Abstract
Background
Vascular leakage is a major feature of acute respiratory distress syndrome (ARDS). We aimed to evaluate the efficacy of FX06, a drug under development that stabilizes interendothelial cell junctions, at reducing vascular leakage during SARS-CoV-2-induced ARDS.
Methods
This multicenter, double-blinded, randomized trial included adults with COVID-19-associated ARDS who had received invasive mechanical ventilation for < 5 days and were randomized to receive either intravenous FX06 (400 mg/d, for 5 days) or its vehicle as placebo. The primary endpoint was the lowering—from day 1 to day 7—of the transpulmonary thermodilution-derived extravascular lung-water index (EVLWi).
Results
Twenty-five patients were randomized to receive FX06 and 24 the placebo. Although EVLWi was elevated at baseline (median [IQR] 15.6 mL/kg [13.5; 18.5]), its declines from day 1 to day 7 were comparable for FX06 recipients and controls (respectively, − 1.9 [− 3.3; − 0.5] vs. − 0.8 [− 5.5; − 1.1] mL/kg; estimated effect − 0.8 [− 3.1; + 2.4], p = 0.51). Cardiac indexes, pulmonary vascular permeability indexes, and fluid balances were also comparable, as were PaO2/FiO2 ratios and durations of mechanical ventilation. Adverse event rates were similar for the 2 groups, although more FX06 recipients developed ventilator-associated pneumonia (16/25 (64%) vs. 6/24 (24%), p = 0.009).
Conclusions
In this unique-dosing–regimen study, FX06 did not lower SARS-CoV-2-induced pulmonary vascular leakage. Future investigations will need to evaluate its efficacy at earlier times during the disease or using other regimens.
Trial registration NCT04618042. Registered 5 November 2020.
Similar content being viewed by others
Introduction
Vascular leakage is a major feature of pathogen-induced acute respiratory distress syndrome (ARDS) [1]. Triggered by inflammation following endothelial and epithelial lesions, it is thought to play an important role in altering gas exchanges. Consequently, the extravascular lung-water index (EVLWi), a marker of pulmonary vascular leakage measured by transpulmonary thermodilution, is independently associated with ARDS patients’ outcomes [2, 3]. Because EVLWi is highly elevated during SARS-CoV-2-induced ARDS [4, 5], which causes high mortality [4, 6], controlling vascular leakage might be of major interest in managing this disease.
FX06, an innovative drug containing fibrin-derived peptide Bβ15-42, stabilizes vascular endothelial (VE)-cadherin–dependent interendothelial cell junctions [7,8,9]. It reduced capillary leakage in several animal models of lipopolysaccharide- or HCl-induced acute lung injury [9, 10] and prolonged survival in a murine model of dengue-virus infection [9]. In a phase II trial conducted on 234 patients suffering from ischemia–reperfusion injuries during acute coronary syndrome, FX06-treated patients had 58% smaller early necrotic core zones [11]. Importantly, adverse events were comparable between groups, indicating the drug’s high safety profile. FX06 was then used as salvage therapy for a patient with severe ARDS following Ebola-virus infection, with a temporal link between its injection and sharply decreased EVLWi [12]. More recently, FX06 (400 mg/d for 4–7 days) was given as compassionate therapy to 6 patients receiving extracorporeal membrane oxygenation (ECMO) for coronavirus disease 2019 (COVID-19) [13]; 4 experienced improvement and 2 died. No clear treatment-related adverse event occurred.
Taken together, those findings indicate that FX06 is well-tolerated by patients and is a potent regulator of vascular leakage during ARDS. We hypothesized that FX06 might limit pulmonary vascular hyperpermeability during ARDS induced by SARS-CoV-2 infection, thereby improving gas exchanges and patients’ outcomes.
Methods
Trial design
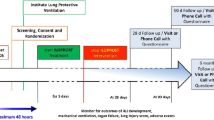
We conducted a multicenter, double-blinded, randomized trial. The independent ethics review board CPP Ouest VI, Brest, France, and the ANSM (Agence Nationale de Sécurité du Médicament et des Produits de Santé) approved the trial protocol (available in Additional file 1). F4-Pharma Ges.m.b.H. (Vienna, Austria) provided FX06. An independent Data- and Safety-Monitoring Committee periodically reviewed safety outcomes, with recruitment interruptions planned after inclusions of 10 and 30 patients. Neither F4-Pharma nor trial sponsors participated in the trial design, data collection, analysis or interpretation, or the writing or submission of the manuscript. The study protocol was registered at ClinicalTrials.gov (NCT04618042).
