Abstract
Synovial sarcoma of the heart is a rare tumor. Herein we would like to report a case of giant intrapericardial cardiac synovial sarcoma that originated from the right ventricle and grew outward near the diaphragm. After making adequate preoperative preparation, we performed the surgery as quickly as possible and resected the tumor completely. Based on the identification of the translocation on chromosome 18 rearrangement, the tumor can be diagnosed as a primary cardiac synovial sarcoma. Through this study, we aim to afford more information about cardiac synovial sarcomas as well as a reference for similar cases.
Similar content being viewed by others
Background
Synovial sarcoma (SS) is malignant and is categorized as a kind of soft tissue sarcoma. It often presents on the limbs [1], especially in the anatomy of the lower limbs near the knee-joint [2]. However, cardiac synovial sarcoma is rare. Herein, we present a case of a patient with a primary cardiac synovial sarcoma (PCSS).
Case presentation
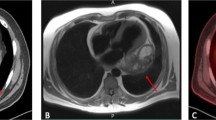
A 35-year-old male patient visited our hospital, complaining of abdominal distention and edema of lower extremities for 1 month. Vital signs recorded included a blood pressure of 90/65mmHg and a heart rate of 110 beats per minute. Based on precordial auscultation, weak and distant heart sounds could be heard on the left chest wall. Upon physical examination, distention of jugular veins and compression edema of the lower extremities showed signs of pericardial tamponade. Upon experimental examination, the blood test results were abnormal, with elevated leukocyte count and reduced erythrocyte count. The electrocardiography showed nodal tachycardia. Transthoracic echocardiography indicated the presence of a giant mass, about 15 cm in size within the pericardium, located between the heart and the diaphragm, and the ventricles and atria were at normal size. The patient’s left ventricular ejection fraction was 63%. The echocardiography also showed massive pericardial effusion and the right heart was severely compressed (Fig. 1A). To relieve the pericardial tamponade, emergency pericardiocentesis was carried out and bloody pericardial effusion was revealed. After about 1000 ml fluid was removed, the patient felt much more comfortable with improved blood pressure, and decreased heart rate. So further examination could be carried out. To investigate more about the mass, we used a three-dimensional cardiac computed tomography (CT) scan and could conclude the presence of a distinct giant mass, about 15cmX20cmX10cm in size, triangular pyramid in shape, partly substantial and partly cystic, adjacent to the ventricular diaphragmatic side of the heart (Fig. 2).
Before undergoing a routine median sternotomy, the patient was well-prepared for surgery. After opening the pericardium and drainage of the bloody pericardial effusion, a giant triangular pyramidal mass, nearly the same size as the heart, was exposed. It was located between the heart and the diaphragm without any connection with the pericardium, but attached to the diaphragmatic side of the right ventricle (Fig. 3A). The pedicle was distant from the right coronary artery and the posterior descending artery. The size of the pedicle was about 4 cm in diameter. The mass was smooth, color-mixed, partly soft, and partly hard, and had a congestive hairy core.
To effectively control the possible perioperative hemorrhage and maintain hemodynamic stability, cardiopulmonary bypass was adopted. The superior and inferior vena cava were cannulated and the ascending aorta was chosen for traditional arterial cannulation. Surgical manipulation was performed on beating heart with normal body temperature, with the assistance of cardiopulmonary bypass. The pedicle of the mass was excised, and the relation between the mass and the cavity of the right ventricle was revealed. After extended excision of the ventricular wall adjacent to the pedicle, the mass was successfully removed en bloc. After that we plugged the defect with fingers to reduce bleeding and used an attractor to increase the clarity of the operative field. A bovine pericardial patch was then used to repair the defect of the right ventricular wall (Fig. 3B). In gross appearance, the mass was about 20cmX15cmX10cm in size, well encapsulated, partly cystic, and partly substantial, and appeared color-mixed (Fig. 4). On histopathological examination, it revealed the presence of tumor cells which were composed of spindle cells, with characteristics of being fasciculate, moderately heterotypic, frequently mitotic, and necrotic (Fig. 5). A strong positive response to cytokeratin AE1/AE3, TLE-1, and BCL-2 by the cells was detected through immunohistochemistry method, and the cells also had incomplete proactiveness to epithelial membrane antigen, WT-1, and ERG. An index of proliferation (immunostaining with Ki67) of about 15% was measured. The fluorescence in-situ hybridization test confirmed the diagnosis of a primary cardiac synovial sarcoma with a positive reaction to the t (X; 18) (p11.2; q11.2) genetic change (Fig. 6).
Postoperative course of this patient was uneventful. On the second day after surgery, he maintained a stable hemodynamics without right ventricular dysfunction, and the tracheal intubation was successfully removed. In-hospital post-operative echocardiography showed no obvious cardiac occupation but a small pericardial effusion of no big significance. The left ventricular ejection fraction was 64% (Fig. 1B). The patient was successfully discharged 10 days post-surgery. According to our follow-up, the patient declined any radiotherapy or chemotherapy for 6 months after discharge. He was diagnosed with a cardiac tumor again by echocardiography, and he received a second resection of cardiac tumor in another hospital, after which, he underwent 6 courses of chemotherapy. However, a more recent echocardiography still showed a tumor which was less than 1 cm in size in his heart. The good news is that the patient has survived more than 1 year and feels well.
