Abstract
Background
Myricetin (MYR) is a common plant flavonoid with antioxidant and anticancer properties. However, the anti-aging effect of MYR on nucleus pulposus cells (NPCs) is still unknown. The study aimed to explore the effect of MYR on the senescence of NPCs.
Methods
Methyl-thiazolyl tetrazolium assay was used to detect NPCs viability. Senescence level was evaluated by senescence-associated β-galactosidase (SA-β-Gal) staining and the expression levels of P21, P16, IL-6 and IL-8. RNA-Sequencing (RNA-seq) technology was used to identify differentially expressed genes (DEGs) between hydrogen peroxide + MYR (HO + MYR) group and HO group, and Gene Ontology (GO) functional was performed to analyze DEGs. A Venn diagram was generated to screen overlap** DEGs related to aging and inflammation, and the role of the promising validated DEG was selected for further investigation by gene functional assays.
Results
HO inhibited NPCs viability and stimulated the senescent phenotype of NPCs, whereas MYR treatment significantly reversed SA-β-gal activity in NPCs. MYR also reduced the expression of p21 and p16 and the secretion of IL-6 and IL-8 induced by HO. RNA-seq screened 421 DEGs. The GO enrichment results showed DEGs were mainly enriched in terms such as "sterol biosynthetic process". We also found SERPINE1 has the highest log2FC abs. Silence of SERPINE1 inhibited HO-induced NPCs senescence, and overexpression of SERPINE1 could limit the anti-aging effect of MYR.
Conclusions
MYR alleviated HO-induced senescence of NPCs by regulating SERPINE1 in vitro.
Similar content being viewed by others
Introduction
Intervertebral disc degeneration (IDD) is an age-related degenerative disease and the main cause of various spinal degenerative diseases such as cervical spondylosis, lumbar disc herniation, and lumbar spinal stenosis. Due to the aging of the population, the prevalence of spinal degenerative diseases is increasing year by year, which not only seriously affects the quality of life of patients, but also brings a great health burden to the society [1]. However, so far the treatment of IDD has not achieved satisfactory results.
Cellular senescence is usually defined as irreversible cell cycle arrest due to replication stress and senescence [2], and the senescence-associated secretory phenotype (SASP) is an important feature of cellular senescence, which secretes a series of cytokines such as pro-inflammatory factors, growth factors, chemokines and proteases [3, 4]. The main feature of IDD is the degradation of the extracellular matrix (ECM), and NPCs, as the main functional cells of IDD, are essential to maintain the homeostasis of ECM [5]. However, senescent NPCs were accompanied by the reduction in Collagen-2, the main component of the ECM, which induced the occurrence of IDD [6], so one of the most important features of IDD is the senescence of NPCs. It has been reported earlier that the degree of cellular senescence was closely related to the IDD grade [7, 8]. Therefore, it is of great significance to explore the mechanism of senescence of NPCs for the exploration of IDD progression.
Myricetin (MYR) is a naturally occurring flavonoid compound widely found in fruits, vegetables and nuts [9]. Several studies have reported that MYR had antioxidant, antiviral, antibacterial and anticancer effects [9, 10]. Studies found that myricetin had anti-photoaging effect [11] and prevented ethanol-induced inflammatory damage [12], and could reduce the occurrence of death by inhibiting the secretion of inflammatory cytokines [13]. However, its anti-aging potential in NPCs has not been intensively investigated so far.
In this study, we identified 421 differentially expressed genes (DEGs) by RNA sequencing (RNA-seq) technology in hydrogen peroxide (HO)-induced senescent NPCs treated with MYR. The Gene Ontology (GO) database was used to enrich the DEGs into the corresponding pathways. Furthermore, we obtained the serine protease inhibitor clade E member 1 (SERPINE1) with the highest log2FC abs, which belongs to one of the serine protease inhibitor family members by Venn diagram. It was finally confirmed that MYR inhibited the senescence of NPCs by regulating the expression of SERPINE1.
