Abstract
Background
Yangqing Chenfei formula (YCF) is a traditional Chinese medicine formula for early-stage silicosis. However, the therapeutic mechanism is unclear. The purpose of this study was to determine the mechanism for the effects of YCF on early-stage experimental silicosis.
Methods
The anti-inflammatory and anti-fibrotic effects of YCF were determined in a silicosis rat model, which was established by intratracheal instillation of silica. The anti-inflammatory efficacy and molecular mechanisms of YCF were examined in a lipopolysaccharide (LPS)/interferon (IFN)-γ-induced macrophage inflammation model. Network pharmacology and transcriptomics were integrated to analyze the active components, corresponding targets, and anti-inflammatory mechanisms of YCF, and these mechanisms were validated in vitro.
Results
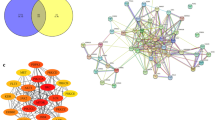
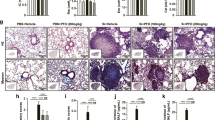
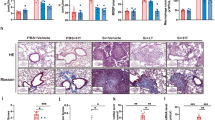
Oral administration of YCF attenuated the pathological changes, reduced inflammatory cell infiltration, inhibited collagen deposition, decreased the levels of inflammatory factors, and reduced the number of M1 macrophages in the lung tissue of rats with silicosis. YCF5, the effective fraction of YCF, significantly attenuated the inflammatory factors induced by LPS and IFN-γ in M1 macrophages. Network pharmacology analysis showed that YCF contained 185 active components and 988 protein targets, which were mainly associated with inflammation-related signaling pathways. Transcriptomic analysis showed that YCF regulated 117 reversal genes mainly associated with the inflammatory response. Integrative analysis of network pharmacology and transcriptomics indicated that YCF suppressed M1 macrophage-mediated inflammation by regulating signaling networks, including the mTOR, mitogen-activated protein kinases (MAPK), PI3K-Akt, NF-κB, and JAK-STAT signaling pathways. In vitro studies confirmed that the active components of YCF significantly decreased the levels of p-mTORC1, p-P38, and p-P65 by suppressing the activation of related-pathways.
Conclusion
YCF significantly attenuated the inflammatory response in rats with silicosis via the suppression of macrophage M1 polarization by inhibiting a “multicomponent-multitarget-multipathway” network.
Similar content being viewed by others
Background
Silicosis, which exhibits high morbidity and mortality rates in develo** countries, is an irreversible occupational respiratory disease caused by long-term inhalation of crystalline silica dust [1, 2]. Silicosis is characterized by chronic inflammation and progressive interstitial fibrosis [3]. The pathogenesis of silicosis is unclear, thus, no effective clinical treatments to retard the progression of silicosis are available [4]. Pulmonary alveolar macrophages are the predominant cells involved in the development of silicosis [5]. When silica enters the lung, it can stimulate macrophages to engulf silica particles, resulting in M1 polarization and the induction of inflammation and tissue damage due to the release of large amounts of pro-inflammatory mediators, including interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), and cyclooxygenase-2 (COX-2) [25]. However, no specific drug is available to treat silicosis, and lung transplantation is the only effective treatment. Thus, novel drugs are urgently needed to suppress the progression of silicosis. YCF is a Chinese medicine for pneumoconiosis patients with yin deficiency and dryness heat syndrome or dryness invading lung syndrome. Previous studies demonstrated that YCF effectively improves lung function and alleviates clinical symptoms, such as coughing, shortness of breath, and dyspnea. The present study suggests that YCF significantly improves lung tissue damage and inhibits inflammation and fibrosis in rats with silicosis. Moreover, YCF can significantly inhibit macrophage M1 polarization in vivo and in vitro. By integrating transcriptomic analysis and network pharmacology, we found that YCF has a variety of active components, including ginsenoside Rg3, platycoside D, cucurbitacin D, which can bind to multiple targets and regulate various signaling pathways, including the mTOR, PI3K/AKT, JAK/STAT, MAPK, and NF-κB signaling pathway. These pathways may be involved in the mechanisms by which YCF inhibits inflammation and suppresses the progression of silicosis.
