Abstract
Background
As a novel necrosis manner, ferroptosis has been increasingly reported to play a role in tumor progression and treatment, however, the specific mechanisms underlying its development in prostate cancer remain unclear. Growing evidence showed that peroxisome plays a key role in ferroptosis. Herein, we identified a novel mechanism for the involvement of ferroptosis in prostate cancer progression, which may provide a new strategy for clinical treatment of prostate cancer.
Methods
Label-Free Mass spectrometry was used to screen and identify candidate proteins after ferroptosis inducer-ML210 treatment. Immunohistochemistry was undertaken to explore the protein expression of AGPS in prostate cancer tissues compared with normal tissues. Co-immunoprecipitation and GST pull-down were used to identify the directly binding of AGPS to MDM2 in vivo and in vitro. CCK8 assay and colony formation assay were used to illustrate the key role of AGPS in the progression of prostate cancer in vitro. The xenograft model was established to verify the key role of AGPS in the progression of prostate cancer in vivo.
Results
AGPS protein expression was downregulated in prostate cancer tissues compared with normal tissues from the first affiliated hospital of Zhengzhou University dataset. Lower expression was correlated with poorer overall survival of patients compared to those with high expression of AGPS. In addition, AGPS can promote ferroptosis by modulating the function of peroxisome-resulting in the lower survival of prostate cancer cells. Furthermore, it was shown that AGPS can be ubiquitinated and degraded by the E3 ligase-MDM2 through the proteasomal pathway. Meanwhile, kinase TrkA can promote the combination of AGPS and MDM2 by phosphorylating AGPS at Y451 site. It was verified that kinase TrkA inhibitor—Larotrectinib can increase the susceptibility of prostate cancer cells to ferroptosis, which leads to the inhibition of prostate cancer proliferation to a great extent in vitro and in vivo.
Conclusion
Based on these findings, we proposed the combination of ferroptosis inducer and TrkA inhibitor to synergistically exert anti-tumor effects, which may provide a new strategy for the clinical treatment of prostate cancer.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the most common malignancy in men, which has the second highest fatality rate among male tumors and accounts for 20% of annually diagnosed cancer [1]. In recent years, with aging and changes in dietary habits, the incidence of PCa has increased rapidly [2,3,4]. Androgen deprivation therapy (ADT) has become the standard of care for patients with advanced PCa [5, 6]. However, PCa cells gradually become insensitive to ADT treatment and eventually develop into castration-resistant PCa (CRPC) [7], and the current radiotherapy or drug treatment of CRPC is ineffective and non-specific [8]. Therefore, the exploration of new potential therapeutic targets for PCa treatment is particularly important.
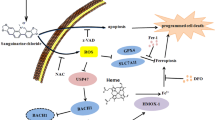
Ferroptosis is a unique iron-dependent and non-apoptotic form of necrosis due to lipid peroxidation. Several studies have reported that ferroptosis is involved in various degenerative diseases and tumors [9, 10]. Recent studies have displayed that peroxisome plays an important role in the occurrence of ferroptosis via lipid peroxidation by promoting the production of PUFAs and inhibiting the reduction of PUFAs [11, 12]. The discovery of the ferroptosis peroxisome-dependent pathway gradually revealed the importance of ether phospholipids (ePLs) in ferroptosis [Full size image
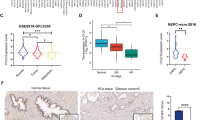
The TCGA data indicated that AGPS was significantly downregulated in PCa tissues (Supplementary Fig. 1b) and the expression differences were more pronounced at higher T stages (Supplementary Fig. 1c). And we also found the different expression of AGPS in different prostate cancer cell lines from the CCLE database (https://sites.broadinstitute.org/ccle/) (Supplementary Fig. 1d). But the mRNA expression seems to be no significantly different in different Gleason scores (Supplementary Fig. 1e). The comparison of 20 pairs of PCa tumor and normal tissues demonstrated that AGPS mRNA expression was not very significantly different between the tumor tissues and normal tissues (Supplementary Fig. 1f), which coordinated with the results in the cell lines (Fig. 1g). Then, we detected the protein levels of AGPS between prostate tissues and the normal tissues, result suggested that AGPS protein expression decreased in prostate cancer compared with the normal tissues (Fig. 1e). Additionally, we examined the protein expression of AGPS in different prostate cancer cell lines by Western blot (Fig. 1f), which lead to the same results.
