Abstract
Background
Alzheimer’s disease (AD) is the major cause of death in adults with Down syndrome (DS). There is an urgent need for objective markers of AD in the DS population to improve early diagnosis and monitor disease progression. NPTX2 has recently emerged as a promising cerebrospinal fluid (CSF) biomarker of Alzheimer-related inhibitory circuit dysfunction in sporadic AD patients. The objective of this study was to evaluate NPTX2 in the CSF of adults with DS and to explore the relationship of NPTX2 to CSF levels of the PV interneuron receptor, GluA4, and existing AD biomarkers (CSF and neuroimaging).
Methods
This is a cross-sectional, retrospective study of adults with DS with asymptomatic AD (aDS, n = 49), prodromal AD (pDS, n = 18) and AD dementia (dDS, n = 27). Non-trisomic controls (n = 34) and patients with sporadic AD dementia (sAD, n = 40) were included for comparison. We compared group differences in CSF NPTX2 according to clinical diagnosis and degree of intellectual disability. We determined the relationship of CSF NPTX2 levels to age, cognitive performance (CAMCOG, free and cued selective reminding, semantic verbal fluency), CSF levels of a PV-interneuron marker (GluA4) and core AD biomarkers; CSF Aβ1–42, CSF t-tau, cortical atrophy (magnetic resonance imaging) and glucose metabolism ([18F]-fluorodeoxyglucose positron emission tomography).
Results
Compared to controls, mean CSF NPTX2 levels were lower in DS at all AD stages; aDS (0.6-fold, adj.p < 0.0001), pDS (0.5-fold, adj.p < 0.0001) and dDS (0.3-fold, adj.p < 0.0001). This reduction was similar to that observed in sporadic AD (0.5-fold, adj.p < 0.0001). CSF NPTX2 levels were not associated with age (p = 0.6), intellectual disability (p = 0.7) or cognitive performance (all p > 0.07). Low CSF NPTX2 levels were associated with low GluA4 in all clinical groups; controls (r2 = 0.2, p = 0.003), adults with DS (r2 = 0.4, p < 0.0001) and sporadic AD (r2 = 0.4, p < 0.0001). In adults with DS, low CSF NPTX2 levels were associated with low CSF Aβ1–42 (r2 > 0.3, p < 0.006), low CSF t-tau (r2 > 0.3, p < 0.001), increased cortical atrophy (p < 0.05) and reduced glucose metabolism (p < 0.05).
Conclusions
Low levels of CSF NPTX2, a protein implicated in inhibitory circuit function, is common to sporadic and genetic forms of AD. CSF NPTX2 represents a promising CSF surrogate marker of early AD-related changes in adults with DS.
Similar content being viewed by others
Background
Down syndrome (DS) has been conceptualized by the International Working Group for Alzheimer’s disease (AD) as a genetically determined form of AD [1] due to the triplication of the gene encoding amyloid precursor protein (APP), among others [2]. AD in DS has a long preclinical phase and a predictable sequence of changes in biomarkers that is strikingly similar (both in order and temporality) to that described in autosomal dominant AD [25]. All adults with DS included in the study had confirmed trisomy of chromosome 21.
CSF collection and biomarker assessment
CSF samples were collected following international consensus recommendations [26] as previously described [27]. Samples had been previously stored at − 80 °C and had not been thawed prior to analysis. Commercially available automated immunoassays were used to determine levels of CSF Aβ1–42 (Lumipulse Aβ1–42, Fujirebio-Europe, Belgium) total tau (Lumipulse G Total Tau, Fujirebio-Europe) and tau phosphorylated at threonine residue 181 (Lumipulse G PTAU 181, Fujirebio-Europe) in the DABNI cohort and SPIN controls [17]. An in-house enzyme-linked immunosorbent assay targeting the N-terminal fragment encoding amino acid 1 to 201 of NPTX2 was performed in the Baltimore Laboratory as previously described [12].
