Abstract
Background
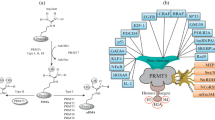
Protein arginine methyltransferases (PRMTs) regulate protein biological activity by modulating arginine methylation in cancer and are increasingly recognized as potential drug targets. Inhibitors targeting PRMTs are currently in the early phases of clinical trials and more candidate drugs are needed. Flavokawain A (FKA), extracted from kava plant, has been recognized as a potential chemotherapy drug in bladder cancer (BC), but its action mechanism remains unclear.
Methods
We first determined the role of a type II PRMT, PRMT5, in BC tissue samples and performed cytological experiments. We then utilized bioinformatics tools, including computational simulation, virtual screening, molecular docking, and energy analysis, to identify the potential use of PRMT5 inhibitors for BC treatment. In vitro and in vivo co-IP and mutation assays were performed to elucidate the molecular mechanism of PRMT5 inhibitor. Pharmacology experiments like bio-layer interferometry, CETSA, and pull-down assays were further used to provide direct evidence of the complex binding process.
Results
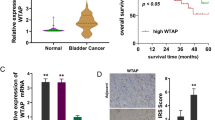
Among PRMTs, PRMT5 was identified as a therapeutic target for BC. PRMT5 expression in BC was correlated with poor prognosis and manipulating its expression could affect cancer cell growth. Through screening and extensive experimental validation, we recognized that a natural product, FKA, was a small new inhibitor molecule for PRMT5. We noticed that the product could inhibit the action of BC, in vitro and in vivo, by inhibiting PRMT5. We further demonstrated that FKA blocks the symmetric arginine dimethylation of histone H2A and H4 by binding to Y304 and F580 of PRMT5.
Conclusions
In summary, our research strongly suggests that PRMT5 is a potential epigenetic therapeutic target in bladder cancer, and that FKA can be used as a targeted inhibitor of PRMT5 for the treatment of bladder cancer.
Similar content being viewed by others
Background
Bladder cancer (BC) is the most common type of urinary cancer worldwide, with high recurrence and mortality [1]. Although numerous novel treatment regimens have been applied in the recent decade, no radical improvement in prognosis has been achieved in clinical practice. To date, local or systemic chemotherapy using widely accepted drugs is still the best therapeutic intervention to assist in surgery for BC treatment [2]. Although immune checkpoint inhibitors (ICIs) have emerged as an effective alternative for managing advanced disease and have shown durable responses in a subset of patients with BC [3], unfortunately, the overall response rate is only approximately 15–25% [4]. To improve the outcome of patients with BC, we need to urgently explore more therapeutic targets and identify appropriate drugs for treating BC [5].
Unbalanced methylation is gradually being accepted as a potential driver in human cancers [6]. Although lysine methyltransferases are widely known to regulate gene expression coupled with BC development, arginine methyltransferases and their roles in BC remain obscure [7]. Protein arginine methyltransferases (PRMTs) are enzymes that transfer a methyl group from S-adenosyl-L-methionine (SAM) to the substrate arginine side chain and can be categorized into different subtypes based on their catalytic routes [8]. Arginine methylation disorder has been reported to be prevalent in breast, lung, and colon cancers and leukemia [9]. Most PRMTs have been implicated in the regulation of cancer-associated epigenetics and chromatin transcription signaling, RNA metabolism, and DNA repair [10]. Recent reports have shown that some PRMTs play indispensable roles in regulating BC and are closely related to the proliferation, invasion, and poor prognosis of the disease [11]. However, no systematic analysis on PRMTs in BC has been performed to date, and the type of PRMT that specifically promotes the development of BC remains unknown.
The roles of PRMT enzymes in numerous diseases have spurred significant interest in targeting them pharmacologically [12]. Among these enzymes, including EPZ015866, EPZ015666, GSK3326595, and LY-283, the development of PRMT5 inhibitors is the most attractive approach [13,14,15,16]. Although some inhibitors have shown an inhibitory effect on PRMT5 enzyme activity and function, their toxicity and therapeutic effect are still being investigated [17].