Subsequently, we selected 89 pairs of PCa tumor and normal tissues for IHC staining and found that the positive rate of AGPS was significantly lower in PCa tumors than in normal tissues (Fig. 1g and h). We then performed a prognostic analysis based on the expression of AGPS in the 89 pairs of cases collected and found a significant association between AGPS and poor prognosis in PCa patients (Fig. 1i, j and k). These results suggest that AGPS is downregulated in PCa tissues and cells. Aditionally, AGPS is associated with better prognosis in PCa patients.
AGPS inhibits the proliferation of prostate cancer cells by promoting the formation of peroxisome and ferroptosis
In order to investigate whether AGPS acts through the ferroptosis in prostate cancer, we conducted colony formation experiments as an initial approach. Specifically, we employed overexpression of AGPS in 22Rv1 cells, which exhibit relatively low AGPS expression levels. Subsequently, we treated the cells with a combination of cellular autophagy inhibitor (CQ,25 μM), ferroptosis inhibitor (Ferrostatin-1,1 μM), and apoptosis inhibitor (Z-VAD-FMK,40 μM), commencing drug administration on the third day. On the seventh day, we assessed the clonal growth of the cells and found that the addition of the ferroptosis inhibitor alone resulted in a significant restoration of cell growth (Supplementary Fig. 2a and b). To investigate the specific role of AGPS in PCa through ferroptosis, we selected three cell lines (i.e., DU145, 22Rv1 and PC-3) for further investigations. DU145 and PC-3 reported relatively higher AGPS expression, whereas 22Rv1 reported a lower expression (Fig. 1f). We knocked down AGPS expression with three different lentivirus packaging short hairpin RNA (shRNA) in DU145 and PC-3 cell lines and over-expressed AGPS in 22Rv1 cell line (Fig. 2a and b, supplementary Fig. 2e, f). Then we examined the sensitivity of PCa cells to ferroptosis inducers. We found that the IC50 of both DU145 and PC-3 cells to ML210 and RSL3 was remarkably decreased after AGPS knockdown (Fig. 2c, supplementary Fig. 2g) as well as increased significantly after AGPS over-expression in 22Rv1 cells, indicating a correlation between AGPS expression and sensitivity to ferroptosis inducers in PCa. We also noticed the expression of peroxisome membrane protein 70 (PMP70) significantly reduced after AGPS knockdown and increased after AGPS over-expression, which suggested a variation in peroxisome production (Fig. 2d and e, Supplementary Fig. 2c, i,j and k). But there were no significant changes in Fe2+ (Supplementary Fig. 2d and h). Our findings reported that when induced with ML210, mitochondria with increased membrane density and atrophy mitochondria reduced after AGPS knockdown through TEM (Transmission electron microscopy) (Fig. 2f, Supplementary Fig. 2l). Similarly, malondialdehyde (MDA), the main product of ferroptosis, was greatly reduced after AGPS knockdown, meanwhile, increased after AGPS over expression (Fig. 2g and Supplementary Fig. 2m). These verified our hypothesis that AGPS could promote the ferroptosis process.