Targeted liquid chromatography mass spectrometry
We monitored a set of 3 proteotypic peptides corresponding to GluA4 by Selected Reaction Monitoring (SRM) as previously described [28]. Briefly, we digested individual CSF samples overnight and spiked isotopically-labeled peptides (Pepotech SRM custom peptides, grade 2, Thermo Fisher Scientific) into each sample. We analyzed an equivalent of 5ul of each sample in a randomized order over a 120-min gradient (0–35% ACN + 0.1%FA) in SRM mode using a triple quadrupole-Qtrap mass spectrometer (5500 QTrap, Sciex, Massachussetts) coupled to a nano-LC chromatography column (300 ul/min, 25-cm C18 column, 75 um I.d., 2 um particle size). We ran BSA technical controls between each sample. We visualized and analyzed transitions using Skyline 3.5. Injection of a pool of all the samples over the duration of the mass spectrometric measurements and monitoring the peak area of the standard peptides confirmed the stability of all peptides over the course of the experiment. We processed the SRM transitions using the dataProcess function of MSstats v3.5 package in R [29] and removed transitions with between run interference (betweenRunInterferenceScore< 0.8). Censored missing values were samples with log base-2 endogenous intensities under the cut-off designated by the MSstats package (10.35). We used the EqualizeMedians function to normalize the transitions and Tukey’s Median Polish to generate a summarized value.
Magnetic resonance imaging (MRI) acquisition and analysis
A high-resolution three-dimensional structural dataset was acquired in a 3 T MRI scanner (Philips 3.T X Series ACHIEVA) with the following parameters: T1-weighted magnetization-prepared rapid gradient-echo, repetition time 8.1 msec, echo time 3.7 msec, 160 slices, matrix size 240 × 234; slice thickness 1 mm, voxel size 0.94 × 0.94 × 1 mm. Cortical thickness reconstruction was performed with Freesurfer package v6 (http://surfer.nmr.mgh.harvard.edu) using a procedure that has been described in detail elsewhere [30], as previously reported [28, 29]. A Gaussian kernel of 15 mm full width at half maximum (FWHM) was applied to the subjects’ cortical thickness maps before further analyses as it is customary in surface-based analyses. From the 75 adults with DS who had available MRI, 14 were excluded due to suboptimal image quality, which included subtle movement artifacts, poor signal to noise ratio, and gradient artifacts. The breakdown of diagnosis in these individuals was as follows: aDS n = 38, pDS n = 15, dDS n = 8.
FDG-PET acquisition and analysis
From the remaining 61 adults with DS with MRI, 42 underwent [18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) scan on an integrated PET-TC system (Philips Gemini TF). The breakdown of diagnosis in these individuals was as follows: aDS n = 28, pDS n = 10, dDS n = 4. Overnight fasting subjects’ plasma glucose was measured and confirmed to be < 180 mg/dL prior to starting the scanning procedure. Data were acquired 50 min after the injection of 259 MBq/ml (7 mCi) of [18F]-FDG. After computerized tomography data was obtained, brain PET was acquired. The reconstruction method was iterative (LOR RAMBLA, 3 iterations and 33 subsets) with a 128 × 128 image size, 2 mm pixel size and 2 mm pixel slice thickness. [18F]-FDG-PET images were intensity-scaled by the reference pons-vermis region [31], spatially normalized to the Freesurfer anatomical space using a PET surfer approach [32] and then smoothed with a 10 mm FWHM kernel in order to obtain similar relative smoothing FDG maps as compared with cortical thickness maps [33, 34]. With this surface-based approach, the reliability of PET effects improves and the inter-individual variance is reduced [35]. All resulting images were visually inspected to check for possible registration errors.
Statistical analysis
Statistical analyses were performed in R version 3.4.3 [36]. Group differences were compared using One-way Analysis of Variance (ANOVA) with post hoc Tukey tests adjusted for multiple testing using the Bonferonni-Hochberg method. Robust logistic regressions were performed using the lm function included in the “robustbase” package that uses MM-type regression estimators to compute robustness weights in the fitting process. All reported r2 values were adjusted for the number of predictors in the model. Where residuals deviated from a Gaussian distribution (Shapiro-Wilk p < 0.05), square root transformed values were used. Outliers were excluded using the 3 × IQR rule. For the neuroimaging analyses we performed Monte-Carlo simulation with 10,000 repeats (family wise error [FWE] correction at p < 0.05). Due to the small size of each clinical group with available neuroimaging, analyses were performed on the complete DS dataset only. The figures show only those results that survived FWE correction. Alpha was set at 0.05 for global analyses and 0.01 for post-hoc analyses.
