Abstract
Background
Critically ill COVID-19 patients may develop acute respiratory distress syndrome and the need for respiratory support, including mechanical ventilation in the intensive care unit. Previous observational studies have suggested early tracheotomy to be advantageous. The aim of this parallel, multicentre, single-blinded, randomized controlled trial was to evaluate the optimal timing of tracheotomy.
Methods
SARS-CoV-2-infected patients within the Region Västra Götaland of Sweden who needed intubation and mechanical respiratory support were included and randomly assigned to early tracheotomy (≤ 7 days after intubation) or late tracheotomy (≥ 10 days after intubation). The primary objective was to compare the total number of mechanical ventilation days between the groups.
Results
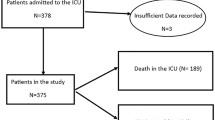
One hundred fifty patients (mean age 65 years, 79% males) were included. Seventy-two patients were assigned to early tracheotomy, and 78 were assigned to late tracheotomy. One hundred two patients (68%) underwent tracheotomy of whom sixty-one underwent tracheotomy according to the protocol. The overall median number of days in mechanical ventilation was 18 (IQR 9; 28), but no significant difference was found between the two treatment regimens in the intention-to-treat analysis (between-group difference: − 1.5 days (95% CI − 5.7 to 2.8); p = 0.5). A significantly reduced number of mechanical ventilation days was found in the early tracheotomy group during the per-protocol analysis (between-group difference: − 8.0 days (95% CI − 13.8 to − 2.27); p = 0.0064). The overall correlation between the timing of tracheotomy and days of mechanical ventilation was significant (Spearman’s correlation: 0.39, p < 0.0001). The total death rate during intensive care was 32.7%, but no significant differences were found between the groups regarding survival, complications or adverse events.
Conclusions
The potential superiority of early tracheotomy when compared to late tracheotomy in critically ill patients with COVID-19 was not confirmed by the present randomized controlled trial but is a strategy that should be considered in selected cases where the need for MV for more than 14 days cannot be ruled out.
Trial registration NCT04412356, registered 05/24/2020.
Graphic abstract

Similar content being viewed by others
Background
Globally, the SARS-CoV-2 pandemic has put health care in a highly demanding situation [1]. Critically ill patients may develop acute respiratory distress syndrome (ARDS) and require respiratory support, including mechanical ventilation (MV), in the intensive care unit (ICU). After an initial period with an endotracheal tube, a tracheotomy is routinely performed to prevent potential airway complications, improve pulmonary secretion clearance, reduce the need for sedation, facilitate weaning from MV and enable speech and oral nutrition. The patient can also be nursed outside the ICU with a tracheostoma. The disadvantages of tracheotomy include early complications such as stomal infection, bleeding, subcutaneous emphysema, cannula obstruction and cannula displacement. Examples of late complications include a persistent stoma, tracheal stenosis, a tracheoesophageal fistula and granuloma formation [2].
The optimal timing of tracheotomy is debated. Several randomized, controlled trials (RCTs) that have evaluated the advantage of early versus late tracheotomy have previously been published; however, these trials did not evaluate COVID-19 patients but rather ICU patients, who represented a broad spectrum of diagnoses [3,4,5,6,7,8,9,10]. In addition, several meta-analyses on this topic have been performed, including an updated Cochrane review in 2015 [2, 11,12,13,14,15,16,17]. In the latter, the authors concluded that there is a lack of data of sufficient quality to define the optimal timing of a tracheotomy. Regarding COVID-19 and tracheotomy, only a few reviews or meta-analyses and no RCTs have been published. Bier-Laning et al. [18] and McGrath et al. [19] reported studies from the first wave of COVID-19 using global institutional tracheotomy protocols and a consensus working group of experts in the field. Their conclusions and recommendations were to have a conservative approach and to delay a potential tracheotomy, and according to McGrath et al. [19], a delay until at least ten days after intubation should be taken.
An important parameter when studying the optimal timing of tracheotomy is the definition of early and late tracheotomy. The variation is substantial in referred studies, with a time span for early tracheotomy between ≤ 48 h to ≤ 21 days, and for late tracheotomy between ≥ 6 days and ≥ 29 days, which induces uncertainty interpreting the results. For the present study, we chose to follow current Swedish guidelines (see “Methods” section), and in the light of the heterogeneity of early and late tracheotomy definitions, we did not see a reason to divert from those guidelines.