Participants
To be eligible for inclusion, patients had to be ≥ 18 year old and receiving invasive mechanical ventilation for < 5 days for polymerase-chain reaction-confirmed SARS-CoV-2-induced ARDS, according to the Berlin definition [14]. Exclusion criteria were mechanical ventilation for > 4 days; participation in another interventional clinical trial; severe renal, hepatic or cardiac insufficiency, or in a moribund state at randomization (see Additional file 1); contraindication for vascular access implantation for transpulmonary thermodilution monitoring; chemotherapy, radiotherapy or immunotherapy for malignancy; pregnancy or lactation; any history of severe allergic drug reaction. Patients taking drugs interfering with inflammation were also excluded, unless the drug’s use during COVID-19 was stated in the hospital center’s written policy.
According to the specifications of emergency consent, randomization was possible without a close relative’s or surrogate’s consent, but informed consent by the patient or patient’s relatives was obtained for all patients.
Treatment allocation
Patients were randomly assigned to receive either FX06 or its vehicle (phosphate-buffered saline) as the placebo. The randomization list was computer-generated with a 1:1 ratio and undisclosed block sizes, stratified by center. Concealment of the study-group assignments used a centralized, secure, interactive, web-based response system (CleanWeb, Telemedicine Technologies S.A.S., Boulogne-Billancourt, France) accessible from each study center. All investigators, statisticians, and data analysts were blinded to arm assignments until the study and analysis were completed.
Interventions
Patients were randomized to receive intravenous FX06, 400 mg/d or the placebo for 5 days. Each dose was administered in two boluses separated by a 10-min interval. The dose regimen chosen was based on the results of previous studies, in animals and humans, that suggested safety and mechanistic engagement with this dosing (additional Methods in Additional file 2). The manufacturer provided each treatment in unrecognizable ready-to-use form (numbered and sealed therapeutic units containing 10 vials of active treatment or placebo solution), stocked in each intensive care unit (ICU) under the supervision of each facility’s pharmacy department.
Patients were monitored using transpulmonary thermodilution systems (EV1000/Volume View, Edwards Lifesciences, Irvine, CA, USA, or PiCCO2, Pulsion Medical Systems, Feldkirchen, Germany), with thermistor-tipped catheters introduced in a femoral artery and an internal jugular vein [2,3,4]. Extravascular lung water and other thermodilution-derived parameters were averaged from three injections of cold physiological saline solution, in supine position, and indexed to the patient’s predicted body weight. Thermodilution measurements were taken before treatment administration during the first 5 days post-inclusion, with a measurement repeated 3 h post-administration on day 2, to detect a possible short-time effect of the drug. For patients receiving venovenous (VV)-ECMO, measurements were taken during a transient diminution of ECMO blood flow to < 2 L/min. Preliminary study results showed that thermodilution parameters were not affected by ECMO blood flow under that level (see Additional file 2: Table S1 and Fig. S1).
For ARDS management, investigators were asked to follow the most recent recommendations from the French Society of Intensive Care Medicine (https://www.srlf.org/rfe-srlf-prise-en-charge-du-syndrome-de-detresse-respiratoire-aigue-sdra-de-ladulte-a-la-phase-initiale/). Specific treatments targeting COVID-19 were discouraged, unless the drug’s use during COVID-19 was stated in the center’s written policy.
Serum interleukin (IL)-6, IL-10, and soluble (s)VE-cadherin were quantified with DuoSet Elisa kits (R&D systems, Minneapolis, MN, USA).