Results
The Table 1 shows the demographic and clinical data for the samples included in the study. The mean age was 52 years (standard deviation 15) across the whole study (n = 168). Compared to controls the mean age was higher in the sporadic AD group (+ 19 years, 95% CI 13 to 25, adj.p < 0.0001), lower in the aDS group (− 14 years, 95% CI 8 to 19; adj.p < 0.0001) and comparable in pDS and dDS (both adj.p > 0.9). As would be expected for an age-related disease such as AD, mean age was lower in aDS than pDS (− 14 years, 95% CI − 7 to − 21; adj.p < 0.0001) and dDS (− 15 years, 95% CI − 9 to − 21; adj.p < 0.0001). The proportion of males:females included in the study was 46:54 and was comparable across diagnostic groups (p = 0.09). The proportion of individuals with severe/profound intellectual disability was comparable across AD stages (p = 0.7). All cognitive measures were lower in sAD compared to controls (all adj.p < 0.0001) and in dDS compared to aDS (all adj.p < 0.0001). CSF biomarker levels were as previously reported [17]. Briefly, compared to controls mean Aβ1–42 levels were lower in all groups (all adj.p < 0.0001) and p-tau and t-tau levels were higher in sAD, pDS and dDS (all adj.p < 0.0001).
CSF NPTX2 levels are similarly reduced in DS and sporadic AD
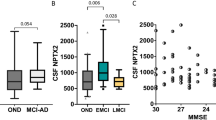
Mean CSF NPTX2 levels were associated with clinical diagnosis (Fig. 1a; n = 168, p < 0.0001). Specifically, post hoc analyses showed that compared to controls, mean NPTX2 levels were 0.5-fold in sporadic AD (− 491 pg/ml, 95% CI −280 to − 702; adj.p < 0.0001), 0.6-fold in aDS (− 400 pg/ml, 95% CI − 196 to − 604; adj.p < 0.0001), 0.5-fold in pDS (− 472, 95% CI − 208 to − 736; adj.p < 0.0001) and 0.3-fold in dDS (− 595, 95% CI − 361 to − 828; adj.p < 0.0001). Mean NPTX2 levels were comparable between sporadic AD and DS (all AD stages adj.p > 0.3) and across AD stages in DS (all adj.p > 0.07).
NPTX2 levels in control, sporadic AD and DS CSF. Box and whisker plots and individual points of the CSF levels of NPTX2 (pg/ml) are grouped (a) by clinical diagnosis and (b) by degree of intellectual disability (DS population only). P-values resulting from one-way ANOVA on square root transformed NPTX2 levels are shown in the top right corner. P-values resulting from Bonferroni-adjusted post-hoc Tukey test are shown where p < 0.05
In adults with DS, CSF NPTX2 levels were not associated with level of intellectual disability (Fig. 1b; n = 94, p = 0.7) and were not associated with any measure of cognitive performance in DS (n = 57–64, all p > 0.07). Mean NPTX2 levels were not associated with age overall (p = 0.6) or at any AD stage (all p > 0.1).
Low CSF NPTX2 levels correlate with low CSF levels of a PV-interneuron specific receptor
We next sought to determine whether the PV-interneuron specific marker, GluA4, shows a coordinated reduction with NPTX2 in the CSF. While CSF GluA4 levels were not associated with clinical diagnosis (Fig. 2a; p = 0.2), low NPTX2 levels correlated with low GluA4 levels in controls (n = 27 r2 = 0.2, p = 0.003), sAD (n = 20, r2 = 0.4, p < 0.0001) and in DS (n = 81, r2 = 0.4, p < 0.0001): The association in DS was apparent at all AD stages; aDS (n = 36, r2 = 0.5, p = 0.04), pDS (n = 18, r2 = 0.5, p = 0.005) and dDS (n = 21, r2 = 0.4, p = 0.02).
GluA4 levels in control, sporadic AD and DS CSF. a Box and whisker plots and individual points of the CSF levels of GluA4 (log2 intensity) grouped by clinical diagnosis. P-value resulting from one-way ANOVA on square root transformed GluA4 levels is shown in the top right corner. b CSF NPTX2 levels are plotted against CSF GluA4 levels with associated linear regression lines shown for controls, sporadic AD and DS groups (see legend)
Low CSF NPTX2 levels correlate with CSF markers of AD pathology in adults with DS
We next sought to determine the relationship between CSF NPTX2 levels and core CSF AD biomarkers in DS. Low NPTX2 levels were associated with low CSF Aβ1–42 levels (Fig. 3a) in all diagnostic groups; aDS (r2 = 0.5, p < 0.0001), pDS (r2 = 0.3, p = 0.0003) and dDS (r2 = 0.6, p = 0.006). Low NPTX2 levels were also associated with p-tau (Fig. 3b) in all diagnostic groups; aDS (r2 = 0.4, p < 0.0001), pDS (r2 = 0.3, p = 0.0004) and dDS (r2 = 0.3, p = 0.001). Similar correlations were observed for CSF t-tau (r2 = 0.3 to 0.4, p < 0.003).
