Abstract
Background
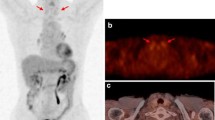
On 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET), diffuse uptake in the thyroid gland is often observed in patients with Hashimoto’s thyroiditis. In this study, we evaluated the factors associated with diffuse uptake by comparing Hashimoto’s thyroiditis patients with or without diffuse uptake in the thyroid.
Methods
A retrospective study was conducted of 18 patients with Hashimoto’s thyroiditis who underwent blood tests, thyroid ultrasonography, and FDG-PET during the period from 2014 to 2015. The patients were divided into two groups: one with diffuse thyroid uptake (group 1, n = 13) and one without diffuse thyroid uptake (group 2, n = 5). Clinical and laboratory parameters, including maximum standardized uptake in the thyroid (SUVmax), which was defined as the higher value obtained in either the right or left thyroid lobe, were compared in the two groups.
Results
The frequency of abnormal findings, such as a rough or heterogeneous pattern, was significantly higher in group 1 (p < 0.01), as were anti-thyroid peroxidase (TPO) antibody titers, anti-thyroglobulin (Tg) antibody titers, and SUVmax (p < 0.01). The frequency of hypothyroidism did not differ significantly in the two groups. Anti-TPO and anti-Tg titers were positively correlated with SUVmax (r = 0.856, p < 0.01 and r = 0.821, p < 0.01, respectively); in univariate analysis, anti-TPO titer was predictive of SUVmax (p < 0.01).
Conclusions
The results of the current study suggest that Hashimoto’s thyroiditis patients with high titers of anti-thyroid antibodies are likely to exhibit intense diffuse FDG uptake in the thyroid, and that thyroid function may be clearly impaired, even in the presence of mild FDG uptake in the thyroid.
Similar content being viewed by others
Background
Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) is a functional imaging technique known as FDG-PET that exploits the typically increased rate of glycolysis in specific cell types, such as malignant tumors and inflammatory tissue [1, 2]. Its use as a method of detecting malignant tumors and foci of infection has recently become more frequent.
With regard to the thyroid, a normal thyroid gland is not visible or shows only low-grade FDG uptake on FDG-PET [3, 4]. However, the increasing use of FDG-PET has led to incidental identification of patients with increased FDG uptake in the thyroid gland. FDG uptake in the thyroid predominantly conforms to one of two patterns: focal and diffuse. The prevalences of incidental focal and diffuse uptake in the thyroid reportedly vary from 0.1 to 4.8% and 0.1 to 4.5%, respectively [5]. Focal uptake in the thyroid suggests the possibility of a malignant tumor, such as papillary thyroid carcinoma, although most well-differentiated thyroid carcinomas are relatively slow-growing and can be FDG-negative [6, 7]. In contrast, diffuse FDG uptake is typically considered to be a result of benign conditions, such as Hashimoto’s thyroiditis or Graves’ disease, among others [8, 9]; however, there have been few reports of diffuse uptake. Furthermore, among patients with Hashimoto’s thyroiditis, not all patients exhibit diffuse uptake.
In the current study, we evaluated the factors associated with diffuse uptake by comparing Hashimoto’s thyroiditis patients with or without diffuse uptake in the thyroid.
Methods
Patients
Records of 18 consecutive attendees (five men, 13 women) with Hashimoto’s thyroiditis who underwent blood tests, thyroid ultrasonography (US), and FDG-PET at our hospital during the period from 2014 to 2015 were examined retrospectively. Clinical diagnosis of Hashimoto’s thyroiditis was made based on the elevation of either anti-thyroid peroxidase (TPO) antibody titer or anti-thyroglobulin (Tg) antibody titer. Additionally, the possibility of neoplastic diseases was considered highly unlikely based on clinical symptoms and ultrasonographic findings, although we did not perform histopathological examinations. Details of the subjects are presented in Table 1. The patients were divided into two groups, one with diffuse thyroid uptake (group 1, n = 13) and one without diffuse thyroid uptake (group 2, n = 5). Clinical and laboratory parameters were investigated, including the maximum standardized uptake value (SUVmax) in the thyroid. The study was approved by the institutional review board of Teikyo University Hospital, Japan (18–036), and the need for written informed consent was waived.