Although the pathogenesis of silicosis remains unclear, increasing evidence suggests that silica-induces persistent pulmonary inflammation, causing tissue damage and fibrosis [26, 27]. In response to silica exposure, activated macrophages polarize into M1 macrophages, which release large amounts of pro-inflammatory mediators, such as IL-1β, IL-6, and TNF-α, that further trigger the inflammatory cascade, leading to tissue damage and fibrosis [28,29,30]. Thus, inhibition of the macrophage-induced inflammatory response may effectively ameliorate silicosis. A previous study demonstrated that bone marrow mesenchymal stem cells exert therapeutic effect in rats with silicosis by ameliorating inflammation and reducing the release of inflammatory cytokines [31]. Moreover, dioscin protects against crystalline silica-induced lung inflammation by suppressing the production of inflammatory factors and inhibiting the activation of macrophages [32]. In our study, YCF treatment significantly inhibited pulmonary inflammation in silica-exposed rats, by reducing inflammatory cell infiltration, and decreasing the secretion of inflammatory chemokines, including TNF-α, IL-1β, and IL-6. YCF also exhibited remarkable anti-fibrotic effects by inhibiting collagen deposition. Moreover, YCF decreased the amount of M1 macrophages in the lung tissue of rats with silicosis. Therefore, we speculate that YCF may exert anti-inflammatory properties via inhibiting M1 macrophage polarization.
To clarify the therapeutic mechanisms of YCF, we separated YCF into five fractions using macroporous resins, and examined the anti-inflammatory effects of these fractions on LPS and IFN-γ-induced M1 macrophages. YCF5 substantially inhibited the production of pro-inflammatory cytokines. These results indicate that YCF5 may be the essential fraction that exerts anti-inflammatory effects on M1 macrophages to attenuate inflammation and delay the progression of silicosis. However, the underlying mechanism of YCF5 attenuation of inflammation via altered macrophage polarization needs to be explored. Thus, we obtained the main active components of YCF5 by MS, network pharmacology and transcriptomics revealed the potential anti-inflammatory mechanisms of YCF5.
Traditional Chinese formulas exert curative effects through multiple compounds and targets with synergistic effects. The holistic and systematic characteristics of network pharmacology are consistent with the “holism concept” in traditional Chinese medicine. Network pharmacology can be used to construct a “compound-target-pathway” network to understand the overall perspective of effective substances and their mechanisms. In recent years, the combination of transcriptomics and network pharmacology has proven to be an effective approach for exploring the therapeutic mechanisms of traditional Chinese prescriptions. Using network pharmacology analysis, we identified the core targets of YCF5, including AKT1, JAK2, MAPK3, STAT3, MYC, EGFR, mTOR, VEGFR, and TNF. These signaling pathways and biological processes, including Toll-like receptor, TNF, and IL-17 signaling pathways, are strongly linked to immunological responses and inflammation.
AKT1, a serine/threonine-protein kinase, is activated through the PI3-kinase pathway to regulate cellular survival signals in response to growth factors and cytokines [33]. The activation of AKT1 accelerates the degradation of IκB and leads to the phosphorylation of NF-κB p65, which promotes the transcriptional activity of NF-κB [34]. Mitogen-activated protein kinase 3 (MAPK3 or ERK) is involved in cell proliferation, growth, migration, metabolism, and transcription [35]. MAPK3 (ERK1) levels are dramatically elevated in the peripheral blood mononuclear cells of patients with silicosis, and crystalline silica may accelerate the release of ROS [36]. ROS further activate the inflammasome through MAPK3 and phosphorylate Ser276 of p65 NF-κB and Ser641 and Ser643 of HIF-1α, to promote the development of silicosis [36,37,38]. JAK2 and STAT3, crucial proteins in the JAK/STAT signaling pathway, play a significant role in regulating macrophage polarization in silicosis; inhibiting the expression of these proteins can delay the progression of silicosis [39]. PI3K-Akt targets mTOR, a crucial component of the rapamycin (mTOR) signaling pathway [40, 41]. Furthermore, mTOR increases autophagy and aggravates the progression of pulmonary fibrosis in silicosis under the regulation of AMP-activated protein kinase (AMPK) [42]. VEGFA, a ligand for the VEGF receptor, plays an important role in angiogenesis and inflammation [43]. Manipulating VEGFA inhibits fibrosis factor release, suppressing the expression of TGF-β and α-SMA in the lung tissue of rats with silicosis [44]. Thus, the targets of YCF5 play vital roles in the inflammatory response, and modulating these targets can delay the progression of silicosis.