Low CSF NPTX2 levels in adults with DS are associated with cortical atrophy and neuronal dysfunction in signature AD brain regions
We also analyzed the relationship between CSF NPTX2 levels and structural and functional brain changes in adults with DS. As shown in Fig. 4, low CSF NPTX2 levels were associated with reduced cortical thickness in the temporal and parietal cortices (top; n = 63, FWE < 0.05) and with reduced glucose metabolism in more widespread regions of the temporal and parietal cortices (bottom; n = 44, FWE < 0.05).
Structural and functional neuroimaging correlates of CSF NPTX2 levels in DS. Correlation of CSF NPTX2 levels and cortical thickness (top) and glucose metabolism (bottom) in all DS participants. Regions in red represent significant correlations (p < 0.05). Only the clusters that survived family-wise error correction p < 0.05 are shown. Scatterplots with linear regression line (black) and standard error (gray) show the correlation between CSF NPTX2 and neuroimaging biomarkers by diagnosis in a representative cluster that survived multiple comparisons, indicated with an asterisk. Regression lines are shown for each clinical group for informative purposes (see legend)
Discussion
Neuronal Pentraxin Receptor 2 (NPTX2) has recently been identified as a novel CSF biomarker of inhibitory circuit dysfunction in sporadic AD [12, 15]. This finding is consistent with studies that report a similar reduction in NPTX2 expression in postmortem sporadic AD and DS brains [12] and indicates that the mechanism underlying these changes is common to both sporadic and genetic forms of AD and that these changes may be reflected in the CSF. As these changes were apparent across all AD stages, CSF NPTX2 may be a useful marker of AD-related changes in adults with DS even prior to symptom onset.
DS cohorts with available CSF are scarce and current biofluid markers for AD in the DS population are restricted to surrogate markers of amyloidosis (CSF Aβ1–42 or amyloid PET tracers) and tau-mediated neurodegeneration (CSF p-tau and t-tau) combined with neuropsychological assessment, which can be confounded by substantial inter-individual variation in intellectual disability. NPTX2 represents a new addition to the DS biomarker arsenal that may be used to detect early signs of AD in this relatively under-studied population. Furthermore, this work opens the door to future studies exploring the potential of CSF NPTX2 as a prognostic marker for late-onset myoclonic epilepsy in Down syndrome and sporadic AD.
The lack of association of NPTX2 levels with intellectual disability suggests that the reduction in NPTX2 levels is not neurodevelopmental. This is supported by the finding that reduced CSF NPTX2 levels were associated with increased cortical atrophy and reduced glucose metabolism in temporal and parietal regions of the brain, regions that are particularly susceptible to AD pathology. Thus, CSF NPTX2 levels inversely correlated with the extent of both neuronal loss and neuronal dysfunction in signature AD regions. Taken together, we propose that changes in CSF NPTX2 levels in adults with DS are related to AD pathophysiology and not to intellectual disability.
Low CSF NPTX2 levels in adults with DS were associated with low CSF GluA4 and low CSF Aβ1–42, a surrogate CSF marker of increased aggregation of brain Aβ1–42. This is the first evidence that a synergized increase in brain Aβ1–42 aggregates and reduced NPTX2 can be measured indirectly in the CSF of adults with DS. NPTX2 is expressed by pyramidal neurons and secreted by axon terminals to mediate activity-dependent strengthening of pyramidal neuron excitatory synapses on GABA-ergic PV interneurons, a mechanism that is dependent upon the AMPA receptor subunit, GluA4 [11]. Histological studies have shown reduced NPTX2 and GluA4 expression in signature AD regions of AD and DS brains [12], regions that also show a reduced number of PV interneurons [37]. This is consistent with the accumulating evidence for reduced inhibitory interneuronal activity in the AD brain [10]. Studies in mice [37,38,39] have shown that low NPTX2 expression at excitatory synapses of PV interneurons combined with brain amyloidosis is critical for inhibitory circuit dysfunction [12].Thus, a hypothesis emerges whereby a loss of NPTX2-GluA4 synapses in regions affected by amyloidosis may lead to inhibitory circuit dysfunction in AD. While extrapolation of data from mouse to human brain should be treated with caution, this could be one plausible explanation for the low CSF NPTX2 levels we report in sporadic AD patients and adults with DS.