Outcomes
The primary endpoint was the EVLWi change, assessed by transpulmonary thermodilution, between day 1 and day 7. Secondary endpoints included the evolution of daily EVLWi, cardiac index, global end-diastolic volume index, and pulmonary vascular permeability index measured by transpulmonary thermodilution for 7 days; daily fluid balance; serum albumin; systolic, diastolic, and mean blood pressures; and heart rate for 7 days; partial oxygen pressure/fraction of inspired oxygen (PaO2/FiO2) ratio and Sequential Organ-Failure Assessment (SOFA) score over 15 days; rate of rescue with VV-ECMO; durations of invasive mechanical ventilation, vasopressor support, and renal replacement therapy over 30 days; Weinberg Radiological Severity score over 30 days [15]; survival at 30 and 60 days; nature and frequency of adverse events. Kinetics of serum d-dimers and C-reactive protein over 7 days were extracted from medical charts afterward. Serum IL-6, IL-10, and sVE-cadherin measurements on days 1 and 7 in available biological samples were added as post hoc measurements.
Statistical analyses
Assuming a baseline (inclusion) mean EVLWi of 13 mL/kg and standard deviation (SD) of 5 mL/kg [16], and a 30% EVLWi decrease in FX06-treated patients compared to controls on day 7 [9, 10, 12], for 80% power and an overall 5% two-sided α-risk, the required sample size was 25 patients/group.
Baseline characteristics are reported as number (%) for categorical variables and median [interquartile range, IQR] for continuous variables. Efficacy endpoints were analyzed according to intention-to-treat principles. Safety endpoints were analyzed for all patients who received at least one assigned-treatment dose.
Missing primary endpoints were replaced by imputation values for patients who died or whose conditions no longer warranted the transpulmonary thermodilution system before 7 days; the last thermodilution value was retained for the primary analyses. Primary endpoints were compared between groups using an adjusted analysis of covariance (ANCOVA) of EVLWi at randomization. Results are expressed in terms of adjusted mean change with 95% confidence interval (CI).
Three sensitivity analyses were computed: complete case analysis, worst-case analysis or using a different statistical method (Mann–Whitney U test). Prespecified subgroup analyses were conducted according to VV-ECMO or EVLWi > 10 mL/kg at inclusion.
Qualitative and quantitative secondary outcome measures were compared between groups using, respectively, Pearson’s Chi-square tests and t tests, or Mann–Whitney U tests. Overall survival was estimated with the Kaplan–Meier method. Longitudinal quantitative endpoints were compared using linear-mixed models with a random effect for subjects. This model was fitted to fixed effect by an interaction between treatment arm and time (since the date of randomization), with the slope parameter estimating the difference between groups. A restricted likelihood maximization-estimation method was used. The p-values associated with the fixed effects were calculated using the analysis of variance (ANOVA) function with Kenward–Roger approximation for calculating the number of degrees of freedom.
Analyses were computed with a 2-sided α risk of 5%. All analyses were performed using R software (R Foundation for Statistical Computing, Vienna, Austria), version 4.0.3.
Results
Forty-nine patients were randomized from November 2020 to April 2021 and retained for analysis (Fig. 1). Their main characteristics are reported in Table 1. They were very severely ill at baseline, with median PaO2/FiO2 ratio at 104, static pulmonary compliance of < 30 mL/cmH2O, and more than one-third of them were on VV-ECMO. One-third were receiving vasopressors. Specific therapies targeting COVID-19 were marginal, except corticosteroids, given to all participants.
Study drug
All but 5 patients received the complete treatment. One patient allocated to receive the placebo was accidentally included in another interventional study; the assigned treatment was interrupted after 3 days. One patient allocated to the FX06 arm stopped treatment after 3 days because of fungal co-infection. Two patients allocated to the placebo arm died on day 3 or day 4. Lastly, one patient’s treatment was accidentally withheld on day 4.
Primary outcome
The primary outcome of EVLWi change between days 1 and 7 did not differ between FX06-treated patients and controls (Fig. 2 and Table 2). EVLWi kinetics and their individual variations were also comparable between groups (Additional file 2: Figs. S2 and S3). Patients’s EVLWis were high at inclusion, comparable for the 2 groups, and remained elevated during the first 7 days. Eight patients—1 FX06 recipient and 7 controls—did not undergo transpulmonary thermodilution on day 7; their last available values were retained for the primary analysis: 3 had died (1 FX06 recipient and 2 controls), 3 had recovered sufficiently to allow removal of their thermodilution catheters, 1 had a catheter infection necessitating its removal, and 1 withdrew consent to participate. Excluding those patients did not affect the primary-analysis results.