A recent meta-analysis concerning the optimal timing of tracheotomy in COVID-19 patients concluded that early tracheotomy, defined as 14 days from intubation or less, implied reduced number of days in MV and days spent at the ICU compared to late tracheotomy (15 days after intubation or later) [20]. No RCTs were, however, available for this meta-analysis. Newly published both prospective [21] and retrospective [22] studies indicate that early tracheotomy implies fewer days of MV and shorter length of stay at the ICU. In accordance to the findings of the latter studies, a retrospective analysis of COVID-19 patients from the first wave of the pandemic performed at our department suggested early tracheotomy to be correlated with a shorter time on MV and, consequently, a shorter ICU stay [23]. To evaluate these findings, an RCT with the primary aim of studying the timing of tracheotomy and subsequent medical consequences was initiated by our group. The hypothesis was that early tracheotomy is beneficial for critically ill patients with COVID-19 in terms of a reduced duration of MV.
Methods
Study design
This was a parallel, multicentre, single-blinded RCT performed in the Region Västra Götaland of Sweden (Additional file 1). The study was approved by the Swedish Ethical Review Authority (Dnr2020-02,372 + 2021–02,700) and was performed in accordance with the Declaration of Helsinki. The study is registered with Clinicaltrials.gov (NCT04412356).
Patients
Patients were consecutively considered for inclusion according to the following criteria:
-
1.
Adult patients (18 years or older).
-
2.
Patients who were intubated due to real-time, reverse transcription polymerase chain reaction (RT–PCR)-verified, SARS-CoV-2 infection with ARDS according to the Berlin definition [24].
-
3.
Patients who were hospitalized at the Sahlgrenska University Hospital in Gothenburg or at two other county hospitals within the Region Västra Götaland of Sweden (Södra Älvsborg Hospital, Borås and NU Hospital group, Trollhättan).
-
4.
Patients in which a need for MV for more than 14 days after intubation could not be ruled out (as assessed by the team of anaesthesiologists at the ICU in agreement with the study coordinators).
Exclusion criteria were as follows:
-
1.
Patients where a tracheotomy performed within 7 days after intubation could be life threatening due to a poor medical condition (as assessed by the team of anaesthesiologists at the ICU in agreement with the study coordinators).
-
2.
Patients with an anatomical abnormality of the neck impeding the tracheotomy procedure (as assessed by the anaesthesiologist or otolaryngologist).
-
3.
Patients with no informed consent.
After matching the inclusion criteria and after the exclusion criteria were used to exclude patients, the intubated patients’ next of kin were informed about the study. If informed consent from the next of kin was given, the patient was included in the study. The inclusion window was 48 h after intubation. After discharge from the ICU, the included patients were contacted to provide their own informed consent retrospectively. If feasible, patients could give their own informed consent prior to intubation. Patients were blinded to the study assignment due to the required anaesthesia while intubated and mechanically ventilated.
Demographics and baseline characteristics are shown in Tables 1 and 2. Age, sex, body mass index (BMI), COVID-19 medication, comorbidities and Simplified Acute Physiology Score 3 (SAPS3) were recorded. SAPS3 is an algorithm frequently used by anaesthesiologists to predict the mortality risk for patients presenting to the ICU by imputing a wide range of medical data, i.e. oxygenation, cardiovascular status and the Glasgow Coma Scale score, all recorded at ICU admission [25].
Randomization
The patients who were included underwent randomized stratification with the allocation to either early (seven days after intubation or less) or late tracheotomy (ten days after intubation or more) in groups of four depending on sex and age (male < 65 years, male ≥ 65 years, female < 65 years and female ≥ 65 years). The reason for the different strata was because there was an anticipated irregularity in the cohort with a predominance of males, 65 years of age or older. An independent statistician (Statistiska Konsultgruppen AB) provided the random allocation sequence using a computerized algorithm. The participants were enrolled and assigned to interventions by the study coordinators (MEO, NP, LH and HB). A tracheotomy was performed with the intention of following the results of the randomization. To increase the protocol compliance, the randomization result was recorded in the patient record, and the anaesthesiologist in charge was informed. Moreover, a specific referral to the Department of Otorhinolaryngology was requested at the time of inclusion for patients randomized to early tracheotomy to promote monitoring of the patient. A power analysis with a target of 80% power and p < 0.05 (sample size of 180 patients, mean difference of 3.0 days, and SD of 6.2) was performed using data from two RCTs similar to the current trial regarding the primary endpoint; however, these studies had more heterogeneous study populations [4, 1).