Material and methods
Cell culture and transfection
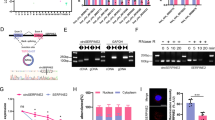
NPCs isolated from mild IDD patients (Pfirrmann grade I–II) were a gift from First Affiliated Hospital, ** genes in aging and inflammation-related genes (Fig. 4A) and found a total of 7 overlap** genes. The expression of the top 5 overlap** genes was then verified by qPCR, and the results proved that the expression trends of these five DEGs were consistent with the RNA-seq results (Fig. 4B). More importantly, SERPINE1 was used for the following studies since SERPINE1 had the highest log2FC abs among the top 5 overlap** genes. Next, we interfered with SERPINE1 expression in NPCs, and the results proved that silencing SERPINE1 was successful (Fig. 4C). Subsequently, by SA-β-gal staining, we found that silencing SERPINE1 reduced the percentage of SA-β-Gal-positive cells (Fig. 4D). Furthermore, by Western blot, we found that silencing SERPINE1 inhibited senescence markers (p21, p16) expression (Fig. 4E). Finally, ELISA was used to detect the effect of silencing SERPINE1 on the secretion of SASP pro-inflammatory factors (IL-6 and IL-8), the results confirmed that silencing of SERPINE1 inhibited the increased expression levels of IL-6 and IL-8. Taken together, the results suggest that silencing SERPINE1 inhibits HO-induced cellular senescence.
Silencing of SERPINE1 regulates HO-induced senescence in NPCs. A Venn diagram analysis of overlap** genes in aging and inflammation-related genes. B qPCR verifies the expression of the top 5 overlap** genes. C The silencing effect of SERPINE1 was detected by qPCR. D SA-β-gal staining was used to detect the level of cellular senescence in two groups of NPCs treated with 100 μM HO for 24 h. E Western blot was used to detect the expression levels of senescence markers (p21, p16) in HO-treated NPCs in si-NC and si-SERPINE1 groups. F The expression levels of IL-6 and IL-8 of NPCs treated with 100 μM HO for 24 h were detected by ELISA. *P < 0.5 VS si-NC group
Overexpression of SERPINE1 inhibits the anti-aging effect of MYR
At the end of the experiment, we explored the effect of SERPINE1 on the anti-aging effect of MYR. First, we verified the transfection efficiency of SERPINE1 overexpression in NPCs. qPCR results showed that SERPINE1 was overexpressed successfully (Fig. 5A). Subsequent SA-β-gal staining (Fig. 5B) and Western blot (Fig. 5C) results confirmed that overexpression of SERPINE1 reversed the MYR-induced decrease in the percentage of SA-β-Gal-positive cells and expression of p16 and p21. Similarly, ELISA results showed that overexpression of SERPINE1 inhibited the expression of MYR-regulated IL-6 and IL-8 (Fig. 5D). Taken together, the results indicated that overexpression of SERPINE1 inhibited the anti-aging effect of MYR.
Overexpression of SERPINE1 affects the anti-aging effect of MYR. A qPCR was used to examine the effect of SERPINE1 overexpression. B SA-β-gal staining was performed to detect senescence levels in three groups of NPCs with 100 μM HO alone for 24 h or co-treated with 100 μM HO for 24 h and 10 μM MYR for 48 h. C Western blot was used to detect the expression levels of p21 and p16 in three groups of NPCs with 100 μM HO alone or co-treated with 100 μM HO and 10 μM MYR. D ELISA was used to detect the expression levels of IL-6 and IL-8 in three groups of NPCs with 100 μM HO alone or co-treated with 100 μM HO for 24 h and 10 μM MYR for 48 h
Discussion
IDD is one of the ancient and common clinical diseases, and cellular senescence is the key inducement of IDD pathogenesis. In the present study, we found that MYR could effectively inhibit HO-induced cellular senescence and the secretion of inflammatory factors of NPCs by regulating SERPINE1.