AGPS inhibits the proliferation of prostate cancer cells by promoting the promotion of peroxisome production and ferroptosis. a AGPS expression in mRNA after transfection with three shRNAs in DU145 and over expression in 22Rv1 cells. * P < 0.05, ** P < 0.01, *** P < 0.001. b AGPS expression in proteins after transfection with three shRNAs in DU145 and over expression in 22Rv1 cells. c. IC50 values of ML210 and RSL3 in DU145 cells after AGPS knockdown and in 22Rv1 cells after AGPS over expression. ** P < 0.01, *** P < 0.001, **** P < 0.0001. d PMP70 protein expression after AGPS knockdown with shRNA in DU145 cells and over expression in 22Rv1 cells. e PMP70 protein immunofluorescence staining after AGPS knockdown with shRNA in DU145 cells and over expression in 22Rv1 cells. f PCa cells were observed by TEM when induced with ML210 after AGPS knockdown. g MDA levels after AGPS knockdown with shRNA in DU145 cells and over expression in 22Rv1 cells. ** P < 0.01. h CCK8 assay on the OD value in 6-well plates after AGPS knockdown with shRNA in DU145 cells and over expression in 22Rv1 cells. n.s., no significance, * P < 0.05, ** P < 0.01. i and j Colony formation assay on colony numbers of the DU145 cells after AGPS knockdown with shRNA and 22Rv1 cell with AGPS over expression. ** P < 0.01. k PCa cells after AGPS knockdown were injected subcutaneously into the right flank of mice. Xenograft growth was measured every other day for 28 days. Tumors in each group at day 28 were harvested and photographed. l Tumor weight in each group. Data represented as mean ± SD (n = 5). **** P < 0.0001. m Tumor volume at each time point. Data represented as mean ± SD (n = 5). n.s., no significance; *P < 0.01, **P < 0.01, ***P < 0.001
The findings derived from the CCK8 and clone formation assays further substantiate that the downregulation of AGPS elicits a stimulatory effect on the proliferation of PCa cells, while the overexpression of AGPS exerts an inhibitory influence on their proliferation (Fig. 2h-j, Supplementary Fig. 2n-p). To further verify the role of AGPS in PCa, we performed a subcutaneous transplantation tumor model using severely immunodeficient mice and found that the tumor progressed by AGPS knockdown (Fig. 2k-m).
Overall, the above results suggested that AGPS can contribute to the development of ferroptosis by promoting peroxisome formation and inhibiting the proliferation of PCa cells.
MDM2 ubiquitinates and modifies AGPS and promotes its degradation
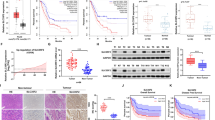
To understand the specific mechanisms involved in the lower expression of AGPS in PCa, we treated PCa cells with the proteasome inhibitor MG132 and the lysosomal inhibitor hydroxychloroquine. We found that AGPS expression was significantly upregulated after MG132 treatment, but no significant changes were observed in hydroxychloroquine-treated cells (Supplementary Fig. 3a). Thus, we speculated that the degradation of AGPS protein might be related to the proteasome pathway. We established the interaction between E3 ligases and AGPS using the Ubibrowser (http://ubibrowser.ncpsb.org/) to predict the possible E3 ligases of AGPS and used the Biogird database (https://thebiogrid.org/) to identify the potentially interacting proteins with AGPS. The combined analysis indicated that MDM2 proteins may interact with and regulate AGPS proteins (Supplementary Fig. 3b). Meanwhile, we transfected different E3 ligases and DUBs in PCa cells, and the level of AGPS protein was significantly decreased in the MDM2 ectopically transfected cells (Fig. 3a), which was consistent with our predictions from the analysis. We then ectopically transfect MDM2 but found no remarkable changes in AGPS at the transcriptional level (Fig. 3b). Subsequently, we found that MDM2 knockdown significantly increased the AGPS expression in a dose-dependent manner (Fig. 3c and d), and the process could be hindered by MG132 treatment (Fig. 3e). This result suggested that MDM2 affects AGPS stability via the ubiquitin–proteasome pathway. Meanwhile, the half-life of AGPS significantly decreased after ectopic expression of MDM2, which confirmed that MDM2 could degrade AGPS (Fig. 3f and g).