Discussion
SS can be dated from original mesenchymal cells. Based on the different composition of epithelial cells and spindle cells, SS can be categorized into 3 broad sections: poorly differentiated, single-phase, or biphasic. With mixed components of epithelial and spindle-cell, the tumor in our case was diagnosed as a biphasic cardiac SS [3]. The t (X; 18) (p11.2; q11.2) genetic change which leads to a merging of the SSX1 or SSX2 gene and the SS18 gene can be a special factor of SS because it has been observed in most cases of SS at present [4].
Cardiac masses are rare diseases and only 10–15% of them are malignant, with angiosarcoma being the most common malignant cardiac tumors [5]. These tumors are usually asymptomatic until they are big enough to cause symptoms [6]. PCSS is an extremely rare malignant entity that could involve the heart’s atrium and ventricles or the pericardium. For intracardiac cases, the right heart accounts for 71% of them and tends to arise mostly from the atrium [7]. And as of 2018, only 36 cases of pericardial synovial sarcoma have been reported in the literature [8]. The tumor discussed in our case, originated from the right ventricle and grew outward toward the diaphragm, which made it even more rare.
PCSS is predominant in male and has a high incidence in their 30s [9]. The clinical presentations of PCSS are nonspecific: dyspnea; heart failure; and pericardial effusion which could lead to cardiac tamponade. Compared with benign heart tumors, PCSS is more likely to present gastrointestinal and systemic symptoms. It can also readily result in hydropericardium and tends to grow bigger [10]. This patient exactly showed abdominal distention and massive bloody pericardial effusion. Chest x-rays images usually can only observe secondary signs of PCSS like cardiomegaly and pleural effusion. In the cases reported in previous studies, echocardiography was the first-line tool to use for diagnosis. Transthoracic bi-dimensional echocardiography has multiple benefits. It is non-invasive, low-cost, and can be performed at the bedside [11]. Also, transesophageal echocardiography can help to assess the tumor more clearly [12], but it is important to keep in mind that transesophageal echocardiography is an invasive procedure. CT is useful for evaluating calcification, and adjacent thoracic structure and checking for obstruction of coronary arteries [13]. Cardiovascular magnetic resonance imaging (cMRI) is more precise than echocardiography in detecting the form and actions of the heart [11]. cMRI can also evaluate the right heart better while echocardiography often neglects that [14]. Besides, cMRI and CT may be able to guide the surgical plan. Coronary angiography or cardiac CT angiography would be useful to delineate the anatomical correlation between coronary arteries and the location of the tumor. If the radiological images show the tumor has invaded coronary arteries, it would be difficult to excise the tumor completely. The final choice of image test depends on the experience of the surgeon and the facility of the hospital. Giving the patient’s symptom of pericardial tamponade, we initially only performed transthoracic bi-dimensional echocardiography. After the pericardiocentesis had relieved the pericardial tamponade, we performed a three-dimensional CT scan to get a clear anatomical relationship between the heart, great vessels, and the tumor, which guided the formulation of surgical plan.
At present, there are no clear protocols and guidelines to treat PCSS given its rarity. Surgical resection is still the most common treatment to serve for biopsy as well as to relieve symptoms in past cases. Complete surgical resection may be related to improved survival, but is usually tough, especially when the cardiac atria and ventricles are extensively involved. Before the operation, a good assessment of the relationship between the tumor and the near structures is necessary, because cardiac involvement may need cardiopulmonary bypass to completely resect the tumor [15]. The choice of operation is also important, median sternotomy has a high risk of postoperative infections and mediastinitis if radiotherapy is necessary [16]. Sometimes minimally invasive cardiac surgery can be an alternative. Adjunctive radiotherapy and chemotherapy have been lined to better survival, and SS seems to be more chemosensitive than most soft tissue sarcomas. Generally, a combination of ifosfamide and doxorubicin is the most commonly used chemotherapy regimen [9]. Targeted therapy is also being considered, Pazopanib is proven to be the only one to treat SS [17]. Heart transplantation is controversial, but some cases reported successful total artificial heart transplantation [18]. Before the patient underwent surgery, we made adequate preoperative preparation. During the operation, after exploring the extent of the tumor, we resected the mass completely. With the help of fluorescence in-situ hybridization testing, we finally diagnosed the tumor as a PCSS.
Most patients with SS had a dismal prognosis and died soon after being diagnosed [19]. Three independent factors (a young patient’s age, a complete resection, and application of adjuvant chemoradiotherapy) showed by an analysis can be associated with improved survival [7]. There is an urgent need for more effective ways to treat SS.