The causes of cellular senescence are mainly divided into two categories, replicative aging and stress-induced premature aging. The former is a programmed death process, while the latter is under the action of some sub-lethal emergencies such as hyperoxia, HO, ultraviolet rays cells age in advance. HO can directly damage DNA and cause premature cell aging [16], so in this study HO was selected as an agent for inducing senescence in NPCs. The concentration of HO used for aging induction of NPCs was not consistent in previous studies [17,18,19], and the major considered parameter was the cell viability, which was generally at 80–90% [17, 18]. Consistently, the concentration (100 μM) we used for aging induction resulted in a ~ 80% viability of NPCs. With the increase in HO dose, cell viability reduced gradually, and only 40% of NPCs was alive after treated with 500 μM HO. Generally, the concentration of HO caused ~ 50% of cell viability was used to induce apoptosis of NPCs [20, 21].
MYR is a flavonol compound with various pharmacological activities such as anti-inflammatory and analgesic, anti-tumor, hypoglycemic, and liver protection [9]. MYR shows abundant resource advantages and huge potential utilization value. Multiple effects of MYR have been reported to depend on the therapeutic dose [22]. Cell viability assay indicated that the higher dose of MYR (40 and 80 μM) significantly inhibited cell growth, while the lower dose groups (10 and 20 μM) showed no cytotoxicity on NPCs. We also found that lower dose of MYR (10 and 20 μM) could not significantly promote the growth of NPCs, but they played positive growth effects on HO-treated NPCs. We inferred that the injured NPCs could not growth as normal, and the potential anti-aging and anti-inflammation effects of MYR helped the injured NPCs to gradually return to a better state. In addition, the positive growth effect of MYR on normal NPCs may be observed by extending the treatment time to 72 h, as a higher viability was observed in 10 μM group treated for 48 h. Further senescent phenotype detection results indicated that MYR could inhibit the senescence of NPCs.
Inflammatory response has always been an important factor affecting the occurrence and development of various degenerative diseases including IDD. It is difficult for the normal immune inflammatory response to act on the intervertebral disc tissue under physiological conditions. However, when disc herniation occurs, herniated disc tissue causes pain responses through the action of inflammatory factors [23]. Previous studies have demonstrated that MYR could inhibit the expression of inflammatory factors [13]. Consistently, we found that MYR inhibited the expression of inflammatory factors IL-6 and IL-8 secreted by HO-induced senescent NPCs.
Next, RNA-seq was used to further explore DEGs in senescent NPCs to uncover the key genes affecting NPC aging. We found a total of 260 up-regulated genes and 161 down-regulated genes compared with the control group. In the GO enrichment analysis of DEGs, DEGs were mainly enriched in the "CXCR chemokine receptor binding" terms, a previous report demonstrated that loss of CXCR7 expression leads to cellular senescence [24]. It indicated that DEGs may play a role by participating in CXCR chemokine receptor binding. At the same time, we searched the GO terms related to aging (aging, senescence) and inflammation (inflammat-), respectively, and a total of 8 related GO biological processes were screened. FOXO4 was enriched in the GO term of "aging", and it has been proved to be involved in IDD [28] and has been found to show pro-angiogenic, growth and migration stimulation and anti-apoptotic activities in recent years [29]. It is also confirmed to be the most reliable biological and prognostic marker for various cancers [30, 31]. More importantly, it is reported that SERPINE1 has showed important regulating role in aging [32, 33] and is also able to regulate inflammatory damage [34], but its role in the senescence of NPCs is still unknown. In this study, we found that the expression of SERPINE1 was significantly decreased in senescent NPCs cotreated with HO and MYR, and interfering with SERPINE1 expression could inhibit the secretion of inflammatory factors in NPCs, which was consistent with previous reports [34]. In addition, studies have shown that SERPINE1 can promote the expression of STAT3 signaling pathway [35], and studies have found that the aging of NPCs was closely related to STAT3 signaling pathway [36]. Therefore, we speculated that SERPINE1 may regulate the aging of NPCs by regulating the STAT3 signaling pathway.
qPCR validation also indicated that EDNRB’s validated log2 FC (abs) value was second only to that of SERPINE1. It is reported that EDNRB is closely correlated with hair graying with aging [37], and deletion of EDNRB leads to delayed development of neural crest cells [38]. Another noteworthy verified DEG is BCL6, which has been shown to regulate cellular senescence [39]. In addition, BCL6 is reported to be a potent inhibitor to suppress the senescence of mouse fibroblasts, and it could induce cyclin D1 expression thus bypassing the senescence response downstream of p53 [40]. The upregulation of the two candidates might also be involved in the anti-aging effect of MYR, which is worth for further studies.