Of the 102 patients (68%) who underwent tracheotomy, sixty-one patients (60%) did so according to the results of the randomization. Twenty-seven patients were included in the early tracheotomy arm, and 34 patients were included in the late tracheotomy arm. Common reasons for not following the intention-to-treat analysis are listed as follows: when patients no longer had an indication for a tracheotomy, when patients were in a poor general condition for surgical intervention, and when patients had a need for an early tracheotomy due to their medical condition. Of 25 patients who were randomized to early tracheotomy but did not undergo the operation within 7 days due to a rapid improvement, sixteen patients (64%) still underwent tracheotomy at a later time due to subsequent deterioration.
The demographics and baseline characteristics in the ITT and PP populations, including age, sex, BMI, COVID-19 medications given, comorbidities and SAPS3, were equally distributed and comparable between the two treatment arms (Tables 1, 2). Men were the majority among the included patients (78.7%). The median age of the whole population was 65.5 years (min/max 20; 84). The overall median number of days in MV was 18 (IQR 9; 28), but no significant difference was found for the primary endpoint between the two treatment regimens in the intention-to-treat analysis (between-group difference: − 1.5 days (95% CI − 5.7 to 2.8; p = 0.5) (Table 3). The correlation between the timing of tracheotomy and the primary endpoint for all tracheotomized patients (n = 102) was, however, significant (Spearman’s correlation of 0.39, p < 0.0001). In the PP analysis, there was a statistically significant mean difference in MV days between the treatment arms of − 8.03 days (95% CI − 13.85 to − 2.27; p = 0.0064) for the whole group and − 7.73 days (95% CI − 14.33 to − 1.13; p = 0.022) for survivors only, in favour of patients allocated to the early tracheotomy group. The secondary efficacy analyses for the ITT and PP population are displayed in Tables 4 and 5.
There were no adverse events during the tracheotomy procedures of which the majority (80%) were performed with open surgical technique. Common reasons for choosing open surgical technique rather than percutaneous were high BMI, high doses of anticoagulants used and/or an effort from otorhinolaryngologists to reduce the workload for anaesthesiologists. There were no significant differences between the early tracheotomy group and late tracheotomy group regarding the type of tracheotomy, total number of days in the ICU, need for reintubation, days from intubation to death in the ICU, death within 90 days, and complications when analysed according to both the ITT and PP principles. The ICU mortality rate for the whole population was 32.7% (n = 49). Three patients died after being discharged from the ICU during the first 90 days after intubation. The percent of missing data not included in the calculations was 0.4%.
Discussion
To the best of our knowledge, this is the first RCT performed in critically ill COVID-19 patients with MV where the primary aim was to clarify the optimal timing for a tracheotomy. Our hypothesis, based on previous studies, that early tracheotomy is beneficial in terms of days in MV could not be confirmed in the main analysis, i.e. according to ITT. The unpredictable course of the disease contributed to the considerable lack of compliance with the random allocation. However, in patients who followed the study protocol a significantly reduced number of days in MV was found for the early tracheotomy arm compared to the late tracheotomy arm. In addition, a strong correlation between early tracheotomy and fewer MV days was found for all patients who underwent a tracheotomy regardless of the results of the randomization.
The optimal timing for tracheotomy has been debated for many years without any clear consensus [17]. When focusing on RCTs, the previously published studies are heterogeneous in many aspects, including the definitions of early and late tracheotomy, primary endpoints, patient populations (e.g. neurological, surgical, postcardiac surgery) and results based on ITT or PP populations. These differences are likely the reason for the diverging results and the difficulty of drawing any firm conclusions. When summarizing results from frequently cited RCTs within the field and with a study population n ≥ 100, early tracheotomy seems to reduce the number of MV days and the ICU stay compared to late tracheotomy [4,5,6,7, The potential superiority of early tracheotomy compared to late tracheotomy in critically ill patients with COVID-19 was not confirmed by the present study. Nevertheless, based on our results and previous studies, early tracheotomy is a strategy that should be considered in selected cases where a need for MV for more than 14 days cannot be ruled out. Further studies are warranted to raise the level of evidence and increase the generalizability within this topic.Conclusions
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Information of the study can also be reached at https://www.researchweb.org/is/vgr/project/276832 (Swedish). On request the corresponding author can provide Swedish language data in English.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- SD:
-
Standard deviation
- CI:
-
Confidence interval
- ARDS:
-
Acute respiratory distress syndrome
- MV:
-
Mechanical ventilation
- ICU:
-
Intensive care unit
- RCT:
-
Randomized controlled trial
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- BMI:
-
Body mass index
- SAPS3:
-
Simplified acute physiology score 3
- DSMB:
-
Data and safety monitoring board
- ITT:
-
Intention-to-treat
- PP:
-
Per-protocol
- CRF:
-
Case report form
- IQR:
-
Inter quartile range
References
Griffin KM, Karas MG, Ivascu NS, Lief L. Hospital preparedness for COVID-19: a practical guide from a critical care perspective. Am J Respir Crit Care Med. 2020;201(11):1337–44.