MDM2 ubiquitinates and modifies AGPS and promotes its degradation. a Western blot of AGPS expression after ectopic transfection with different E3 or DUBs. b qPCR of the AGPS expression after ectopic transfection with MDM2. c AGPS expression after MDM2 knockdown. n.s. no significance. d Western blot of the AGPS expression after ectopic transfection with different doses of MDM2. e Western blot of the AGPS expression after ectopic transfection with MDM2 and treatment with or without MG132 (20 μM) for 8 h. f Western blot analysis of AGPS protein expression in PC-3 cells after AGPS knockdown followed by treatment with cycloheximide (CHX) (50 μg/mL) for 0-, 4-, 8-, 12-, 16-, or 20- h. g The protein bands were quantified and normalized to the band intensity at the 0-h time point. * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001. h Ubiquitination experiment on the changes in AGPS ubiquitination levels after ectopic transfection with MDM2 and treatment with MG132 (20 μM) for 8 h. i, j Ectopia and endogenic MDM2 and AGPS binding. k Ubiquitination experiment on the changes in AGPS ubiquitination levels after ectopic transfection with MDM2 and different lysis mutant-type of UB
Next, we examined the ubiquitination level of AGPS after ectopic transfection with MDM2 and found that MDM2 overexpression increased the ubiquitination level of AGPS, indicating that MDM2 could alter the ubiquitination level of AGPS (Fig. 3h). On a molecular level, we examined the possible interactions between MDM2 and AGPS. The co-immunoprecipitation (co-IP) assay confirmed that both ectopically expressed and endogenous AGPS and MDM2 interacted with each other (Fig. 3i and j). To investigate the type of ubiquitin linkage on AGPS, we mutated the lysine (K) and arginine (R) residues on ubiquitin and evaluated their effects on AGPS ubiquitination after MDM2 transfection. Results demonstrated that K6R-, K11R-, K27R-, K29R-, K33R- and K63R-ubiquitin could trigger MDM2-mediated AGPS ubiquitination, but the lysine 48 (K48) R-ubiquitin largely inhibited the ubiquitination of AGPS (Fig. 3k). These results also support the notion that MDM2 could mediate AGPS ubiquitination and degradation. To further verify our conclusion, we overexpressed AGPS with or without MDM2 overexpression (Supplementary Fig. 3c). As expected, we observed that mitochondria with increased membrane density and atrophy mitochondria increased significantly, and the phenomenon was reversed by MDM2 overexpression (Supplementary Fig. 3d and e). Similarly, the level of MDA was reversed by the overexpression of MDM2 (Supplementary Fig. 3f). On the contrary, the CCK8 and clone formation assays indicated that AGPS overexpression inhibited tumor cell growth, and concomitant overexpression of MDM2 resulted in significantly higher PCa cell activity as compared to the experimental group overexpressing AGPS alone (Supplementary Fig. 3g-i).
Collectively, our experiments revealed that AGPS, a new substrate for MDM2, can be ubiquitinated and degraded by E3 ligase MDM2.
AGPS binds to MDM2 at the C-terminal amino acid sequence of the F443-F455 segment
To verify the specific binding motifs of AGPS and MDM2, we generated GST recombinant proteins for AGPS and MDM2 (Fig. 4a and b). Except for the full length, the two truncated MDM2 recombinant proteins contained the domains of p53 binding domain or not (Fig. 4a). GST pull-down assay reported that the p53 binding domain could bind to AGPS (Fig. 4c), while the C-terminus of AGPS could bind to the C-terminus of MDM2 (Fig. 4d). To verify this, we simulated the spatial structures of AGPS and MDM2 proteins using the Z-DOCK software and subsequently performed molecular docking for the two proteins. The high Z-score suggests a strong binding of the two proteins (Fig. 4e). Moreover, the result of the molecular docking revealed the amino acids in the C-terminus of AGPS that could be specific binding sites for MDM2. This included F443, L447, K448, F450, Y451, I452, K454, and F455.