Data analysis
Serum levels of thyroid stimulating hormone (TSH), free T4, Tg, anti-Tg antibody, and anti-TPO antibody were measured. Thyroid US was performed close to the time of FDG-PET examination. SUVmax in the thyroid was recorded as the higher value obtained from either the right or the left thyroid lobe.
PET/computed tomography
All PET scans were performed on a PET/computed tomography (CT) system (Biograph 40 True Point, Siemens Health Care, Erlangen, Germany). FDG was supplied via a commercial delivery system (Nihon Medi-Physics Co., Ltd., Tokyo, Japan). All patients were fasted for at least 8 h before the injection of 165.5–352.5 MBq of FDG. Imaging was performed 1 h after injection and 2 h after injection. Three-dimensional scanning data were obtained from the top of the skull through to the pelvis, with a 3-min acquisition time per bed position. The studies were reconstructed using a vendor-supplied iterative reconstruction algorithm.
Laboratory tests
Serum levels of TSH, free T3, and free T4 were measured by using a chemiluminescence enzyme immunoassay (Lumipulse Presto, Fujirebio Co., Tokyo, Japan). Serum Tg, anti-Tg titer, and anti-TPO titer were measured with an electrochemiluminescence immunoassay (Elecsys Tg, Elecsys Anti-TPO, and Elecsys Anti-Tg, respectively; Roche Diagnostics K. K., Tokyo, Japan). The reference ranges for these laboratory tests at our institution were 0.5–5.0 mIU/mL for TSH, 2.3–4.0 pg/mL for free T3, 0.9–1.7 ng/dL for free T4, 0–33.7 ng/mL for serum Tg, 0–27 IU/mL for anti-Tg titer, and 0–15 IU/mL for anti-TPO titer. US examinations were performed with an 8.0-MHz linear phased-array probe (Aplio-XG, Canon Medical Systems Corporation, Tokyo, Japan). FDG uptake was evaluated by two board-certified radiologists (TK and HE).
Statistical analysis
All comparisons between the two groups were analyzed using the nonparametric Mann-Whitney U test. Differences in prevalence between men and women were evaluated by using the χ2 test. Correlations were assessed by using Spearman’s correlation coefficient analysis. All statistical analyses were performed with R version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria). Two-tailed p values < 0.05 were considered significant.
Results
Comparison of clinical and laboratory parameters
Table 2 shows comparisons of clinical and laboratory parameters between patients with or without diffuse thyroid uptake of FDG. Anti-Tg titer and anti-TPO titer were significantly higher in group 1 than in group 2. Serum free T4 was also significantly higher in patients with diffuse thyroid uptake, but it was similar in groups 1 and 2 after excluding those undergoing replacement therapy consisting of levothyroxine. No significant differences in other parameters were detected. The patients in group 1 were more likely to exhibit diffusely swollen thyroids with a rough and/or heterogeneous pattern on thyroid US.
Associations between thyroid SUVmax and thyroid autoantibodies
Associations between thyroid SUVmax and thyroid autoantibodies were evaluated in all subjects. Thyroid SUVmax was positively correlated with both anti-TPO titer (r = 0.856, p < 0.01) and anti-Tg titer (r = 0.821, p < 0.01). Notably, however, anti-TPO and anti-Tg titers were also strongly correlated with each other (r = 0.607, p < 0.05). Therefore, univariate analysis of SUVmax was performed for each autoantibody. Anti-TPO titer was a significant predictor of thyroid SUVmax in univariate analysis, but anti-Tg titer was not (Table 3). After transformation into Z-scores, anti-TPO titer yielded a β-coefficient of 0.851 (p < 0.01).
Discussion
In this study, we evaluated patients with Hashimoto’s thyroiditis, which was diagnosed based on the elevation of either anti-TPO titer or anti-Tg titer; Hashimoto’s thyroiditis patients with high titers of anti-thyroid antibodies were likely to exhibit intense diffuse FDG uptake in the thyroid. In contrast, the frequency of hypothyroidism was similar in patients with or without diffuse uptake in the thyroid.
