formation assay results indicated that NCALD overexpression reduced TNBC cell proliferation and colony formation abilities (Fig. 5C, D). Additionally, the apoptosis rate was remarkably increased and the number of cells in G1 phase was increased after NCALD overexpression (Fig. 5F, Additional file 1: Fig. S5A). On the other hand, NCALD knockdown led to significantly increased proliferation, colony formation abilities of TNBC cells (Fig. 5C, E). Moreover, the apoptosis rate was decreased and the number of the cells in G1 phase was less in NCALD knockdown cells than that in control cells (Fig. 5F, Additional file 1: Fig. S5A). Additionally, transwell assay and wound healing assay revealed that NCALD overexpression significantly reduced TNBC cell migration and invasion abilities (Fig. 5G, Additional file 1: Fig. S5B), while NCALD knockdown led to increased migration and invasion abilities in TNBC cells (Fig. 5H, Additional file 1: Fig. S5B). These data demonstrated that NCALD served as a suppressive functional factor in TNBC progression.
The overexpression of NCALD inhibits TNBC cell proliferation and migration in vitro. A-B The efficiency of overexpression and knockdown of NCALD were confirmed by qRT-PCR (A) and Western blot (B) in MDA-MB-231 and MDA-MB-468 cells. C–E The effects of NCALD overexpression and knockdown on the proliferation of MDA-MB-231 and MDA-MB-468 cells were examined by MTT assay (C) and colony formation assays (D-E). F Flow cytometry was performed to measure the effect of NCALD overexpression and knockdown on apoptosis. G Transwell and invasion were used to evaluate the motility of MDA-MB-231 and MDA-MB-468 cells transfected with NCALD-overexpressing vector or control vector (G) and si-NC or si-MIDEAS-AS1 (H). I Western blot analysis of p-ERK1/2, p-NF-κB and TNF-β protein levels in MDA-MB-231 and MDA-MB-468 cells transfected with NCALD overexpression plasmid. J Western blot analysis of p-ERK1/2, p-NF-κB and TNF-β protein levels in MDA-MB-231 and MDA-MB-468 cells transfected with transfected with si-NC or si-NCALD. Data were shown as mean ± SD. (*p < 0.05, ** p < 0.01, *** p < 0.001)
Previous literature reported that NCALD might be related to ERK1/2 signaling pathway, NF-κB signaling pathway, TGF-β signaling pathway and immune response pathway in ovarian cancer [35]. We further investigated the potential molecular mechanism caused by the change of NCALD in TNBC cells. Western blot analysis indicated that overexpression of NCALD would significantly inhibited phosphorylate p-65, but not TGF-β and, p-ERK1/2 in MDA-MB-231 and MDA-MB-468 cells (Fig. 5I), while NCALD knockdown brought about opposite results (Fig. 5J). Collectively, our results demonstrated that NCALD inhibited TNBC cell proliferation, migration, and invasion by suppressing NF-κB signaling pathways.
MIDEAS-AS1 regulates TNBC progression through regulating the expression of NCALD
Our previous results have showed that the association between MIDEAS-AS1 and MATR3 played significant role in initiating NCALD transcription. To further confirm whether MIDEAS-AS1 exerts tumor-suppressive functions via modulating NCALD, we co-transfected si-NCALD and MIDEAS-AS1-overexpressed plasmid in TNBC cell lines. The MTT and colony formation experiments indicated that NCALD knockdown remarkably rescued the proliferation ability of MDA-MB-231 and MDA-MB-468 cells inhibited by the overexpression of MIDEAS-AS1 (Fig. 6A-B). Moreover, the transwell and wound healing assays showed that NCALD knockdown could partially recover the cell migration and invasion abilities reduced by MIDEAS-AS1-overexpression (Fig. 6C-D). Moreover, Western blot analysis revealed that NF-κB pathway-related proteins were decreased after overexpression of MIDEAS-AS1, and that was increased after co-transfection of MIDEAS-AS1 overexpression plasmid and si-MATR3 (Fig. 6E). Taken together, MIDEAS-AS1 associates with MATR3 to initiate NCALD transcription and inhibits NF-κB signaling pathway, which further affects the TNBC progression.
MIDEAS-AS1 exerts its function by regulating the NCALD expression and downstream signaling. A-B The effects of co-transfected with MIDEAS-AS1 overexpression vector or empty vector together with si-NCALD or si-NC on the proliferation of MDA-MB-231 and MDA-MB-468 cells by MTT assay (A) and colony formation assays (B). C-D Transwell and wound-healing assays were used to evaluate the motility of MDA-MB-231 and MDA-MB-468 cells of co-transfected with MIDEAS-AS1 overexpression vector or empty vector together with si-NCALD or si-NC. E Western blot analysis of the corresponding signaling in MDA-MB-231 and MDA-MB-468 cells co-transfected with MIDEAS-AS1 overexpression vector or empty vector together with si-MATR3 or si-NC. Data were shown as mean ± SD. (*p < 0.05, ** p < 0.01, *** p < 0.001)
MIDEAS-AS1 overexpression inhibits TNBC progression and metastasis in vivo
In order to further evaluate the biological function of MIDEAS-AS1 in vivo, a subcutaneous xenograft model was first constructed. The MDA-MB-231 cells stably transfected with MIDEAS-AS1 overexpressing vectors or control vectors were subcutaneously injected to the flanks of nude mice. The result showed that the tumor weight and tumor volume were significantly inhibited in xenografts of MIDEAS-AS1 overexpressing group compared with those in the control group (Fig. 7A–C). Furthermore, immunohistochemistry (IHC) assays confirmed that MIDEAS-AS1 overexpression caused decreased Ki67 expression and increased NCALD expression, but did not affect MATR3 expression (Fig. 7D-E). In addition, we further constructed pulmonary metastasis model through intravenously injecting MDA-MB-231 cells stably expressing MIDEAS-AS1 or control vectors to compare the metastatic abilities in vivo. The results showed that mice injected with MIDEAS-AS1-overexpressing TNBC cells had no or fewer metastatic foci compared with the control group (Fig. 7F–H). Together, these results indicated that MIDEAS-AS1 played a critical role in inhibiting TNBC progression and metastasis.
MIDEAS-AS1 overexpression inhibits tumor formation in nude mice xenograft models. A MDA-MB-231 cells were stably transfected with the MIDEAS-AS1 overexpression plasmid or control plasmid and inoculated subcutaneously into nude mice. Compared with the control plasmid group, MIDEAS-AS1 overexpression inhibited tumor growth. B-C Tumor weight (B) Tumor volume (mm3) (C) were significantly decreased in the MIDEAS-AS1 overexpression group. D Immunohistochemistry with a Ki67-specific antibody was performed in the tumor. The results showed that MIDEAS-AS1 overexpression led to reduced expression of Ki67. Scale bars, 50 μm. E Representative images MATR3, NCALD staining in the tumor. Immunohistochemical staining showed that MIDEAS-AS1 overexpression led to increased expression of NCALD, while MATR3 was not affected. Scale bars, 50 μm. F-G Stably transfected MDA-MB-231 cells were injected into the tail veins of nude mice (n = 5). Representative images of lungs (F) and HE staining of lungs (H) isolated from mice. MIDEAS-AS1 overexpression resulted in a decreased number of lung metastatic colonies (G). I Schematic diagram depicting mechanisms involved in the effect of MIDEAS-AS1. Data were shown as mean ± SD. (*p < 0.05, ** p < 0.01, *** p < 0.001)