AGPS binds to MDM2 at the C-terminal amino acid sequence of the F443-F455 segment. Schematic diagrams depicting a set of recombinant protein constructs for a GST-MDM2 and b GST- AGPS. c Western blot analysis of AGPS proteins in DU145 whole-cell lysate pulled down by GST or GST-MDM2 recombinant proteins. d Western blot analysis of MDM2 proteins in DU145 whole-cell lysate pulled down by GST or GST-AGPS recombinant proteins. e Schematic diagrams depicting the potential binding motif of AGPS with MDM2 protein, molecular dock with AGPS (AlphaFold: AF-O00116-F1) and MDM2(6KZU). f Western blot analysis of ectopically expressed Flag-tagged AGPS protein in DU145 cells transfected with wild-type or mutant AGPS. g Western blot analysis of AGPS protein expression in DU145 cells after ectopically expressed wild type or mutant AGPS followed by treatment of CHX (50 μg/mL) for 0-, 4-, 8-, 12-, 16-, or 20- h. h Protein bands were quantified and normalized to the band intensity at the 0-h time point. * P < 0.05, ** P < 0.01, *** P < 0.001. i Co-IP analysis of binding of Flag-tagged WT AGPS or mutant AGPS with ectopically expressed Myc-tagged MDM2 in DU145 cells treated with MG132 (20 μM) for 8 h. j Western blot analysis of ubiquitination levels in ectopically expressed Flag-tagged WT AGPS or mutant AGPS with ectopically expressed Myc-tagged MDM2 in DU145 cells treated with MG132 (20 μM) for 8 h
To validate the binding sites, we constructed the AGPS delete mutant of AGPS F443-F455 region and transfected the mutant AGPS into PCa cells. We found that the protein expression and half-life of mutated AGPS were significantly higher or longer than wild-type AGPS (Fig. 4f–h). In contrast, there seems less binding of mutated AGPS with MDM2and the level of ubiquitination of AGPS by MDM2 also significantly reduced after AGPS mutation (Fig. 4i and j). These results suggest that the binding of mutated AGPS and MDM2 was greatly reduced, which resulted in a decline in ubiquitination and degradation of AGPS. In summary, these experimental results identified the specific binding sites of AGPS and MDM2 and revealed the regulatory relationship between them.
Phosphorylation modification of the Y451 site leads to the accumulation of AGPS in prostate cancer cells
The expression of MDM2 did not significantly change in PCa (Supplementary Fig. 4a), but its substrate, AGPS, was significantly downregulated. Interestingly, a study previously reported that the ubiquitination modification and degradation function of MDM2 for AR was affected by phosphorylation modification [21]. The research proposed the function of MDM2 ubiquitination modification in a phosphorylation-dependent manner. To validate that the phosphorylation of AGPS could affect the binding of AGPS and MDM2 and lead to a change in AGPS expression, we first treated the cells with lambda protein phosphatase (λpp) to eliminate the function of phosphatase. We found that the expression of AGPS increased after λpp treatment, and this effect was removed after combination treatment with MG132 (Fig. 5a). Subsequently, we transfected both AGPS and MDM2 and found MDM2 can no longer degrade AGPS after λpp treatment (Fig. 5b). Likewise, the level of ubiquitination modification of AGPS by MDM2 significantly decreased after λpp treatment (Fig. 5c). These results suggested that the ubiquitination regulation of AGPS might be influenced by the phosphorylation of AGPS.