Unlike NPTX2, GluA4 levels were not significantly reduced in CSF of adults with DS compared to controls, making NPTX2 a potentially more valuable CSF biomarker in DS. Based on these findings, CSF NPTX2 can be added to the growing list of biomarkers that show changes similar, both in direction and magnitude, to those described in sporadic or autosomal dominant AD [4, 20, 40]. The potential of CSF NPTX2 as a novel biomarker of interneuronal dysfunction can now be extended to DS, a genetic form of AD that targets the same cortical regions as the sporadic and autosomal dominant forms [5].
CSF Aβ1–42, t-tau and p-tau are excellent diagnostic markers in sporadic AD and in the DS population and we do not expect NPTX2 to improve upon those markers. Rather, this study supports the increasing literature that suggests CSF NPTX2 as a surrogate marker of underlying interneuronal circuit dysfunction in sporadic and, now genetic, forms of AD even at the preclinical stage. Further studies in longitudinal cohorts are needed to explore the full potential of CSF NPTX2. The capacity of NPTX2 as an early prognostic marker would be one potential use worth exploring for example. Alternatively, as tau and Aβ are common drug targets in AD clinical trials, a surrogate measure of synaptic dysfunction that does not directly measure the drug target levels would be a useful addition to the biomarker arsenal.
In previous studies in sporadic AD, CSF NPTX2 levels correlated better with cognitive performance than the core AD biomarkers [12]. Furthermore, in genetic frontotemporal dementia, low CSF NPTX2 levels predicted subsequent decline in phonemic verbal fluency and Clinical Dementia Rating scale [41]. In contrast, here we report that reduced CSF NPTX2 levels were not associated with any measure of cognitive performance in adults with DS. That being said, the high variability in intellectual disability in the DS population can complicate cognitive assessment and therefore these neuropsychological tests could only be performed in individuals with mild or moderate intellectual disability, which resulted in a limited sample size. Thus, the potential relationship between CSF NPTX2 levels and cognitive performance in the DS population warrants further study in a larger population. On the other hand, there is evidence that the temporal onset of cognitive decline may differ in DS compared to autosomal dominant AD [4], thus underscoring the importance of considering the neurodevelopmental differences in the DS population when interpreting the cognitive results.
The association of increased CSF NPTX2 levels with increased CSF p-tau levels in the DS population could indicate that widespread neuronal loss at later disease stages may lead to increased NPTX2 clearance into the CSF and may mask a reduction in NPTX2 synapses. Nevertheless, the inverse relationship we report between CSF NPTX2 levels and a neuroimaging marker of cortical atrophy, suggests that neurodegeneration has a discernable but limited confounding effect on CSF NPTX2 levels.
A limitation of this study is the cross-sectional design; longitudinal studies are needed to fully establish the prognostic value of NPTX2 in both sporadic AD and in DS. Furthermore, these findings warrant replication in an independent DS cohort. Exploration of the potential of NPTX2 as a blood biomarker would also be valuable to minimize the use of lumbar puncture in people with DS.
Conclusions
Here we report that CSF NPTX2 levels are reduced in adults with DS compared to cognitively normal, non-trisomic controls even prior to AD onset. Similar reductions were observed in the CSF of sporadic AD patients and have been previously reported in postmortem AD and DS brains [12, 15]. Substantial evidence from animal studies suggests that such changes may be associated with reduced inhibitory inter-neuronal activity. While further functional studies are needed, this could explain the correlation we report between low CSF NPTX2 and low CSF GluA4, the predominant AMPA receptor subunit in PV interneurons. In conclusion, this study shows for the first time that NPTX2 could be a valuable CSF biomarker of AD-related inhibitory neuronal circuit dysfunction in adults with DS even prior to symptom onset.