De Leyn P, Bedert L, Delcroix M, Depuydt P, Lauwers G, Sokolov Y, Van Meerhaeghe A, Van Schil P. Tracheotomy: clinical review and guidelines. Eur J Cardio Thorac Surg Off J Eur Assoc Cardio Thorac Surg. 2007;32(3):412–21.
Barquist ES, Amortegui J, Hallal A, Giannotti G, Whinney R, Alzamel H, MacLeod J. Tracheostomy in ventilator dependent trauma patients: a prospective, randomized intention-to-treat study. J Trauma. 2006;60(1):91–7.
Diaz-Prieto A, Mateu A, Gorriz M, Ortiga B, Truchero C, Sampietro N, Ferrer MJ, Mañez R. A randomized clinical trial for the timing of tracheotomy in critically ill patients: factors precluding inclusion in a single center study. Crit Care (Lond, Engl). 2014;18(5):585.
Koch T, Hecker B, Hecker A, Brenck F, Preuß M, Schmelzer T, Padberg W, Weigand MA, Klasen J. Early tracheostomy decreases ventilation time but has no impact on mortality of intensive care patients: a randomized study. Langenbeck’s Arch Surg. 2012;397(6):1001–8.
Rumbak MJ, Newton M, Truncale T, Schwartz SW, Adams JW, Hazard PB. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Crit Care Med. 2004;32(8):1689–94.
Terragni PP, Antonelli M, Fumagalli R, Faggiano C, Berardino M, Pallavicini FB, Miletto A, Mangione S, Sinardi AU, Pastorelli M, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303(15):1483–9.
Trouillet JL, Luyt CE, Guiguet M, Ouattara A, Vaissier E, Makri R, Nieszkowska A, Leprince P, Pavie A, Chastre J, et al. Early percutaneous tracheotomy versus prolonged intubation of mechanically ventilated patients after cardiac surgery: a randomized trial. Ann Intern Med. 2011;154(6):373–83.
Young D, Harrison DA, Cuthbertson BH, Rowan K. Effect of early vs late tracheostomy placement on survival in patients receiving mechanical ventilation: the TracMan randomized trial. JAMA. 2013;309(20):2121–9.
Zheng Y, Sui F, Chen XK, Zhang GC, Wang XW, Zhao S, Song Y, Liu W, **n X, Li WX. Early versus late percutaneous dilational tracheostomy in critically ill patients anticipated requiring prolonged mechanical ventilation. Chin Med J. 2012;125(11):1925–30.
Hosokawa K, Nishimura M, Egi M, Vincent JL. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care (Lond, Engl). 2015;19:424.
Deng H, Fang Q, Chen K, Zhang X. Early versus late tracheotomy in ICU patients: a meta-analysis of randomized controlled trials. Medicine. 2021;100(3):e24329.
Wang R, Pan C, Wang X, Xu F, Jiang S, Li M. The impact of tracheotomy timing in critically ill patients undergoing mechanical ventilation: a meta-analysis of randomized controlled clinical trials with trial sequential analysis. Heart Lung J Crit Care. 2019;48(1):46–54.
Adly A, Youssef TA, El-Begermy MM, Younis HM. Timing of tracheostomy in patients with prolonged endotracheal intubation: a systematic review. Eur Arch Otorhinolaryngol. 2018;275(3):679–90.
Siempos II, Ntaidou TK, Filippidis FT, Choi AMK. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(2):150–8.
Chorath K, Hoang A, Rajasekaran K, Moreira A. Association of early vs late tracheostomy placement with pneumonia and ventilator days in critically ill patients: a meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147(5):450–9.
Andriolo BN, Andriolo RB, Saconato H, Atallah ÁN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2015;1(1):Cd007271.
Bier-Laning C, Cramer JD, Roy S, Palmieri PA, Amin A, Añon JM, Bonilla-Asalde CA, Bradley PJ, Chaturvedi P, Cognetti DM, et al. Tracheostomy during the COVID-19 pandemic: comparison of international perioperative care protocols and practices in 26 countries. Otolaryngol Head Neck Surg. 2020;164:1136–47.
McGrath BA, Brenner MJ, Warrillow SJ, Pandian V, Arora A, Cameron TS, Añon JM, Hernández Martínez G, Truog RD, Block SD, et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8(7):717–25.