Overall, the present study found that MYR was able to alleviate HO-induced senescence of NPCs by regulating the expression of SERPINE1 in vitro, providing a promising candidate molecule to reverse the senescence of NPCs in vivo. The study also identified and verified other candidate DEGs in MYR treating group, which helps to further investigate the multiple mechanisms of MYR.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Gille O, Bouloussa H, Mazas S, Vergari C, Challier V, Vital JM, et al. A new classification system for degenerative spondylolisthesis of the lumbar spine. Eur Spine J. 2017;26(12):3096–105. https://doi.org/10.1007/s00586-017-5275-4.
Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010;24(22):2463–79. https://doi.org/10.1101/gad.1971610.
Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–68. https://doi.org/10.1371/journal.pbio.0060301.
Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133(6):1019–31. https://doi.org/10.1016/j.cell.2008.03.039.
Wu X, Liu Y, Guo X, Zhou W, Wang L, Shi J, et al. Prolactin inhibits the progression of intervertebral disc degeneration through inactivation of the NF-kappaB pathway in rats. Cell Death Dis. 2018;9(2):98. https://doi.org/10.1038/s41419-017-0151-z.
Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle. 2016;15(13):1674–84. https://doi.org/10.1080/15384101.2016.1152433.
Tang N, Dong Y, Chen C, Zhao H. Anisodamine maintains the stability of intervertebral disc tissue by inhibiting the senescence of nucleus pulposus cells and degradation of extracellular matrix via interleukin-6/janus kinases/signal transducer and activator of transcription 3 pathway. Front Pharmacol. 2020;11:519172. https://doi.org/10.3389/fphar.2020.519172.
Machino M, Yukawa Y, Imagama S, Ito K, Katayama Y, Matsumoto T, et al. Age-related and degenerative changes in the osseous anatomy, alignment, and range of motion of the cervical spine: a comparative study of radiographic data from 1016 patients with cervical spondylotic myelopathy and 1230 asymptomatic subjects. Spine. 2016;41(6):476–82. https://doi.org/10.1097/BRS.0000000000001237.
Song X, Tan L, Wang M, Ren C, Guo C, Yang B, et al. Myricetin: a review of the most recent research. Biomed Pharmacother. 2021;134:111017. https://doi.org/10.1016/j.biopha.2020.111017.
Gupta G, Siddiqui MA, Khan MM, Ajmal M, Ahsan R, Rahaman MA, et al. Current pharmacological trends on myricetin. Drug Res (Stuttg). 2020;70(10):448–54. https://doi.org/10.1055/a-1224-3625.
Jung SK, Lee KW, Kim HY, Oh MH, Byun S, Lim SH, et al. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem Pharmacol. 2010;79(10):1455–61. https://doi.org/10.1016/j.bcp.2010.01.004.
Ahmad SB, Rashid SM, Wali AF, Ali S, Rehman MU, Maqbool MT, et al. Myricetin (3,3('),4('),5,5('),7-hexahydroxyflavone) prevents ethanol-induced biochemical and inflammatory damage in the liver of Wistar rats. Hum Exp Toxicol. 2022. https://doi.org/10.1177/09603271211066843.
Agraharam G, Girigoswami A, Girigoswami K. Myricetin: a multifunctional flavonol in biomedicine. Curr Pharmacol Rep. 2022;8(1):48–61. https://doi.org/10.1007/s40495-021-00269-2.
Rui G, Sun N, Hu B, Lin S, Wang Z, Lin Q. Upregulated plant homeodomain finger protein 6 promotes extracellular matrix degradation in intervertebral disc degeneration based on microarray analysis. Spine. 2020;45(19):E1216–24. https://doi.org/10.1097/BRS.0000000000003549.
Lian B, Pei YC, Jiang YZ, Xue MZ, Li DQ, Li XG, et al. Truncated HDAC9 identified by integrated genome-wide screen as the key modulator for paclitaxel resistance in triple-negative breast cancer. Theranostics. 2020;10(24):11092–109. https://doi.org/10.7150/thno.44997.