Kava has been used for treating inflammatory bladder diseases for more than 100 years, and chalcones are the main classes of compounds identified from kava extracts, including flavokawain A, B, and C [18, 19]. These chalcones have also been found to have strong anti-tumor activity against various cancers, such as colon, lung, gastric, and breast cancers [20,21,22,23,24,25]. FKA, in particular, has a unique anti-cancer activity against urinary tract tumors [26]. Studies have demonstrated that FKA has the strongest anti-BC activity among the kava extracts discovered till date [27].
In previous studies, FKA could induce apoptosis in BC cells via the involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathways [27]. In addition, FKA could induce a G2-M arrest in the bladder and prostate cancer cells [47, 48]. The combination of PRMT5 inhibitors with other regimens has also received great attention. NCT02783300 is evaluating PRMT5 inhibitors with immunotherapy like PD-1 antibody and NCT04794699 is testing the synthetic lethal effect of PRMT5 inhibitor with MTAP defect [49]. In our study, we also determined the combined effect of PRMT5 with some clinically used drugs. Inhibiting PRMT5 increased the chemotherapeutic effect of cisplatin and gemcitabine, and co-treatment of PRMT5 and EGFR inhibitor significantly inhibited cancer cell growth. The encouraging results of this work indicated that PRMT5 was a suitable target for develo** combined therapies with other drugs [50]. Further exploring its synergetic effect with PARP inhibitors, immunotherapeutic antibodies, or chemotherapeutic drugs is worthy for further research.
Conclusions
In conclusion, we systematically analyzed the role of the PRMT family in BC and confirmed that PRMT5 was highly correlated with the malignant properties of BC and could be an ideal epigenetic therapeutic target. As the first natural small molecule inhibitor of PRMT5, FKA had a strong inhibitory effect on BC and warrants further development to translate it into clinical applications.
Availability of data and materials
The data in the current study are available from the corresponding author upon request.
Abbreviations
- BC:
-
Bladder cancer
- CETSA:
-
Cellular thermal shift assay
- FKA:
-
flavokawain A
- GC:
-
Gemcitabine and cisplatin
- PRMT:
-
protein arginine methyltransferase
- SAM:
-
S-adenosyl methionine
- TGCA:
-
Cancer genome atlas program
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Facchini G, Cavaliere C, Romis L, Mordente S, Facchini S, Iovane G, et al. Advanced/metastatic bladder cancer: current status and future directions. Eur Rev Med Pharmacol Sci. 2020;24:11536–52.
Lavoie JM, Sridhar SS, Ong M, North S, Alimohamed N, McLeod D, et al. The rapidly evolving landscape of first-line targeted therapy in metastatic urothelial cancer: a systematic review. Oncologist. 2021;26:e1381–e94.
van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;26:1839–44.
Segovia C, San Jose-Eneriz E, Munera-Maravilla E, Martinez-Fernandez M, Garate L, Miranda E, et al. Inhibition of a G9a/DNMT network triggers immune-mediated bladder cancer regression. Nat Med. 2019;25:1073–81.
Kim H, Ronai ZA. PRMT5 function and targeting in cancer. Cell Stress. 2020;4:199–215.
Lawson ARJ, Abascal F, Coorens THH, Hooks Y, O'Neill L, Latimer C, et al. Extensive heterogeneity in somatic mutation and selection in the human bladder. Science. 2020;370:75–82.
Fuhrmann J, Clancy KW, Thompson PR. Chemical biology of protein arginine modifications in epigenetic regulation. Chem Rev. 2015;115:5413–61.
Guccione E, Richard S. The regulation, functions and clinical relevance of arginine methylation. Nat Rev Mol Cell Biol. 2019;20:642–57.
Zhu F, Rui L. PRMT5 in gene regulation and hematologic malignancies. Genes Dis. 2019;6:247–57.
Zhang L, Shao G, Shao J, Zhao J. PRMT5-activated c-Myc promote bladder cancer proliferation and invasion through up-regulating NF-kappaB pathway. Tissue Cell. 2022;76:101788.
Wu Q, Schapira M, Arrowsmith CH, Barsyte-Lovejoy D. Protein arginine methylation: from enigmatic functions to therapeutic targeting. Nat Rev Drug Discov. 2021;20:509–30.