Several sensitivity analyses were computed: analyzing the primary outcome for patients with ECMO vs without; most severely ill (EVLWi > 16 mL/kg) vs less severely ill; or indexing the dose received above vs below 4.2 mg/kg/d (median dose received). All failed to detect any significant FX06 effect on EVLWi (not shown). Interestingly, EVLWi measured before and 3 h after FX06 bolus injections was very similar (median variation − 0.02 [IQR − 0.57; 0.55], n = 22).
Secondary outcomes
The Pulmonary Vascular Permeability Index (PVPI) was also very high during the first 7 days and did not differ between groups (Additional file 2: Fig. S4). Cardiac index and global end-diastolic volume index (GEDVI) were also comparable (not shown). Daily fluid balance remained positive during the first week, with comparable levels for the 2 groups (Additional file 2: Fig. S5). Serum albumin, another marker of vascular leakage, was very low at inclusion (median 23 [IQR 19; 26] g/L; n = 48); it remained stable with no between-group differences during the first week.
PaO2/FiO2 remained very low during the first 15 days (Additional file 2: Fig. S6), and very few patients survived to be extubated on 30 days (Table 2). Interestingly, the Weinberg Radiological Severity score decreased less for FX06 recipients (estimated effect 0.13 [95% CI 0.07–0.18]; p < 0.001). However, for a post hoc analysis taking into account missing values and mortality as a competing risk by imputing the last score available for survivors and a score of 12 after the patient died, score kinetics did not differ between groups (Additional file 2: Fig. S7). Three patients (2 FX06 recipients and 1 control) received VV-ECMO rescue therapy after inclusion.
Although catecholamine-free days were comparable for the 2 groups (Table 2), FX06 recipients had significantly lower systolic and diastolic blood pressure values after day 4 (Additional file 2: Fig. S8). FX06 did not affect heart rate (not shown).
The baseline SOFA score, reflecting the extent of multiple organ failure, was high and remained stable for both groups for 15 days (Additional file 2: Fig. S9). Finally, survival was comparable for both groups, with 34/49 (69%) day-60 survivors (Table 2 and Additional file 2: Fig. S10). C-reactive protein and serum d-dimers, elevated at baseline, remained stable and comparable. Serum cytokine measurements were available for 24 patients. For both groups at inclusion, IL-6 was elevated and continued to rise during the first week, while IL-10 levels were also high and declined slightly during the first week. FX06 recipients had slightly higher baseline sVE-cadherin values that remained comparable to those of the placebo group thereafter.
Safety
Adverse event rates were comparable for the 2 groups (Table 3). Although overall secondary infections were not more common in FX06 recipients, they did develop more episodes of microbiologically confirmed ventilator-associated pneumonia [17].
Discussion
In this multicenter, double-blinded, randomized trial, FX06 did not alter the thermodilution-measured EVLWi evolution during SARS-CoV-2-induced ARDS. Other markers of pulmonary vascular leakage, e.g., patients’ functional outcomes reflecting pulmonary function and 60-day survival, were also not affected. Although elevated at baseline, circulating markers of inflammation and endothelial lesions were comparable for the 2 groups. Despite their similar rates of serious adverse events, FX06 was associated with higher rates of ventilator-associated pneumonia.
Inflammation-induced pulmonary vascular leakage is widely diffused during severe SARS-CoV-2 infection. Autopsies of COVID-19 patients revealed markedly elevated lung weights [18, 19] and disruption of interendothelial VE-cadherin-dependent junctions [7] and to dampen neutrophil recruitment in the lung in two models of lipopolysaccharide- or HCl-induced acute lung injury, indicating a risk of impaired bacterial clearance [10]. Importantly, incubation of FX06 with monocytes or alveolar macrophages did not impact their in vitro activation and capacity to release pro-inflammatory cytokines. Moreover, on the contrary, FX06 was shown to enhance bacterial clearance and ultimately survival in a model of secondary Pseudomonas aeruginosa infection [10]. Although this potential effect of FX06 needs to be better clarified, these findings indicate the need for close monitoring of secondary infections in future evaluations of it.