Availability of data and materials
The data analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AD:
-
Alzheimer’s disease
- aDS:
-
Asymptomatic AD in the context of DS
- CAMCOG:
-
Cambridge Cognition Examination
- CSF:
-
Cerebrospinal fluid
- DABNI:
-
Down Alzheimer Barcelona Neuroimaging Initiative
- dDS:
-
AD dementia in the context of DS
- DS:
-
Down syndrome
- FDG:
-
[18F]-fluorodeoxyglucose
- FWE:
-
Family wise error
- FWHM:
-
Full width at half maximum
- GABA:
-
Gamma-Aminobutyric acid
- MRI:
-
Magnetic resonance imaging
- NPTX2:
-
Neuronal pentraxin-2
- pDS:
-
Prodromal AD in the context of DS
- PET:
-
Positron emission tompgraphy
- PV:
-
Parvalbumin
- SPIN:
-
Sant Pau Initiative for Neurodegeneration
- SRM:
-
Selected Reaction Monitoring
References
Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–29.
Wiseman FK, Pulford LJ, Barkus C, Liao F, Portelius E, Webb R, Chavez-Gutierrez L, Cleverley K, Noy S, Sheppard O, et al. Trisomy of human chromosome 21 enhances amyloid-beta deposition independently of an extra copy of APP. Brain. 2018;141:2457–74.
Bateman RJ, **ong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Marcus DS, Cairns NJ, **e X, Blazey TM, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804.
Fortea J, Vilaplana E, Carmona-Iragui M, Benejam B, Videla L, Barroeta I, Fernandez S, Altuna M, Pegueroles J, Montal V, et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet. 2020;395:1988–97.
Neale N, Padilla C, Fonseca LM, Holland T, Zaman S. Neuroimaging and other modalities to assess Alzheimer’s disease in Down syndrome. Neuroimage Clin. 2018;17:263–71.
Schweber MS. Alzheimer’s disease and Down syndrome. Prog Clin Biol Res. 1989;317:247–67.
McCarron M, McCallion P, Reilly E, Mulryan N. A prospective 14-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2014;58:61–70.
Sinai A, Mokrysz C, Bernal J, Bohnen I, Bonell S, Courtenay K, Dodd K, Gazizova D, Hassiotis A, Hillier R, et al. Predictors of age of diagnosis and survival of Alzheimer’s disease in Down syndrome. J Alzheimers Dis. 2018;61:717–28.
Hithersay R, Startin CM, Hamburg S, Mok KY, Hardy J, Fisher EMC, Tybulewicz VLJ, Nizetic D, Strydom A. Association of Dementia with Mortality among Adults with Down syndrome Older than 35 years. JAMA Neurol. 2018;1;76(2):152–160.
Palop JJ, Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17:777–92.
Chang MC, Park JM, Pelkey KA, Grabenstatter HL, Xu D, Linden DJ, Sutula TP, McBain CJ, Worley PF. Narp regulates homeostatic scaling of excitatory synapses on parvalbumin-expressing interneurons. Nat Neurosci. 2010;13:1090–7.
**ao MF, Xu D, Craig MT, Pelkey KA, Chien CC, Shi Y, Zhang J, Resnick S, Pletnikova O, Salmon D, et al. NPTX2 and cognitive dysfunction in Alzheimer’s disease. Elife. 2017;6:e23798.
Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604.
Pelkey KA, Barksdale E, Craig MT, Yuan X, Sukumaran M, Vargish GA, Mitchell RM, Wyeth MS, Petralia RS, Chittajallu R, et al. Pentraxins coordinate excitatory synapse maturation and circuit integration of parvalbumin interneurons. Neuron. 2015;85:1257–72.
Galasko DR, **ao M, Xu D, Smirnov D, Salmon DP, Dewit N, Vanbrabant J, Jacobs D, Vanderstichele H, Vanmechelen E, Worley P. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimer’s Dement. 2019;5:871–82.
Alcolea D, Clarimon J, Carmona-Iragui M, Illan-Gala I, Morenas-Rodriguez E, Barroeta I, Ribosa-Nogue R, Sala I, Sanchez-Saudinos MB, Videla L, et al. The Sant Pau initiative on Neurodegeneration (SPIN) cohort: a data set for biomarker discovery and validation in neurodegenerative disorders. Alzheimers Dement (N Y). 2019;5:597–609.