Ji Y, Fang Y, Cheng B, Li L, Fang X. Tracheostomy timing and clinical outcomes in ventilated COVID-19 patients: a systematic review and meta-analysis. Crit Care (Lond, Engl). 2022;26(1):40.
Tsonas AM, Botta M, Horn J, Brenner MJ, Teng MS, McGrath BA, Schultz MJ, Paulus F, Serpa Neto A. Practice of tracheostomy in patients with acute respiratory failure related to COVID-19-insights from the PRoVENT-COVID study. Pulmonology. 2022;28(1):18–27.
Navaratnam AV, Gray WK, Wall J, Takhar A, Day J, Tatla T, Batchelor A, Swart M, Snowden C, Marshall A, et al. Utilisation of tracheostomy in patients with COVID-19 in England: patient characteristics, timing and outcomes. Clin Otolaryngol. 2022. https://doi.org/10.1111/coa.13913.
Pauli N, Eeg-Olofsson M, Bergquist H. Tracheotomy in COVID-19 patients: a retrospective study on complications and timing. Laryngoscope Investig Otolaryngol. 2021;6(3):446–52.
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR. SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345–55.
Vagionas D, Vasileiadis I, Rovina N, Alevrakis E, Koutsoukou A, Koulouris N. Daily sedation interruption and mechanical ventilation weaning: a literature review. Anaesthesiol Intensive Ther. 2019;51(5):380–9.
Nieszkowska A, Combes A, Luyt CE, Ksibi H, Trouillet JL, Gibert C, Chastre J. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005;33(11):2527–33.
Frithiof R, Rostami E, Kumlien E, Virhammar J, Fällmar D, Hultström M, Lipcsey M, Ashton N, Blennow K, Zetterberg H, et al. Critical illness polyneuropathy, myopathy and neuronal biomarkers in COVID-19 patients: a prospective study. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2021;132(7):1733–40.
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32.
Fan E, Beitler JR, Brochard L, Calfee CS, Ferguson ND, Slutsky AS, Brodie D. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816–21.
Grieco DL, Bongiovanni F, Chen L, Menga LS, Cutuli SL, Pintaudi G, Carelli S, Michi T, Torrini F, Lombardi G, et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit Care (Lond, Engl). 2020;24(1):529.
Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, Thompson BT, Hardin CC. Reply to Yaroshetskiy et al.: Acute respiratory distress syndrome in COVID-19: do all these patients definitely require intubation and mechanical ventilation? Am J Respir Criti Care Med. 2020;202(10):1481–2.
Chiumello D, Busana M, Coppola S, Romitti F, Formenti P, Bonifazi M, Pozzi T, Palumbo MM, Cressoni M, Herrmann P, et al. Physiological and quantitative CT-scan characterization of COVID-19 and typical ARDS: a matched cohort study. Intensive Care Med. 2020;46(12):2187–96.
Acknowledgements
The authors are grateful for the Swedish Research Council funding for clinical research in medicine, ALF (Agreement concerning research and education of doctors). The authors express their appreciation to Statistiska Konsultgruppen AB for statistical support, and Kai Knudsen, Anders Ebenfelt, Mogens Bove and Petter Olsson for valuable contributions.
Funding
Open access funding provided by University of Gothenburg. Grants from the Swedish state under an agreement between the Swedish government and county councils.
Author information
Authors and Affiliations
Contributions
MEO: conceptualization, funding, validation, investigation, visualization, methodology, writing original draft, project administration, and writing—review and editing. HB involved in conceptualization, supervision, funding, validation, investigation, visualization, methodology, project administration, and writing—review and editing. NP involved in conceptualization, funding, validation, investigation, visualization, methodology, project administration, and writing—review and editing. LH involved in data curation, validation, investigation, methodology, project administration, and writing—review and editing. MG, MB, CL, KL, JJ, EL, and HL involved in validation, investigation, methodology, writing—review and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Swedish Ethical Review Authority (Dnr2020-02372 + 2021-02700) and was performed in accordance with the Declaration of Helsinki.
Consent for publication
Either patients gave their informed consent prior to intubation, or the patients next of kin gave their informed consent. After discharge from the ICU surviving patients gave their written informed consent retrospectively.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. TTCOV19 Research Protocol.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eeg-Olofsson, M., Pauli, N., Hafsten, L. et al. TTCOV19: timing of tracheotomy in SARS-CoV-2-infected patients: a multicentre, single-blinded, randomized, controlled trial. Crit Care 26, 142 (2022). https://doi.org/10.1186/s13054-022-04005-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-022-04005-0