Itahana K, Campisi J, Dimri GP. Mechanisms of cellular senescence in human and mouse cells. Biogerontology. 2004;5(1):1–10. https://doi.org/10.1023/b:bgen.0000017682.96395.10.
Du J, Xu M, Kong F, Zhu P, Mao Y, Liu Y, et al. CB2R attenuates intervertebral disc degeneration by delaying nucleus pulposus cell senescence through AMPK/GSK3beta pathway. Aging Dis. 2022;13(2):552–67. https://doi.org/10.14336/AD.2021.1025.
Lin J, Du J, Wu X, Xu C, Liu J, Jiang L, et al. SIRT3 mitigates intervertebral disc degeneration by delaying oxidative stress-induced senescence of nucleus pulposus cells. J Cell Physiol. 2021;236(9):6441–56. https://doi.org/10.1002/jcp.30319.
He J, Zhang A, Song Z, Guo S, Chen Y, Liu Z, et al. The resistant effect of SIRT1 in oxidative stress-induced senescence of rat nucleus pulposus cell is regulated by Akt-FoxO1 pathway. 2019. Biosci Rep. https://doi.org/10.1042/BSR20190112.
Tian Y, Bao Z, Ji Y, Mei X, Yang H. Epigallocatechin-3-gallate protects H(2)O(2)-induced nucleus pulposus cell apoptosis and inflammation by inhibiting cGAS/Sting/NLRP3 activation. Drug Des Dev Ther. 2020;14:2113–22. https://doi.org/10.2147/DDDT.S251623.
Lin H, Wang Y, **g K, Wu T, Niu Y, Wei J. Nuclear factor erythroid-2 related factor 2 inhibits human disc nucleus pulpous cells apoptosis induced by excessive hydrogen peroxide. Rev Assoc Med Bras. 2020;66(7):986–91. https://doi.org/10.1590/1806-9282.66.7.986.
Peng S, Fang C, He H, Song X, Zhao X, Zou Y, et al. Myricetin exerts its antiviral activity against infectious bronchitis virus by inhibiting the deubiquitinating activity of papain-like protease. Poult Sci. 2022;101(3):101626. https://doi.org/10.1016/j.psj.2021.101626.
Iwabuchi S, Ito M, Chikanishi T, Azuma Y, Haro H. Role of the tumor necrosis factor-alpha, cyclooxygenase-2, prostaglandin E2, and effect of low-intensity pulsed ultrasound in an in vitro herniated disc resorption model. J Orthop Res. 2008;26(9):1274–8. https://doi.org/10.1002/jor.20525.
Hoy JJ, Kallifatidis G, Smith DK, Lokeshwar BL. Inhibition of androgen receptor promotes CXC-chemokine receptor 7-mediated prostate cancer cell survival. Sci Rep. 2017;7(1):3058. https://doi.org/10.1038/s41598-017-02918-3.
Liu Q, Tan Z, **e C, Ling L, Hu H. Oxidative stress as a critical factor might involve in intervertebral disc degeneration via regulating NOXs/FOXOs. J Orthop Sci. 2021. https://doi.org/10.1016/j.jos.2021.09.010.
Alvarez-Garcia O, Matsuzaki T, Olmer M, Masuda K, Lotz MK. Age-related reduction in the expression of FOXO transcription factors and correlations with intervertebral disc degeneration. J Orthop Res. 2017;35(12):2682–91. https://doi.org/10.1002/jor.23583.
Deng B, Ren JZ, Meng XQ, Pang CG, Duan GQ, Zhang JX, et al. Expression profiles of MMP-1 and TIMP-1 in lumbar intervertebral disc degeneration. Genet Mol Res. 2015;14(4):19080–6. https://doi.org/10.4238/2015.December.29.16.
Declerck PJ, Gils A. Three decades of research on plasminogen activator inhibitor-1: a multifaceted serpin. Semin Thromb Hemost. 2013;39(4):356–64. https://doi.org/10.1055/s-0033-1334487.