Alcolea D, Pegueroles J, Muñoz L, Camacho V, López-Mora D, Fernández-León A, Bastard NL, Huyck E, Nadal A, Olmedo V, et al. Agreement between 18F-Florbetapir PET imaging and cerebrospinal fluid Abeta1–42, Abeta1–40, tTau and pTau measured on the LUMIPULSE G fully automated platform. bioRxiv. 2018. https://doi.org/10.1101/476937.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Fortea J, Carmona-Iragui M, Benejam B, Fernandez S, Videla L, Barroeta I, Alcolea D, Pegueroles J, Munoz L, Belbin O, et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 2018;17(10):860–9.
Carmona-Iragui M, Balasa M, Benejam B, Alcolea D, Fernandez S, Videla L, Sala I, Sanchez-Saudinos MB, Morenas-Rodriguez E, Ribosa-Nogue R, et al. Cerebral amyloid angiopathy in Down syndrome and sporadic and autosomal-dominant Alzheimer’s disease. Alzheimers Dement. 2017;13:1251–60.
Carmona-Iragui M, Santos T, Videla S, Fernandez S, Benejam B, Videla L, Alcolea D, Blennow K, Blesa R, Lleo A, Fortea J. Feasibility of lumbar puncture in the study of cerebrospinal fluid biomarkers for Alzheimer’s disease in subjects with Down syndrome. J Alzheimers Dis. 2017;55:1489–96.
Kaufman AS, Kaufman NL. Manual for the Kaufman Brief Intelligence Test. Circle Pines: American Guidance Service; 1990.
Esteba-Castillo S, Dalmau-Bueno A, Ribas-Vidal N, Vila-Alsina M, Novell-Alsina R, Garcia-Alba J. Adaptation and validation of CAMDEX-DS (Cambridge examination for mental disorders of older people with Down’s syndrome and others with intellectual disabilities) in Spanish population with intellectual disabilities. Rev Neurol. 2013;57:337–46.
Sala I, Illan-Gala I, Alcolea D, Sanchez-Saudinos MB, Salgado SA, Morenas-Rodriguez E, Subirana A, Videla L, Clarimon J, Carmona-Iragui M, et al. Diagnostic and prognostic value of the combination of two measures of verbal memory in mild cognitive impairment due to Alzheimer’s disease. J Alzheimers Dis. 2017;58:909–18.
Peiffer DA, Le JM, Steemers FJ, Chang W, Jenniges T, Garcia F, Haden K, Li J, Shaw CA, Belmont J, et al. High-resolution genomic profiling of chromosomal aberrations using Infinium whole-genome genoty**. Genome Res. 2006;16:1136–48.
Teunissen CE, Tumani H, Bennett JL, Berven FS, Brundin L, Comabella M, Franciotta D, Federiksen JL, Fleming JO, Furlan R, et al. Consensus guidelines for CSF and blood biobanking for CNS biomarker studies. Mult Scler Int. 2011;2011:246412.
Alcolea D, Martinez-Lage P, Sanchez-Juan P, Olazaran J, Antunez C, Izagirre A, Ecay-Torres M, Estanga A, Clerigue M, Guisasola MC, et al. Amyloid precursor protein metabolism and inflammation markers in preclinical Alzheimer disease. Neurology. 2015;85:626–33.
Lleo A, Nunez-Llaves R, Alcolea D, Chiva C, Balateu-Panos D, Colom-Cadena M, Gomez-Giro G, Munoz L, Querol-Vilaseca M, Pegueroles J, et al. Changes in synaptic proteins precede Neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid. Mol Cell Proteomics. 2019;18:546–60.
Choi M, Chang CY, Clough T, Broudy D, Killeen T, MacLean B, Vitek O. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics. 2014;30:2524–6.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5.
Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, Foster NL, Weiner MW, Jagust WJ. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–18.
Greve DN, Salat DH, Bowen SL, Izquierdo-Garcia D, Schultz AP, Catana C, Becker JA, Svarer C, Knudsen GM, Sperling RA, Johnson KA. Different partial volume correction methods lead to different conclusions: an (18)F-FDG-PET study of aging. Neuroimage. 2016;132:334–43.
La Joie R, Perrotin A, Barre L, Hommet C, Mezenge F, Ibazizene M, Camus V, Abbas A, Landeau B, Guilloteau D, et al. Region-specific hierarchy between atrophy, hypometabolism, and beta-amyloid (Abeta) load in Alzheimer’s disease dementia. J Neurosci. 2012;32:16265–73.
Bejanin A, Viard A, Chetelat G, Clarys D, Bernard F, Pelerin A, de La Sayette V, Eustache F, Desgranges B. When higher activations reflect lower deactivations: a PET study in Alzheimer’s disease during encoding and retrieval in episodic memory. Front Hum Neurosci. 2012;6:107.