Conclusions
FX06 did not lower thermodilution-derived EVLWi during severe ARDS induced by SARS-CoV-2 infection. Whether other time-lines for its administration or other dosing regimens might be more efficient remains to be determined.
Availability of data and materials
All deidentified individual participant’s data collected for our study “FX06 to rescue acute respiratory distress syndrome during COVID-19 pneumonia. A randomized clinical trial” will be shared beginning with publication with no end date. These data will be available to researchers to who provide a methodologically sound proposal for the purposes of achieving specific aims outlined in that proposal. Proposals should be directed to the corresponding author via email: nicolas.brechot@aphp.fr and will be reviewed by the senior authors of the study. Requests to access data to undertake hypothesis-driven research will not be unreasonably withheld. To gain access, data requesters will need to sign a data-access agreement and to confirm that data will be used only for the agreed purpose for which access was granted.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- ECMO:
-
Extracorporeal membrane oxygenation
- EVLWi:
-
Extravascular lung-water index
- (s)VE-cadherin:
-
(soluble) vascular endothelial cadherin
- SARS-CoV-2:
-
Severe acute respiratory syndrome–coronavirus-2
- COVID-19:
-
Coronavirus disease 2019
References
Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–63.
Tagami T, Ong MEH. Extravascular lung water measurements in acute respiratory distress syndrome: why, how, and when? Curr Opin Crit Care. 2018;24:209–15.
Jozwiak M, Silva S, Persichini R, Anguel N, Osman D, Richard C, et al. Extravascular lung water is an independent prognostic factor in patients with acute respiratory distress syndrome*. Crit Care Med. 2013;41:472–80.
Shi R, Lai C, Teboul J-L, Dres M, Moretto F, De Vita N, et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: the PiCCOVID study. Crit Care Lond Engl. 2021;25:186.
Rasch S, Schmidle P, Sancak S, Herner A, Huberle C, Schulz D, et al. Increased extravascular lung water index (EVLWI) reflects rapid non-cardiogenic oedema and mortality in COVID-19 associated ARDS. Sci Rep. 2021;11:11524.
Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–18.
Yakovlev S, Gao Y, Cao C, Chen L, Strickland DK, Zhang L, et al. Interaction of fibrin with VE-cadherin and anti-inflammatory effect of fibrin-derived fragments. J Thromb Haemost JTH. 2011;9:1847–55.
Bach TL, Barsigian C, Yaen CH, Martinez J. Endothelial cell VE-cadherin functions as a receptor for the beta15-42 sequence of fibrin. J Biol Chem. 1998;273:30719–28.
Gröger M, Pasteiner W, Ignatyev G, Matt U, Knapp S, Atrasheuskaya A, et al. Peptide Bbeta(15–42) preserves endothelial barrier function in shock. PLoS ONE. 2009;4: e5391.
Matt U, Warszawska JM, Bauer M, Dietl W, Mesteri I, Doninger B, et al. Bbeta(15–42) protects against acid-induced acute lung injury and secondary pseudomonas pneumonia in vivo. Am J Respir Crit Care Med. 2009;180:1208–17.
Atar D, Petzelbauer P, Schwitter J, Huber K, Rensing B, Kasprzak JD, et al. Effect of intravenous FX06 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction results of the F.I.R.E. (Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury) trial. J Am Coll Cardiol. 2009;53:720–9.
Wolf T, Kann G, Becker S, Stephan C, Brodt H-R, de Leuw P, et al. Severe Ebola virus disease with vascular leakage and multiorgan failure: treatment of a patient in intensive care. Lancet Lond Engl. 2015;385:1428–35.
Adam EH, Schmid B, Sonntagbauer M, Kranke P, Zacharowski K, Meybohm P. Fibrin-derived peptide Bβ15-42 (FX06) as salvage treatment in critically ill patients with COVID-19-associated acute respiratory distress syndrome. Crit Care. 2020;24:574.
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307:2526–33.