Jevric M, Matic IZ, Krivokuca A, Dordic Crnogorac M, Besu I, Damjanovic A, et al. Association of uPA and PAI-1 tumor levels and 4G/5G variants of PAI-1 gene with disease outcome in luminal HER2-negative node-negative breast cancer patients treated with adjuvant endocrine therapy. BMC Cancer. 2019;19(1):71. https://doi.org/10.1186/s12885-018-5255-z.
Nakatsuka E, Sawada K, Nakamura K, Yoshimura A, Kinose Y, Kodama M, et al. Plasminogen activator inhibitor-1 is an independent prognostic factor of ovarian cancer and IMD-4482, a novel plasminogen activator inhibitor-1 inhibitor, inhibits ovarian cancer peritoneal dissemination. Oncotarget. 2017;8(52):89887–902. https://doi.org/10.18632/oncotarget.20834.
Sotiropoulos GP, Kotopouli M, Karampela I, Christodoulatos GS, Antonakos G, Marinou I, et al. Circulating plasminogen activator inhibitor-1 activity: a biomarker for resectable non-small cell lung cancer? J BUON. 2019;24(3):943–54.
Kortlever RM, Bernards R. Senescence, wound healing and cancer: the PAI-1 connection. Cell Cycle. 2006;5(23):2697–703. https://doi.org/10.4161/cc.5.23.3510.
Wang Y, Lim R, Nie G. Elevated circulating HtrA4 in preeclampsia may alter endothelial expression of senescence genes. Placenta. 2020;90:71–81. https://doi.org/10.1016/j.placenta.2019.12.012.
Wang T, Lu H, Li D, Huang W. TGF-beta1-mediated activation of SERPINE1 is involved in hemin-induced apoptotic and inflammatory injury in HT22 cells. Neuropsychiatr Dis Treat. 2021;17:423–33. https://doi.org/10.2147/NDT.S293772.
Chen S, Li Y, Zhu Y, Fei J, Song L, Sun G, et al. SERPINE1 overexpression promotes malignant progression and poor prognosis of gastric cancer. J Oncol. 2022. https://doi.org/10.1155/2022/2647825.
Ashraf S, Santerre P, Kandel R. Induced senescence of healthy nucleus pulposus cells is mediated by paracrine signaling from TNF-alpha-activated cells. FASEB J. 2021;35(9):e21795. https://doi.org/10.1096/fj.202002201R.
Iida M, Tazaki A, Yajima I, Ohgami N, Taguchi N, Goto Y, et al. Hair graying with aging in mice carrying oncogenic RET. Aging Cell. 2020;19(11):e13273. https://doi.org/10.1111/acel.13273.
Druckenbrod NR, Epstein ML. Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development. 2009;136(18):3195–203. https://doi.org/10.1242/dev.031302.
Chen J, Wang M, Guo M, **e Y, Cong YS. miR-127 regulates cell proliferation and senescence by targeting BCL6. PLoS ONE. 2013;8(11):e80266. https://doi.org/10.1371/journal.pone.0080266.
Shvarts A, Brummelkamp TR, Scheeren F, Koh E, Daley GQ, Spits H, et al. A senescence rescue screen identifies BCL6 as an inhibitor of anti-proliferative p19(ARF)-p53 signaling. Genes Dev. 2002;16(6):681–6. https://doi.org/10.1101/gad.929302.
Acknowledgements
We are grateful for the technical support provided by Ma'anshan Institute of Rehabilitation, Shanghai University of Traditional Chinese Medicine in RNA-sequencing.
Funding
This work was supported by Natural Science Foundation of Fujian Province (No. 2020J01957).
Author information
Authors and Affiliations
Contributions
CW and WX designed the study. RC and XZhang carried out experiments and analyzed data. RC and XZhu collected the data. RC and XZhang drafted the manuscript. All authors contributed to the revision of manuscript and approved the final version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Table S1: Primer sequences used for qPCR.
Additional file 2
. Table S2: Fold change of all 421 DEGs.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, R., Zhang, X., Zhu, X. et al. Myricetin alleviated hydrogen peroxide-induced cellular senescence of nucleus pulposus cell through regulating SERPINE1. J Orthop Surg Res 18, 143 (2023). https://doi.org/10.1186/s13018-022-03463-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03463-0