Phosphorylation modification of the Y451 site leads to the accumulation of AGPS in prostate cancer cells. a Western blot analysis of AGPS expression after MG132 (20 μM), λpp(1U/ml,2 h), or a combination. b Western blot analysis of ectopically expressed Flag-tagged AGPS protein in DU145 cells transfected with AGPS followed by treatment with λpp(1U/ml,2 h). c Western blot analysis of ubiquitination levels in ectopically expressed Flag-tagged AGPS and ectopically expressed Myc-tagged MDM2 in DU145 cells treated with MG132 (20 μM) for 8 h or λpp (1U/ml,2 h). d Co-IP analysis of binding of Flag-tagged AGPS with ectopically expressed Myc-tagged MDM2 in DU145 cells treated with λpp(1U/ml,2 h). e Western blot analysis of ectopically expressed Flag-tagged AGPS protein in DU145 cells transfected with Flag-tagged WT AGPS or mutant AGPS with or without λpp (1U/ml,2 h) treatment. f Alignment of amino acid sequences of the F443-F455 region of the AGPS protein. g Schematic diagrams depicting the potential binding and Z score of ADPS and MDM2 protein binding after AGPS Y451 site mutation to A. h Western blot analysis of ectopically expressed Flag-tagged AGPS protein in DU145 cells transfected with wild-type AGPS or AGPS Y451A mutant followed treatment with MG132 (20 μM) for 8 h. i Western blot analysis of AGPS protein expression in DU145 cells after ectopically expressed wild-type or mutant AGPS followed by treatment with CHX (50 μg/mL) for 0-, 4-, 8-, 12-, 16-, or 20- h. j Protein bands were quantified and normalized to the band intensity at the 0-h time point. * P < 0.05, ** P < 0.01. k Co-IP analysis of binding of Flag-tagged WT AGPS or mutant AGPS with ectopically expressed Myc-tagged MDM2 in DU145 cells treated with MG132 (20 μM) for 8 h. l Western blot analysis of ubiquitination levels in ectopically expressed Flag-tagged WT AGPS or mutant AGPS with ectopically expressed Myc-tagged MDM2 in DU145 cells treated with MG132 (20 μM) for 8 h
Additionally, Co-IP results confirm that the binding of AGPS and MDM2 was affected by λpp (Fig. 5d). We transfected AGPS△F443-F455 and wild-type AGPS in PCa cells and treated the cells with λpp. The result indicated λpp only affected the wild-type AGPS and not the mutated AGPS (Fig. 5e), which verified that AGPS might be modified by phosphorylation at the binding site with MDM2, thus affecting the binding of the two proteins. Among these binding sites, we found that Y451 could be modified by phosphorylation and is highly conserved in evolution (Fig. 5f). Consequently, we speculated that the phosphorylation modification of Y451 was the primary cause. To confirm this speculation, we used the Z-DOCK software to simulate the spatial conformation of AGPS after the phosphorylation of the Y451 site and used this model to dock again with MDM2. Result showed that the binding of the two proteins was greatly increased after docking, and the Z-score significantly increased (Fig. 5g), which indicated that the phosphorylation of Y451 promoted the binding between AGPS and MDM2. To verify this, we constructed an Y451-mutated AGPS and found that the expression and half-life of AGPS significantly increased (Fig. 5h-j). Similarly, the level of AGPS ubiquitination was significantly reduced after simultaneous overexpression of MDM2 after the Y451 mutation (Fig. 5l). Co-IP experiments revealed that the binding of AGPS to MDM2 was significantly weakened after mutation at Y451 (Fig. 5k), which explained the reduced ubiquitination level and protein accumulation of AGPS. Our findings confirmed that AGPS can be phosphorylated at Y451, thus enhancing its binding to MDM2 and AGPS accumulation.
TrkA promotes the ubiquitinated degradation of AGPS by MDM2 by modifying the phosphorylation of AGPS
To future illustrate the regulation mechanism, we screened several common kinases from both databases that might modify the phosphorylation of AGPS at the Y451 position (The sequence of AGPS used was obtained from two separate databases: https://services.healthtech.dtu.dk/service.php?NetPhos-3.1 and www.phosphonet.ca), including EGFR/ERBB4, Ack/TNK2, Met/MSTIR, PDGFR/CFFIP, and Trk/NTRK1. Only the TrkA inhibitor, Larotrectinib, but not any other inhibitor could significantly increase the expression of endogenous AGPS (Fig. 6a). Interestingly, we conducted an intersection analysis between these kinases and AGPS-related proteins in the Biogrid database, revealing that solely TrkA exhibited a connection with AGPS (Supplementary Fig. 4b). This finding further substantiates our observed phenomenon. Furthermore, we discovered that TrkA is overexpressed and phosphorylated in prostate cancer, suggesting a potential association with disease progression. Consequently, we selected the TrkA inhibitor Larotrectinib for further investigation. Also, Co-IP results suggested that Larotrectinib could inhibit the phosphorylation of AGPS tyrosine (Fig. 6b), which was consistent with our previous results that TrkA can modify the phosphorylation of AGPS.