Bréchot N, Demondion P, Santi F, Lebreton G, Pham T, Dalakidis A, et al. Intra-aortic balloon pump protects against hydrostatic pulmonary oedema during peripheral venoarterial-extracorporeal membrane oxygenation. Eur Heart J Acute Cardiovasc Care. 2018;7:62–9.
Wang H, Cui N, Su L, Long Y, Wang X, Zhou X, et al. Prognostic value of extravascular lung water and its potential role in guiding fluid therapy in septic shock after initial resuscitation. J Crit Care. 2016;33:106–13.
Luyt C-E, Sahnoun T, Gautier M, Vidal P, Burrel S, Pineton de Chambrun M, et al. Ventilator-associated pneumonia in patients with SARS-CoV-2-associated acute respiratory distress syndrome requiring ECMO: a retrospective cohort study. Ann Intensive Care. 2020;10:158.
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383:120–8.
Wichmann D, Sperhake J-P, Lütgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268–77.
D’Agnillo F, Walters K-A, **ao Y, Sheng Z-M, Scherler K, Park J, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med. 2021;13:eabj7790.
Michalick L, Weidenfeld S, Grimmer B, Fatykhova D, Solymosi PD, Behrens F, et al. Plasma mediators in patients with severe COVID-19 cause lung endothelial barrier failure. Eur Respir J. 2021;57:2002384.
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–8.
Rauch A, Dupont A, Goutay J, Caplan M, Staessens S, Moussa M, et al. Endotheliopathy Is Induced by Plasma From Critically Ill Patients and Associated With Organ Failure in Severe COVID-19. Circulation. 2020;142:1881–4.
Teuwen L-A, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–91.
Giannotta M, Trani M, Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–54.
Bergt S, Gruenewald M, Beltschany C, Grub A, Neumann T, Albrecht M, et al. The Fibrin-Derived Peptide Bβ15-42 (FX06) Ameliorates Vascular Leakage and Improves Survival and Neurocognitive Recovery: Implications From Two Animal Models of Cardiopulmonary Resuscitation. Crit Care Med. 2016;44:e988-995.
RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med. 2020;
Petzelbauer P, Zacharowski PA, Miyazaki Y, Friedl P, Wickenhauser G, Castellino FJ, et al. The fibrin-derived peptide Bbeta15-42 protects the myocardium against ischemia-reperfusion injury. Nat Med. 2005;11:298–304.
Roesner JP, Petzelbauer P, Koch A, Mersmann J, Zacharowski PA, Boehm O, et al. The fibrin-derived peptide Bbeta15-42 is cardioprotective in a pig model of myocardial ischemia-reperfusion injury. Crit Care Med. 2007;35:1730–5.
Tagami T, Kushimoto S, Yamamoto Y, Atsumi T, Tosa R, Matsuda K, et al. Validation of extravascular lung water measurement by single transpulmonary thermodilution: human autopsy study. Crit Care. 2010;14:R162.
Wollborn J, Hassenzahl LO, Reker D, Staehle HF, Omlor AM, Baar W, et al. Diagnosing capillary leak in critically ill patients: development of an innovative scoring instrument for non-invasive detection. Ann Intensive Care. 2021;11:175.
van de Weg CAM, Pannuti CS, van den Ham H-J, de Araújo ESA, Boas LSV, Felix AC, et al. Serum angiopoietin-2 and soluble VEGF receptor 2 are surrogate markers for plasma leakage in patients with acute dengue virus infection. J Clin Virol Off Publ Pan Am Soc Clin Virol. 2014;60:328–35.
Clajus C, Lukasz A, David S, Hertel B, Lichtinghagen R, Parikh SM, et al. Angiopoietin-2 is a potential mediator of endothelial barrier dysfunction following cardiopulmonary bypass. Cytokine. 2012;60:352–9.
Vassiliou AG, Keskinidou C, Jahaj E, Gallos P, Dimopoulou I, Kotanidou A, et al. ICU Admission Levels of Endothelial Biomarkers as Predictors of Mortality in Critically Ill COVID-19 Patients. Cells. 2021;10:186.
Spadaro S, Fogagnolo A, Campo G, Zucchetti O, Verri M, Ottaviani I, et al. Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit Care. 2021;25:74.