Duncan KW, Rioux N, Boriack-Sjodin PA, Munchhof MJ, Reiter LA, Majer CR, et al. Structure and property guided Design in the Identification of PRMT5 tool compound EPZ015666. ACS Med Chem Lett. 2016;7:162–6.
Chan-Penebre E, Kuplast KG, Majer CR, Boriack-Sjodin PA, Wigle TJ, Johnston LD, et al. A selective inhibitor of PRMT5 with in vivo and in vitro potency in MCL models. Nat Chem Biol. 2015;11:432–7.
AbuHammad S, Cullinane C, Martin C, Bacolas Z, Ward T, Chen H, et al. Regulation of PRMT5-MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma. Proc Natl Acad Sci U S A. 2019;116:17990–8000.
Bonday ZQ, Cortez GS, Grogan MJ, Antonysamy S, Weichert K, Bocchinfuso WP, et al. LLY-283, a potent and selective inhibitor of arginine methyltransferase 5, PRMT5, with antitumor activity. ACS Med Chem Lett. 2018;9:612–7.
Vinet M, Suresh S, Maire V, Monchecourt C, Nemati F, Lesage L, et al. Protein arginine methyltransferase 5: a novel therapeutic target for triple-negative breast cancers. Cancer Med. 2019;8:2414–28.
Abu N, Ho WY, Yeap SK, Akhtar MN, Abdullah MP, Omar AR, et al. The flavokawains: uprising medicinal chalcones. Cancer Cell Int. 2013;13:102.
Bian T, Corral P, Wang Y, Botello J, Kingston R, Daniels T, et al. Kava as a clinical nutrient: promises and challenges. Nutrients. 2020;12:3044.
Hseu YC, Lin RW, Shen YC, Lin KY, Liao JW, Thiyagarajan V, et al. Flavokawain B and doxorubicin work synergistically to impede the propagation of gastric Cancer cells via ROS-mediated apoptosis and autophagy pathways. Cancers (Basel). 2020;12:2475.
Karimi-Sales E, Mohaddes G, Alipour MR. Chalcones as putative hepatoprotective agents: preclinical evidence and molecular mechanisms. Pharmacol Res. 2018;129:177–87.
Li J, Zheng L, Yan M, Wu J, Liu Y, Tian X, et al. Activity and mechanism of flavokawain A in inhibiting P-glycoprotein expression in paclitaxel resistance of lung cancer. Oncol Lett. 2020;19:379–87.
Phang CW, Abd Malek SN, Karsani SA. Flavokawain C exhibits anti-tumor effects on in vivo HCT 116 xenograft and identification of its apoptosis-linked serum biomarkers via proteomic analysis. Biomed Pharmacother. 2021;137:110846.
Li X, Pham V, Tippin M, Fu D, Rendon R, Song L, et al. Flavokawain B targets protein neddylation for enhancing the anti-prostate cancer effect of Bortezomib via Skp2 degradation. Cell Commun Signal. 2019;17:25.
Wang J, Qi Q, Zhou W, Feng Z, Huang B, Chen A, et al. Inhibition of glioma growth by flavokawain B is mediated through endoplasmic reticulum stress induced autophagy. Autophagy. 2018;14:2007–22.
Liu Z, Xu X, Li X, Liu S, Simoneau AR, He F, et al. Kava chalcone, flavokawain a, inhibits urothelial tumorigenesis in the UPII-SV40T transgenic mouse model. Cancer Prev Res (Phila). 2013;6:1365–75.
Zi X, Simoneau AR. Flavokawain a, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005;65:3479–86.
Tang Y, Simoneau AR, **e J, Shahandeh B, Zi X. Effects of the kava chalcone flavokawain a differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev Res (Phila). 2008;1:439–51.
Wang K, Zhang W, Wang Z, Gao M, Wang X, Han W, et al. Flavokawain a inhibits prostate cancer cells by inducing cell cycle arrest and cell apoptosis and regulating the glutamine metabolism pathway. J Pharm Biomed Anal. 2020;186:113288.