Using transcriptomic analysis, we identified 117 reversal genes for YCF5 inhibition of M1 macrophage polarization. These reversal genes are primarily involved in immunological and inflammatory responses, including the regulation of interleukins and tumor necrosis factor production. By integrating network pharmacology and transcriptomic analysis, we constructed a PPI network of YCF5 targets and reversal genes. The anti-inflammatory mechanisms of YCF5 were associated with various targets, including AKT1, TNF, TRP53, IL6, MAPK3, and JUN, and signaling pathways, including the including PI3K-Akt, MAPK, TNF, JAK-STAT, mTOR, NF-κB, and AMPK signaling pathways. These signaling pathways play critical roles in regulating inflammation. For example, MAPKs and NF-κB regulate the expression of pro-inflammatory factors [22]. The activation of mTORC1 also influences the inflammatory response [23]. The targets enriched in these pathways are related to 81 active components, which may be the main active components of YCF. In vitro, we confirmed the activity of YCF5 and the active components of YCF, including ginsenoside Rg3, Ginsenoside Rd, and Cucurbitacin D, which suppressed the activation of these signaling pathways.
Conclusions
Our study demonstrated that YCF treatment improved the pathological changes, inflammatory response, and fibrosis in rats with silicosis, probably by inhibiting M1 macrophage polarization. Moreover, network pharmacology, transcriptomics, molecular docking, and in vitro experiments showed that YCF contains multiple effective compounds with various targets that exert anti-inflammatory effects by inhibiting the pathway networks, such as mTOR, MAPK, and NF-κB signaling pathways. Although this study provides an explanation for the anti-silicosis effects of YCF, there are several limitations to this study. For instance, 101 active compounds identified in YCF5 exert anti-inflammatory effects; however, the identity of the specific active substance of YCF with beneficial effects on silicosis is not known. In future work, we will perform the effective-constituent compatibility-based analysis to identify the critical ingredients from YCF and then form the effective-constituent compatibility (ECC) of JCF, which has the potential bioactive equivalent of JCF [45]. Moreover, we will explore the anti-silicosis mechanisms and potential targets of the ECC of YCF.
Availability of data and materials
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
References
Lopes-Pacheco M, Bandeira E, Morales MM. Cell-based therapy for silicosis. Stem Cells Int. 2016;2016:5091838.
Martínez González C, Prieto González A, García Alfonso L, Fernández Fernández L, Moreda Bernardo A, Fernández Álvarez R, et al. Silicosis in artificial quartz conglomerate workers. Arch Bronconeumol. 2019;55:459–64.
Xue C, Wu N, Fan Y, Ma J, Ye Q. Distinct metabolic features in the plasma of patients with silicosis and dust-exposed workers in China: a case-control study. BMC Pulm Med. 2021;21:91.
Leung CC, Yu ITS, Chen W. Silicosis. Lancet Lond Engl. 2012;379:2008–18.
Chen S, Tang K, Hu P, Tan S, Yang S, Yang C, et al. Atractylenolide III alleviates the apoptosis through inhibition of autophagy by the mTOR-dependent pathway in alveolar macrophages of human silicosis. Mol Cell Biochem. 2021;476:809–18.
Fan M, **ao H, Song D, Zhu L, Zhang J, Zhang X, et al. A novel N-arylpyridone compound alleviates the inflammatory and fibrotic reaction of silicosis by inhibiting the ASK1-p38 pathway and regulating macrophage polarization. Front Pharmacol. 2022;13: 848435.
Hu S, Zhao H, Al-Humadi NH, Yin XJ, Ma JKH. Silica-induced apoptosis in alveolar macrophages: evidence of in vivo thiol depletion and the activation of mitochondrial pathway. J Toxicol Environ Health A. 2006;69:1261–84.
Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596–606.
Cruz FF, Horta LFB, de Maia A, Lopes-Pacheco M, da Silva AB, Morales MM, et al. Dasatinib reduces lung inflammation and fibrosis in acute experimental silicosis. PloS ONE. 2016;11: e0147005.
Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–62.
Carneiro PJ, Clevelario AL, Padilha GA, Silva JD, Kitoko JZ, Olsen PC, et al. Bosutinib therapy ameliorates lung inflammation and fibrosis in experimental silicosis. Front Physiol. 2017;8:159.
Lv W, Booz GW, Wang Y, Fan F, Roman RJ. Inflammation and renal fibrosis: recent developments on key signaling molecules as potential therapeutic targets. Eur J Pharmacol. 2018;820:65–76.
Zughaier SM, Zimmer SM, Datta A, Carlson RW, Stephens DS. Differential induction of the toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun. 2005;73:2940–50.
Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–68.
Fan M, Li X, Gao X, Dong L, **n G, Chen L, et al. LPS induces preeclampsia-like phenotype in rats and HTR8/SVneo cells dysfunction through TLR4/p38 MAPK pathway. Front Physiol. 2019;10:1030.
Prescott JA, Mitchell JP, Cook SJ. Inhibitory feedback control of NF-κB signalling in health and disease. Biochem J. 2021;478:2619–64.
Wu L-J, He X-Y, Wang W-X, Liang J, Zhang Y-D, Liang J-T, et al. Dahuang Zhechong pills suppress silicosis fibrosis progression via p38 MAPK/TGF-β1/Smad pathway in vitro. Evid Based Complement Altern Med ECAM. 2021;2021:6662261.
Zhao H, **e Y, Wang J, Li X, Li J. Pulmonary rehabilitation can improve the functional capacity and quality of life for pneumoconiosis patients: a systematic review and meta-analysis. BioMed Res Int. 2020;2020:6174936.
Hemmati AA, Nazari Z, Samei M. A comparative study of grape seed extract and vitamin E effects on silica-induced pulmonary fibrosis in rats. Pulm Pharmacol Ther. 2008;21:668–74.
Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–9.
Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–70.
Ren Q, Guo F, Tao S, Huang R, Ma L, Fu P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed Pharmacother Biomed Pharmacother. 2020;122: 109772.
Zhou M, Xu W, Wang J, Yan J, Shi Y, Zhang C, et al. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine. 2018;35:345–60.
Benmerzoug S, Rose S, Bounab B, Gosset D, Duneau L, Chenuet P, et al. STING-dependent sensing of self-DNA drives silica-induced lung inflammation. Nat Commun. 2018;9:5226.
Hoy R, Chambers DC. Silicosis: an ancient disease in need of a dose of modern medicine. Respirol Carlton Vic. 2020;25:464–5.
Li C, Lu Y, Du S, Li S, Zhang Y, Liu F, et al. Dioscin exerts protective effects against crystalline silica-induced pulmonary fibrosis in mice. Theranostics. 2017;7:4255–75.
Yang M, Wang D, Gan S, Wang B, Yu L, **e Y, et al. Triiodothyronine ameliorates silica-induced pulmonary inflammation and fibrosis in mice. Sci Total Environ. 2021;790: 148041.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95.
Joshi GN, Goetjen AM, Knecht DA. Silica particles cause NADPH oxidase-independent ROS generation and transient phagolysosomal leakage. Mol Biol Cell. 2015;26:3150–64.
Wei J, Zhao Q, Yang G, Huang R, Li C, Qi Y, et al. Mesenchymal stem cells ameliorate silica-induced pulmonary fibrosis by inhibition of inflammation and epithelial-mesenchymal transition. J Cell Mol Med. 2021. https://doi.org/10.1111/jcmm.16621.
Du S, Li C, Lu Y, Lei X, Zhang Y, Li S, et al. Dioscin alleviates crystalline silica-induced pulmonary inflammation and fibrosis through promoting alveolar macrophage autophagy. Theranostics. 2019;9:1878–92.