Dupont A, Rauch A, Staessens S, Moussa M, Rosa M, Corseaux D, et al. Vascular Endothelial Damage in the Pathogenesis of Organ Injury in Severe COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:1760–73.
Flemming S, Burkard N, Renschler M, Vielmuth F, Meir M, Schick MA, et al. Soluble VE-cadherin is involved in endothelial barrier breakdown in systemic inflammation and sepsis. Cardiovasc Res. 2015;107:32–44.
Yu W-K, McNeil JB, Wickersham NE, Shaver CM, Bastarache JA, Ware LB. Vascular endothelial cadherin shedding is more severe in sepsis patients with severe acute kidney injury. Crit Care. 2019;23:18.
Loosen G, Conrad AM, Hagman M, Essert N, Thiel M, Luecke T, et al. Transpulmonary thermodilution in patients treated with veno-venous extracorporeal membrane oxygenation. Ann Intensive Care. 2021;11:101.
Herner A, Lahmer T, Mayr U, Rasch S, Schneider J, Schmid RM, et al. Transpulmonary thermodilution before and during veno-venous extra-corporeal membrane oxygenation ECMO: an observational study on a potential loss of indicator into the extra-corporeal circuit. J Clin Monit Comput. 2020;34:923–36.
Acknowledgements
We are very grateful to Prof. Peter Radermacher, Prof. Jean Chastre, and Dr. Florence Caniou-Poitrine, who were responsible for the independent safety monitoring of the study. We also thank Dr Fanny Charbonnier-Beaupel and Dr Annick Tibi, for their constant help with pharmacology, technical, and organizational issues. We thank Edwards Lifesciences S.A., USA, for providing thermodilution systems.
Funding
The sponsor was Assistance Publique–Hôpitaux de Paris (Direction de la Recherche Clinique et de l’Innovation). Funding sources were donations from Sanofi SA, Paris, France; Bouygues SA, Paris, France; F4-Pharma Ges.m.b.H., Vienna, Austria, and Charles-Henri Hirsch. F4-Pharma Ges.m.b.H., Vienna, Austria, provided the drug. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
NB had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. NB, EG, LB, DH, SG were involved in concept and design. NB, EG, LB, DH, MHD, GF, LLG, TF, LLF, JH, PA helped in acquisition, analysis, or interpretation of data. NB, EG, SG, LB, CEL, AC, JH, PA drafted the manuscript. NB, EG, LB, CEL, AC, SG, JH, PA contributed to critical revision of the manuscript for important intellectual content. LB, DH, MHD helped in statistical analyses. NB, EG obtained funding. NB, JH, PA were involved in administrative, technical, or material support and supervision. All authors contributed to the revising of the manuscript and read and approved its final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The independent ethics review board CPP Ouest VI, Brest, France, approved the trial protocol (CPP 1306-EudraCT 2020-002056-20/RIPH 20.06.15.56627). The trial was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Conference on Harmonisation–Good Clinical Practice (ICH-GCP) guideline, the Quality Management Standards for Drug Clinical Trials of the French Food and Drug Administration (ANSM, Agence Nationale de Sécurité du Médicament et des Produits de Santé). According to the specifications of emergency consent, randomization without a close relative or surrogate consent could be performed, but informed consent by the patient or patient’s relatives was obtained for all patients.
Consent for publication
Not applicable.
Competing interests
Dr Bréchot is on the F4-Pharma advisory board, without any financial competing interest. He received a grant from the French Ministry of Health for another study evaluating FX06. He receives fees from Findimmune, outside the scope of this study. Dr Luyt reported receiving grants from French Ministry of Health during the conduct of the study; personal fees from Bayer Healthcare, Carmat, Faron, Merck Sharp & Dohme, ThermoFisher Brahms, and BioMérieux, outside the submitted work. Dr. Combes reports receiving grant support and lecture fees from Maquet and Baxter, and consulting fees from Hemovent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. The study protocol.
Additional file 2
. The supplementary data.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guérin, E., Belin, L., Franchineau, G. et al. FX06 to rescue SARS-CoV-2-induced acute respiratory distress syndrome: a randomized clinical trial. Crit Care 27, 331 (2023). https://doi.org/10.1186/s13054-023-04616-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04616-1