Liu Z, Song L, **e J, Simoneau AR, Uchio E, Zi X. Chemoprevention of urothelial cell carcinoma tumorigenesis by dietary Flavokawain a in UPII-mutant ha-ras transgenic mice. Pharmaceutics. 2022;14:496.
Abu N, Mohameda NE, Tangarajoo N, Yeap SK, Akhtar MN, Abdullah MP, et al. In vitro toxicity and in vivo immunomodulatory effects of Flavokawain a and Flavokawain B in Balb/C mice. Nat Prod Commun. 2015;10:1199–202.
Romero C, Lambert LJ, Sheffler DJ, De Backer LJS, Raveendra-Panickar D, Celeridad M, et al. A cellular target engagement assay for the characterization of SHP2 (PTPN11) phosphatase inhibitors. J Biol Chem. 2020;295:2601–13.
Cancer Genome Atlas Research N, Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet. 2013;45:1113–20.
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559.
Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics. 2013;14:7.
Kurtova AV, **ao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517:209–13.
Kamoun A, de Reynies A, Allory Y, Sjodahl G, Robertson AG, Seiler R, et al. A consensus molecular classification of muscle-invasive bladder Cancer. Eur Urol. 2020;77:420–33.
Blanc RS, Richard S. Arginine methylation: the coming of age. Mol Cell. 2017;65:8–24.
Kalev P, Hyer ML, Gross S, Konteatis Z, Chen CC, Fletcher M, et al. MAT2A inhibition blocks the growth of MTAP-deleted Cancer cells by reducing PRMT5-dependent mRNA splicing and inducing DNA damage. Cancer Cell. 2021;39:209–24 e11.
Zheng Y, Dai M, Dong Y, Yu H, Liu T, Feng X, et al. ZEB2/TWIST1/PRMT5/NuRD multicomplex contributes to the epigenetic regulation of EMT and metastasis in colorectal carcinoma. Cancers (Basel). 2022;14:3426.
Bhattacharjee S, Rehman I, Basu S, Nandy S, Richardson JM, Das BB. Interplay between symmetric arginine dimethylation and ubiquitylation regulates TDP1 proteostasis for the repair of topoisomerase I-DNA adducts. Cell Rep. 2022;39:110940.
Zhang J, Fan X, Zhou Y, Chen L, Rao H. The PRMT5-LSD1 axis confers slug dual transcriptional activities and promotes breast cancer progression. J Exp Clin Cancer Res. 2022;41:191.
Tang Y, Dong L, Zhang C, Li X, Li R, Lin H, et al. PRMT5 acts as a tumor suppressor by inhibiting Wnt/beta-catenin signaling in murine gastric tumorigenesis. Int J Biol Sci. 2022;18:4329–40.
Huang L, Zhang XO, Rozen EJ, Sun X, Sallis B, Verdejo-Torres O, et al. PRMT5 activates AKT via methylation to promote tumor metastasis. Nat Commun. 2022;13:3955.
Antonysamy S, Bonday Z, Campbell RM, Doyle B, Druzina Z, Gheyi T, et al. Crystal structure of the human PRMT5:MEP50 complex. Proc Natl Acad Sci U S A. 2012;109:17960–5.
Pastore F, Bhagwat N, Pastore A, Radzisheuskaya A, Karzai A, Krishnan A, et al. PRMT5 inhibition modulates E2F1 methylation and gene-regulatory networks leading to therapeutic efficacy in JAK2(V617F)-mutant MPN. Cancer Discov. 2020;10:1742–57.
Chen Y, Shao X, Zhao X, Ji Y, Liu X, Li P, et al. Targeting protein arginine methyltransferase 5 in cancers: roles, inhibitors and mechanisms. Biomed Pharmacother. 2021;144:112252.
Bai X, Zhai Z, Zhao X, Li R, Liang L, ** Y, et al. Discovery of novel PRMT5 inhibitors bearing a methylpiperazinyl moiety. Future Med Chem. 2022;14:1071–86.
Abumustafa W, Zamer BA, Khalil BA, Hamad M, Maghazachi AA, Muhammad JS. Protein arginine N-methyltransferase 5 in colorectal carcinoma: insights into mechanisms of pathogenesis and therapeutic strategies. Biomed Pharmacother. 2022;145:112368.