TrkA promotes the ubiquitinated degradation of AGPS by MDM2 by modifying the phosphorylation of AGPS. a Western blot analysis of endogenous AGPS proteins in DU145 and 22Rv1 cells followed by treatment with different phosphatase inhibitors (Merestinib, 10 μM for 24 h; AG1295,10 μM for 2 h; AIM-100, 10 μM overnight; Dacomitinib, 2 μM for 24 h; Larotrectinib, 20 nM overnight). b Co-IP analysis of the phosphorylation levels of Flag-tagged AGPS in DU145 cells after being treated with MG132 (20 μM) for 8 h and Larotrectinib. c Western blot analysis of TrkA and p-TrkA expression in DU145, 22Rv1 and RWPE-1 cells. d Analysis of correlation between IHC staining of AGPS and p-TrkA proteins in different Gleason score PCa patient specimens. e Western blot analysis of AGPS protein expression in DU145 and 22Rv1 cells with or without Larotrectinib treatment. f Western blot analysis of ubiquitination levels in ectopically expressed Flag-tagged AGPS and ectopically expressed Myc-tagged MDM2 in DU145 cells treated with Larotrectinib. g Western blot analysis of ubiquitination levels in ectopically expressed Flag-tagged AGPS and ectopically expressed Myc-tagged MDM2 in DU145 cells treated with MG132 (20 μM) for 8 h or with Larotrectinib. h Western blot analysis of AGPS protein expression in DU145 cells with or without CHX (50 μg/mL) treatment for 0-, 4-, 8-, 12-, 16-, or 20- h. i Protein bands were quantified and normalized to the band intensity at the 0-h time point. * P < 0.05, ** P < 0.01, *** P < 0.001. j Co-IP analysis of binding of Flag-tagged AGPS with ectopically expressed Myc-tagged MDM2 in DU145 cells after NTRK1 knockdown. k Western blot analysis of ubiquitination levels in ectopically expressed Flag-tagged AGPS with ectopically expressed Myc-tagged MDM2 in DU145 cells after NTRK1 knockdown and treated with MG132 (20 μM) for 8 h
Interestingly, it has been reported that TrkA is aberrantly activated in PCa and the phosphorylation level is positively correlated with the Gleason score [22, 23]. We examined the phosphorylation level of TrkA in PCa cells and also found that p-TrkA was significantly higher in PCa cells than in RWPE-1 cells (Fig. 6c). Immunohistochemistry in prostate tissues also indicated the expression of p-TrkA gradually increased with increasing Gleason score. On the contrary, AGPS expression is a negative correlation with the Gleason score and p-TrkA level (Fig. 6d, Supplementary Fig. 4c). Meanwhile, we found that the expression of AGPS was significantly elevated with p-TrkA inhibitor Larotrectinib (Fig. 6e and f). Also, the ubiquitination level of AGPS was significantly reduced and AGPS protein half-life significantly increased after Larotrectinib treatment which was consistent with our previous findings (Fig. 6g-i). To further corroborate our findings, we knocked down NTRK1 (Supplementary Fig. 4d) and found that the ubiquitination level of AGPS and the binding of AGPS and MDM2 were significantly reduced (Fig. 6j, k). This indicated that TrkA phosphorylation modification of AGPS promoted the binding of AGPS and MDM2, leading to the degradation of AGPS. Likewise, the protein half-life of AGPS was increased (Supplementary Fig. 4e and f).
Overall, TrkA is aberrantly activated in PCa and modifies the phosphorylation of AGPS at Y451, which promotes the binding of AGPS and MDM2 and significantly downregulates AGPS expression in PCa.