Conclusion
We report a rare case where the tumor was completely resected surgically and the patient has survived more than 1 year. We want to provide some data to promote research on the treatment of PCSS.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
- SS:
-
Synovial sarcoma
- PCSS:
-
Primary cardiac synovial sarcoma
- CT:
-
Computed tomography
- cMRI:
-
Cardiovascular magnetic resonance imaging
References
Vlenterie M, Ho VK, Kaal SE, Vlenterie R, Haas R, van der Graaf WT. Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br J Cancer. 2015;113(11):1602–6.
Gazendam AM, Popovic S, Munir S, Parasu N, Wilson D, Ghert M. Synovial sarcoma: a clinical review. Curr Oncol (Toronto Ont). 2021;28(3):1909–20.
Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology. 2014;46(2):95–104.
Vlenterie M, Hillebrandt-Roeffen MH, Flucke UE, Groenen PJ, Tops BB, Kam** EJ, et al. Next generation sequencing in synovial sarcoma reveals novel gene mutations. Oncotarget. 2015;6(33):34680–90.
Poonia A, Mishra R, Giridhara P, Arora YK. Left atrial angiosarcoma: a rare Cardiac Tumor at an uncommon site. J Cardiovasc Echography. 2020;30(1):38–40.
Ceresa F, Calarco G, Franzì E, Patanè F. Right atrial lipoma in patient with Cowden syndrome. Interact Cardiovasc Thorac Surg. 2010;11(6):803–4.
Coli A, Cassano A, Novello M, Ranelletti FO, Lauriola L. Primary cardiac synovial sarcoma: a review correlating outcomes with surgery and adjuvant therapy. J Card Surg. 2019;34(11):1321–7.
Luo Y, Gong K, **e T, Liu R, Guo H, Wang L, et al. Case Report: a Young Man with Giant Pericardial Synovial Sarcoma. Front Cardiovasc Med. 2022;9:829328.
Zhou AL, Halub ME, Gross JM, Shou BL, Kilic A. Massive primary cardiac synovial sarcoma of the left atrium: a case report. J Cardiothorac Surg. 2022;17(1):76.
Wang JG, Li NN. Primary cardiac synovial sarcoma. Ann Thorac Surg. 2013;95(6):2202–9.
Scicchitano P, Sergi MC, Cameli M, Miglioranza MH, Ciccone MM, Gentile M et al. Primary soft tissue sarcoma of the heart: an emerging chapter in Cardio-Oncology. Biomedicines. 2021;9(7).
Tyebally S, Chen D, Bhattacharyya S, Mughrabi A, Hussain Z, Manisty C, et al. Cardiac tumors: JACC CardioOncology State-of-the-art review. JACC CardioOncology. 2020;2(2):293–311.
Kassop D, Donovan MS, Cheezum MK, Nguyen BT, Gambill NB, Blankstein R, et al. Cardiac masses on Cardiac CT: a review. Curr Cardiovasc Imaging Rep. 2014;7(8):9281.
Cazalbou S, Chong Fah Shen V, Petermann A, Eyharts D, Fournier P, Cariou E et al. What is the most useful imaging parameter to explore the prognostic value of the right ventricular function at the time of multimodality cardiovascular imaging? Echocardiography (Mount Kisco, NY). 2020;37(8):1233–42.
Ceresa F, Sansone F, Rinaldi M, Patanè F. Left atrial paraganglioma: diagnosis and surgical management. Interact Cardiovasc Thorac Surg. 2010;10(6):1047–8.
Endo Y, Nakamura Y, Kuroda M, Nakanishi Y, Ito Y, Hori T, et al. Treatment of malignant primary cardiac lymphoma with tumor resection using minimally invasive cardiac surgery. J Cardiothorac Surg. 2018;13(1):97.
Desar IME, Fleuren EDG, van der Graaf WTA. Systemic treatment for adults with synovial sarcoma. Curr Treat Options Oncol. 2018;19(2):13.
Bruckner BA, Abu Saleh WK, Al Jabbari O, Copeland JG, Estep JD, Loebe M, et al. Total Artificial Heart Implantation after Excision of Right Ventricular Angiosarcoma. Tex Heart Inst J. 2016;43(3):252–4.
Püsküllüoglu M, Kruczak A, Mularz K, Rozmus M, Harazin-Lechowska A, Pietruszka A, et al. Primary cardiac atrial sarcomas. Report of two histologically different cases and review of the literature. Pol J Pathology: Official J Pol Soc Pathologists. 2021;72(4):358–69.
Acknowledgements
Not applicable.
Funding
The research received no specific grants from any funding agency in the public, commercial, or non-for-profit sectors.
Author information
Authors and Affiliations
Contributions
B.W. was responsible for data collection and manuscript writing, L.L. provided the idea and was mainly responsible for reviewing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors obtained informed consent for publication from the patient.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, B., Liu, L. Complete resection of a giant intrapericardial cardiac synovial sarcoma. J Cardiothorac Surg 19, 243 (2024). https://doi.org/10.1186/s13019-024-02725-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02725-8