Yasuda T. Activation of Akt leading to NF-κB up-regulation in chondrocytes stimulated with fibronectin fragment. Biomed Res Tokyo Jpn. 2011;32:209–15.
Peng H-B, Wang R-X, Deng H-J, Wang Y-H, Tang J-D, Cao F-Y, et al. Protective effects of oleanolic acid on oxidative stress and the expression of cytokines and collagen by the AKT/NF-κB pathway in silicotic rats. Mol Med Rep. 2017;15:3121–8.
Lu N, Malemud CJ. Extracellular signal-regulated kinase: a regulator of cell growth, inflammation, chondrocyte and bone cell receptor-mediated gene expression. Int J Mol Sci. 2019;20:3792.
Zhao Y, Xu G, Li H, Chang M, **ong C, Tao Y, et al. Genome-wide mRNA profiling identifies the NRF2-regulated lymphocyte oxidative stress status in patients with silicosis. J Occup Med Toxicol Lond Engl. 2021;16:40.
Harijith A, Ebenezer DL, Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front Physiol. 2014;5:352.
Korbecki J, Simińska D, Gąssowska-Dobrowolska M, Listos J, Gutowska I, Chlubek D, et al. Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int J Mol Sci. 2021;22:10701.
Tang Q, **ng C, Li M, Jia Q, Bo C, Zhang Z. Pirfenidone ameliorates pulmonary inflammation and fibrosis in a rat silicosis model by inhibiting macrophage polarization and JAK2/STAT3 signaling pathways. Ecotoxicol Environ Saf. 2022;244: 114066.
Sun Y, Qin H, Zhang H, Feng X, Yang L, Hou D-X, et al. Fisetin inhibits inflammation and induces autophagy by mediating PI3K/AKT/mTOR signaling in LPS-induced RAW264.7 cells. Food Nutr Res. 2021. https://doi.org/10.29219/fnr.v65.6355.
Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4:64.
Li S, Li C, Pang X, Zhang J, Yu G, Yeo AJ, et al. Metformin attenuates silica-induced pulmonary fibrosis by activating autophagy via the AMPK-mTOR signaling pathway. Front Pharmacol. 2021;12: 719589.
Shibuya M. VEGF-VEGFR system as a target for suppressing inflammation and other diseases. Endocr Metab Immune Disord Drug Targets. 2015;15:135–44.
Wu Q, Han L, Gui W, Wang F, Yan W, Jiang H. MiR-503 suppresses fibroblast activation and myofibroblast differentiation by targeting VEGFA and FGFR1 in silica-induced pulmonary fibrosis. J Cell Mol Med. 2020;24:14339–48.
Li J, Ma J, Tian Y, Zhao P, Liu X, Dong H, et al. Effective-component compatibility of Bufei Yishen formula II inhibits mucus hypersecretion of chronic obstructive pulmonary disease rats by regulating EGFR/PI3K/mTOR signaling. J Ethnopharmacol. 2020;257: 112796.
Acknowledgements
This work was financially supported by Special Project of Traditional Chinese Medicine Research of Henan Province (20-21ZYZD01), National Natural Science Fund of China (81973822).
Funding
The research is supported by Special Project of Traditional Chinese Medicine Research of Henan Province (20-21ZYZD01), Zhengzhou Science and Technology Collaborative Innovation Project (XTCX2021-05), National Natural Science Fund of China (81973822).
Author information
Authors and Affiliations
Contributions
JL, PZ, and YT designed the outline of the study. XT conceived study and draft manuscript. XT, YW, and RH performed experiments, data analysis. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the First Affiliated Hospital of Henan University of Chinese Medicine's Experimental Animal Ethics Committee (Zhengzhou, China).
Consent for publication
All authors consent to the publication of this work in Chinese Medicine.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Tian, X., Wei, Y., Hou, R. et al. Yangqing Chenfei formula alleviates silica-induced pulmonary inflammation in rats by inhibiting macrophage M1 polarization. Chin Med 18, 79 (2023). https://doi.org/10.1186/s13020-023-00787-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13020-023-00787-9