Previous studies suggested that Hashimoto’s thyroiditis is the most frequent cause of diffuse FDG uptake in the thyroid. Yasuda et al. [10] reported that, in a sample of 36 patients with diffuse FDG uptake, there were seven with hypothyroidism and 27 who were positive for anti-TPO antibody. In a sample of 45 patients, Kim et al. [11] detected 10 patients with hypothyroidism and six with anti-TPO or anti-Tg antibodies. Karantanis et al. [12] evaluated 133 patients with diffuse uptake in the thyroid, in most of whom the indication for FDG-PET was oncology imaging, and reported that 63 (47.4%) had prior clinical diagnoses of hypothyroidism or autoimmune thyroiditis. This percentage was significantly higher than that of their control group (13/133; 9.8%). In an evaluation of 137 patients with diffuse uptake, Lee et al. [13] found that 76 (55.5%) were positive for anti-microsomal antibody. This frequency was higher than that of patients without diffuse uptake (64/1925; 3.3%).
The mechanisms underlying diffuse FDG uptake in the thyroid have not yet been clarified. Glucose utilization by normal thyrocytes appears to be dependent on TSH. However, in the current study, which evaluated only patients with Hashimoto’s thyroiditis, serum TSH and the frequency of hypothyroidism were similar regardless of diffuse FDG uptake. In fact, one patient in the present study required 100 μg levothyroxine despite low FDG uptake in the thyroid. However, Lee et al. [13] evaluated 2062 patients with or without diffuse uptake and found that TSH was significantly higher in patients with diffuse uptake. Therefore, the limited sample size in our study might underpower the results with respect to serum TSH and the frequency of hypothyroidism. Conversely, Yoshida et al. [14] evaluated 70 autopsied cases and reported that positive serum anti-thyroid antibodies in subjects without overt thyroid disease may indicate the existence of lymphocytic infiltration in the thyroid gland. In addition, inflammatory cells were shown to exhibit increased expression of glucose transporter isoforms when activated [15]. This indicates that thyroid FDG uptake may be associated with the degree of lymphocyte infiltration into the thyroid, rather than residual thyroid function, and higher SUVmax and high titers of anti-TPO antibody may indicate a higher degree of lymphocytic infiltration. Furthermore, in the current study, Hashimoto’s thyroiditis patients with high titers of anti-TPO antibody were more likely to exhibit diffusely swollen thyroids with a heterogeneous pattern on US. In a previous study, high anti-TPO titer was highly indicative of the degree of hypoechoic pattern in autoimmune thyroiditis [16]. That result is concordant with the results of the current study. Further studies are necessary to clarify the relationships between FDG uptake, the degree of chronic thyroiditis, and the degree of hypothyroidism.
Anti-TPO antibody was predictive of SUVmax in the present study. However, Karantanis et al. [12] studied 21 patients with diffuse uptake and no prior history of thyroid disease and reported no correlation between SUVmax and TSH or anti-TPO titer. The discrepancy between their results and those of the current study may be partially due to differing backgrounds of the subjects in the two studies. The present study only included patients with serologically diagnosed Hashimoto’s thyroiditis, while Karantanis et al. [12] included patients with other diseases. Several previous studies have evaluated clinical backgrounds in patients with diffuse uptake in the thyroid [10,11,12,13], but the current study is unique in that only patients with Hashimoto’s thyroiditis were included. Additionally, in the clinical setting, diffuse FDG uptake may be observed in a patient without thyroid hormone replacement therapy, when his or her anti-TPO antibody titer is high.
The primary limitation of the present study was its small sample size. Notably, the relationship between higher SUVmax and high titers of anti-TPO antibody was observed in this study. However, additional studies with larger sample sizes are required to evaluate the relationship between TSH and diffuse FDG uptake. Another limitation of the present study was the method used for clinical diagnosis of Hashimoto’s thyroiditis. We evaluated only clinical symptoms and ultrasonographic findings but did not perform fine needle aspiration, to exclude the possibility of neoplastic diseases (e.g., thyroid lymphoma).
Conclusions
The results of the present study suggest that patients with Hashimoto’s thyroiditis and high titers of anti-thyroid antibodies are likely to exhibit intense diffuse FDG uptake in the thyroid. However, the frequency of hypothyroidism was similar in patients with or without diffuse uptake in the thyroid. Therefore, it should be borne in mind that thyroid function may be clearly impaired even in the presence of only mild FDG uptake in the thyroid.