Larotrectinib promotes the sensitivity of prostate cancer cells to ferroptosis by inhibiting the phosphorylation of TrkA
In recent years, the application of ferroptosis attracted a lot of attention in tumor therapy, some studies reported the use of ferroptosis inducers RSL3 and Erastin in the treatment of PCa [24]. Therefore, we speculate Larotrectinib could promote the accumulation of AGPS and exert a positive effect on ferroptosis inducers in tumor therapy. To validate our claim, we treated the cells with ML210 and Larotrectinib alone or in combination. We noticed different degrees of elevation of AGPS with the combination application of both drugs (Fig. 7a, Supplementary Fig. 5b). Also, we found that the prostate cancer cells were much more sensitive to ferroptosis inducer when combination administration of Larotrectinib (Fig. 7b, Supplementary Fig. 5c). In addition, PMP70 level and membrane density, and atrophy mitochondria reduced were greatly increased in the combination application of ML210 and Larotrectinib (Fig. 7c-f, Supplementary Fig. 5e). Also, MDA level also increased when combination application of ML210 and Larotrectinib (Fig. 7g, Supplementary Fig. 5f). Thus, we conclude that Larotrectinib-induced accumulation of AGPS could significantly increase the sensitivity of PCa cells to ferroptosis, and this was more pronounced when ML210 was used concomitantly.
Larotrectinib promotes the sensitivity of prostate cancer cells to ferroptosis by inhibiting the phosphorylation of TrkA. a Western blot analysis of AGPS protein expression in DU145 and 22Rv1 cells with or without ML210 (10 μM for 8 h) and Larotrectinib (20 nM, overnight) treatment. b The sensitive of ferroptosis in DU145 and 22Rv1 cells with or without ML210 and Larotrectinib treatment. ** P < 0.01, *** P < 0.001. c, d PMP70 staining in DU145 and 22Rv1 cells with or without ML210 and Larotrectinib treatment. ** P < 0.01, *** P < 0.001, **** P < 0.0001. e, f Pca cells were observed by TEM with or without ML210 and Larotrectinib treatment. ** P < 0.01, *** P < 0.001, **** P < 0.0001. g MDA level after treatment with ML210 and/or Larotrectinib in DU145 and 22Rv1 cells. * P < 0.05, ** P < 0.01. h CCK8 essay with the OD values in 6-wells plate after treatment with ML210 and/or Larotrectinib in DU145 and 22Rv1 cells. ** P < 0.01, *** P < 0.001, **** P < 0.0001. i Colony formation assay displayed the DU145 and 22Rv1 cell colony numbers after treatment with ML210 and/or Larotrectinib. j Organoid culture treated with ML210 and/or Larotrectinib from Pca patient tissues. k PCa cells were injected subcutaneously into the right flank of mice after treatment with ML210(50 mg/kg) and/or Larotrectinib (300 mg/kg) every other 24 h. Xenograft growth was measured every other day for 28 days. Tumors in each group at day 28 were harvested and photographed. l Tumor weight in different groups. Data represented as mean ± SD (n = 5). **P < 0.01, **** P < 0.0001. m Tumor volume at each time point. Data represented as mean ± SD (n = 5). n.s., no significance; *P < 0.01, **P < 0.01, ***P < 0.001, **** P < 0.0001
Next, we examined the effect of both drugs on cell proliferation. The CCK8 assay and colony formation results suggested that the inhibition of cell proliferation by the combination of both drugs was significantly higher than the individual drug treatments (Fig. 7h and i, Supplementary Fig. 5a, h and i). To further verify the therapeutic efficacy of the two drugs in vivo, we established organoid culture from prostate cancer tissues, when cultured with different drugs, the result showed that the sphere area of organoids generated through the synergistic application of two drugs exhibits a significantly reduced magnitude compared to the individual drug effects, aligning with the outcomes obtained from our in vitro experimental investigations (Fig. 7j, Supplementary Fig. 5j).Finally, we performed a subcutaneous transplantation tumor model using severely immune-deficient mice and found that the tumor suppression effect of Larotrectinib in combination with ML210 was much greater than that of the individual drug treatments (Fig. 7k-m).
In conclusion, Larotrectinib can increase the sensitivity of PCa cells to ferroptosis by promoting the accumulation of AGPS, thereby enhancing the therapeutic effect of ferroptosis inducers on tumors. This provides new ideas for the clinical treatment of PCa.