Greve DN, Svarer C, Fisher PM, Feng L, Hansen AE, Baare W, Rosen B, Fischl B, Knudsen GM. Cortical surface-based analysis reduces bias and variance in kinetic modeling of brain PET data. Neuroimage. 2014;92:225–36.
R-Core-Team. R: a language and environment for statistical computing. In Book R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2018.
Brady DR, Mufson EJ. Parvalbumin-immunoreactive neurons in the hippocampal formation of Alzheimer’s diseased brain. Neuroscience. 1997;80:1113–25.
Kamenetz F, Tomita T, Hsieh H, Seabrook G, Borchelt D, Iwatsubo T, Sisodia S, Malinow R. APP processing and synaptic function. Neuron. 2003;37:925–37.
Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer abeta oligomer bound to cellular prion protein. Neuron. 2013;79:887–902.
Wisniewski KE, Wisniewski HM, Wen GY. Occurrence of neuropathological changes and dementia of Alzheimer’s disease in Down’s syndrome. Ann Neurol. 1985;17:278–82.
van der Ende EL, **ao M, Xu D, Poos JM, Panman JL, Jiskoot LC, Meeter LH, Dopper EG, Papma JM, Heller C, et al. Neuronal pentraxin 2: a synapse-derived CSF biomarker in genetic frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2020;91:612–21.
Acknowledgements
We thank the participants and their families for their generosity. We also acknowledge the Fundació Catalana Síndrome de Down for global support; Soraya Torres, and Laia Muñoz for laboratory and sample handling; Sílvia Valldeneu for administrative support; and Lidia García Losada for nursing management.
Funding
This work was supported by the Fundació Catalana Síndrome de Down and Fundació Víctor Grífols i Lucas and research grants from Institute of Health Carlos III (ISCIII), Spain (PI18/00327 to OB, PI17/01019 to JF; PI16/01825 to RB; PI17/01895 to AL and PI18/0335 to MC-I and CIBERNED Program 1, Alzheimer Disease to AL and SIGNAL study, www.signal.es) and the National Institutes of Health, US (NIA grants R35NS097966 to PFW, R01AG056850-01A1, R21AG056974 and R01AG061566 to JF). This work has also been supported by the Fundació La Marató de TV3 (20141210 to JF and 044412 to RB), the Fundació Bancaria Obra Social La Caixa (DABNI project to RB), Fundación BBVA (to AL) and by the Department of Health Generalitat de Catalunya the Department of Health, Generalitat de Catalunya PERIS and SGR programs (SLT006/17/125 to DA, SLT002/16/00099 to RNL, SLT006/17/00119 to JF 2017 and SGR 2017 00547 to the “Grup de Recerca en Demències: Sant Pau".) and partly funded by Fondo Europeo de Desarrollo Regional (FEDER), Unión Europea, “Una manera de hacer Europa”. OB is contractually supported by the Miguel Servet Type II fellowship (CP18/00011; ISCIII, FEDER). The funders of this study played no role in the design of the study, collection, analysis or interpretation of data or writing the manuscript.
Author information
Authors and Affiliations
Contributions
OB, MX, DX, MC-I, JP, BB, SF, LV, IB, RNL, VM, EV, JC, DA and RB contributed to the acquisition and analysis of data. OB, MX, MC-I, JP, BB, JC, AL, PW and JF drafted a significant portion of the manuscript and figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Sant Pau Ethics Committee following the standards for medical research in humans recommended by the Declaration of Helsinki and reported to the Minister of Justice according to the Spanish law for research in people with intellectual disabilities. All participants gave signed, informed consent for the interventions described, inclusion of samples, neuroimaging and demographic data.
Consent for publication
Not applicable.
Competing interests
OB, AL, JF and DA declare a filed patent application (pending) to the European Patent Office for Markers of synaptopathy neurodegenerative disease (does not relate to data included in this manuscript). MX, DX and PW are co-founders of CogNext.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Belbin, O., **ao, MF., Xu, D. et al. Cerebrospinal fluid profile of NPTX2 supports role of Alzheimer’s disease-related inhibitory circuit dysfunction in adults with Down syndrome. Mol Neurodegeneration 15, 46 (2020). https://doi.org/10.1186/s13024-020-00398-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13024-020-00398-0