Abbreviations
- CT:
-
Computed tomography
- FDG:
-
18F-fluorodeoxyglucose
- PET:
-
Positron emission tomography
- SUVmax:
-
Maximum standardized uptake value
- Tg:
-
Hyroglobulin
- TPO:
-
Thyroid peroxidase
- TSH:
-
Thyroid stimulating hormone
- US:
-
Ultrasonography
References
Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med. 1991;32:623–48 discussion 649-50.
Zhuang H, Alavi A. 18-fluorodeoxyglucose positron emission tomographic imaging in the detection and monitoring of infection and inflammation. Semin Nucl Med. 2002;32:47–59.
Nakamoto Y, Tatsumi M, Hammoud D, Cohade C, Osman MM, Wahl RL. Normal FDG distribution patterns in the head and neck: PET/CT evaluation. Radiology. 2005;234:879–85.
Tsubaki F, Kurata S, Tani J, Sumi A, Fujimoto K, Abe T. Clinical significance of patterns of increased [18F]-FDG uptake in the thyroid gland: a pictorial review. Jpn J Radiol. 2018;36:181–93.
Soelberg KK, Bonnema SJ, Brix TH, Hegedus L. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid. 2012;22:918–25.
Nayan S, Ramakrishna J, Gupta MK. The proportion of malignancy in incidental thyroid lesions on 18-FDG PET study: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2014;151:190–200.
Saif MW, Tzannou I, Makrilia N, Syrigos K. Role and cost effectiveness of PET/CT in management of patients with cancer. Yale J Biol Med. 2010;83:53–65.
Kurata S, Ishibashi M, Hiromatsu Y, Kaida H, Miyake I, Uchida M, et al. Diffuse and diffuse-plus-focal uptake in the thyroid gland identified by using FDG-PET: prevalence of thyroid cancer and Hashimoto's thyroiditis. Ann Nucl Med. 2007;21:325–30.
Boerner AR, Voth E, Theissen P, Wienhard K, Wagner R, Schicha H. Glucose metabolism of the thyroid in Graves’ disease measured by F-18-fluoro-deoxyglucose positron emission tomography. Thyroid. 1998;8:765–72.
Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, et al. Chronic thyroiditis: diffuse uptake of FDG at PET. Radiology. 1998;207:775–8.
Kim TY, Kim WB, Ryu JS, Gong G, Hong SJ, Shong YK. 18F-fluorodeoxyglucose uptake in thyroid from positron emission tomogram (PET) for evaluation in cancer patients: high prevalence of malignancy in thyroid PET incidentaloma. Laryngoscope. 2005;115:1074–8.
Karantanis D, Bogsrud TV, Wiseman GA, Mullan BP, Subramaniam RM, Nathan MA, et al. Clinical significance of diffusely increased 18F-FDG uptake in the thyroid gland. J Nucl Med. 2007;48:896–901.
Lee JY, Choi JY, Choi Y-H, Hyun SH, Moon SH, Jang SJ, et al. Diffuse thyroid uptake incidentally found on18F-Fluorodeoxyglucose positron emission tomography in subjects without Cancer history. Korean J Radaol. 2013;14:501–9.
Yoshida H, Amino N, Yagawa K, Uemura K, Satoh M, Miyai K, et al. Association of serum antithyroid antibodies with lymphocytic infiltration of the thyroid gland: studies of seventy autopsied cases. J Clin Endocrinol Metab. 1978;46:859–62.
Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P, et al. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Investig. 2007;37:282–90.
Raber W, Gessl A, Nowotny P, Vierhapper H. Thyroid ultrasound versus antithyroid peroxidase antibody determination: a cohort study of four hundred fifty-one subjects. Thyroid. 2002;12:725–31.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Availability of data and materials
All data analyzed in this study are included in this published article.
Author information
Authors and Affiliations
Contributions
NE, KM, and TI were involved in study design, acquisition of data, analysis and interpretation of data and drafting and revising the manuscript. MS, TK, HE, EO, MO, KT and HO were involved in data collection and manuscript drafting. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional review board of Teikyo University Hospital, Japan (18–036). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The need for written informed consent was waived.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Edo, N., Morita, K., Sakamoto, M. et al. Correlation between anti-thyroid peroxidase antibody levels and diffuse thyroid uptake of 18F-fluorodeoxyglucose in Hashimoto’s thyroiditis: a retrospective study. Thyroid Res 11, 14 (2018). https://doi.org/10.1186/s13044-018-0058-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13044-018-0058-5