Orben F, Lankes K, Schneeweis C, Hassan Z, Jakubowsky H, Krauss L, et al. Epigenetic drug screening defines a PRMT5 inhibitor-sensitive pancreatic cancer subtype. JCI Insight. 2022;7:e151353.
Acknowledgements
We would like to thank Editage for English language editing.
Funding
This work was supported by the National Nature Science Fund in China (Grants No. 81672525).
Author information
Authors and Affiliations
Contributions
SL: Conceptualization, writing, in vitro experiments, visualization. ZL: Bioinformatics analysis. CP: In vivo experiments. ZZ: Data curation, validation. CK: Funding acquisition. LY: Supervision. XL: Conceptualization, methodology, investigation, editing. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study design was approved (Approval No. [2022]102) by the Institutional Ethics Committees at the First Affiliate Hospital of China Medical University. All participants in the study provided informed consent before specimens were collected. All experiments on animals were conducted in accordance with the institutional guidelines approved (IACUC Issue No. KT2022608) by the Animal Care and Use Committee of China Medical University.
Consent for publication
All authors have agreed to publish this manuscript.
Competing interests
There are no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Methods. Supplementary Table.
Correlation between expression of PRMT5 clinicopathological parameters in 60 cases of bladder cancer patients. Fig. S1. (a) PRMT5 expression correlated with BLCA gene signatures in the TCGA dataset. (b) PRMT5 expression correlated with BLCA marker genes in the TCGA dataset. (c) Kaplan–Meier analysis of survival prognosis based on PRMT5 expression in Imvigor datasets. (d) IHC analysis of PRMT5 in different stage BC. (e) Western blot analysis of PRMT5 expression and histone methylation level in BC tissues and adjacent normal bladder tissues. Statistical analysis of protein expression was shown on the right side. Fig. S2. (a) Cell viability assay performed by treating FKA in urothelial cell SV-HUC-1. (b) Cell viability assay performed after treatment with FKB, and FKB IC50 were calculated in T24 (left) and UMUC3 (right). Data are represented as mean ± SD in three replications. (c) Cell viability assay performed after treatment with FKC, and FKC IC50 were calculated in T24 (left) and UMUC3 (right). Data are represented as mean ± SD in three replications. (d) PRMT5 expression changed with different concentrations of FKB treatment times in T24 (upper) and UMUC3 (lower). (e) PRMT5 expression changed with different concentrations of FKC treatment times in T24 (upper) and UMUC3 (lower). (f) Different PRMT expression changed with different concentrations of FKA in T24 (left) and UMUC3 (right). Fig. S3. (a) Cell apoptosis measured by knocking down PRMT5 expression, FKA treatment, and supplied FKA in PRMT5 overexpressed UMUC3 using flow cytometry. (b) Apoptosis rates for replicated assays were counted. (c) Cell viability assay performed after treatment with FKA or PRMT5 shRNA combined with cetuximab in T24 (left) and UMUC3 (right). Data are represented as mean ± SD in five replications. (d) Functional pathway enrichment of predicted FKA downstream targets. (e) Bladder cancer regulon genes changes after supplying FKA in UMUC3 measured by PCR, and the fold changes were standardized and normalized by log10. Fig. S4. (a) Bio-Layer Interferometry detecting the combination and dissociation constants of FKA and human recombinant PRMT5. (b) Fluorescent report CETSA assay confirmed that 25 μM FKA treatment could inhibit PRMT5 degradation when heated, thus enhancing PRMT5 stability in UMUC3. (c) Fluorescent report CETSA assay performed for PRMT5 expression when treated with FKA in wild-type and Y304A/ F580A mutated PRMT5 in UMUC3 cells. (d) Conservation of PRMT5 Y304 and F580 in different species.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, S., Liu, Z., Piao, C. et al. Flavokawain A is a natural inhibitor of PRMT5 in bladder cancer. J Exp Clin Cancer Res 41, 293 (2022). https://doi.org/10.1186/s13046-022-02500-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13046-022-02500-4