Abstract
Background
An increasing number of studies have shown that dysregulated miR-589-3p is associated with multiple diseases. However, the role of miR-589-3p in osteogenic differentiation of periodontal ligament stem cells (PDLSCs) remains unknown. This study aimed to explore the biological function and potential molecular mechanism of miR-589-3p in osteogenic differentiation of PDLSCs.
Methods
GSE159508 was downloaded from Gene Expression Omibus (GEO, http://www.ncbi.nlm.nih.gov/geo/). Differentially expressed miRNAs between osteogenic induction PDLSCs versus non-induction PDLSCs were obtained by R software. miR-589-3p mimic and miR-589-3p inhibitor and corresponding negative control were obtained and to identify the role of miR-589-3p in osteogenic differentiation of PDLSCs. ALP staining and ARS were used to evaluate ALP activity and mineralization, respectively. The targeted binding relationship between miR-589-3p and ATF1 was predicted and verified by target prediction analysis and dual-luciferase assay. Furthermore, the functional mechanism based on miR-589-3p and ATF1 in osteogenic differentiation of PDLSCs was further investigated through rescue experiments.
Results
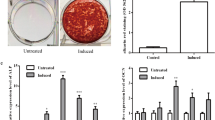
According to the cut-off criteria with log 2 FC > 1.0 and P < 0.05, 514 differentially expressed miRNAs were identified between osteogenic induction and non-induction PDLSCs, including 309 upregulated miRNAs and 205 downregulated miRNAs. Compared with control PDLSCs, miR-589-3p expression level was notably increased in PDLSCs that underwent osteogenic induction. The overexpression of miR-589-3p promoted the cell viability of PDLSCs, while the low expression of miR-589-3p had the opposite effect. The dual luciferase reporter assay verified that ATF1 was proved to be a direct target of miR-589-3p in PDLSCs. And overexpressed miR-589-3p reduced the expression of ATF1. Overexpression of miR-589-3p enhanced the osteogenic capacity of PDLSCs, as demonstrated by increases in ALP activity, matrix mineralization, and RUNX2, OCN and OSX expression. In addition, the rescue experiments confirmed that overexpressed ATF1 restored the effects of overexpressed miR-589-3p on cell proliferation and osteogenic differentiation of PDLSCs.
Conclusion
miR-589-3p could down-regulate the expression of ATF1, thereby promote the proliferation and osteogenic differentiation of PDLSCs. This finding may provide a new therapeutic target for molecular therapy of periodontitis.
Similar content being viewed by others
Background
The periodontal ligament has stem cells that have the ability to regenerate lost periodontal tissues [1,2,3]. Orthodontic tooth movement progresses by a combination of periodontal ligament tissue and alveolar bone remodeling processes [4, 5]. Periodontitis is the most common types of diseases that cause bone destruction [6]. Periodontitis, tooth replantation, and repair of bone defects around implants all require periodontal tissue bone regeneration [7].
Periodontal ligament stem cells (PDLSCs) a new population of mesenchymal stem cells (MSCs), exhibited the ability to repair alveolar bone defects in periodontitis [8]. Also, there is need to understand molecular mechanism involved in PDLSCs osteogenic differentiation [9].
MicroRNAs (miRNAs) belong to a class of non-coding RNAs. Mechanically, miRNAs bind to complementary sites on the 3′-untranslated region (3′-UTR) of mRNAs and cause mRNA degradation or translational suppression, thereby inhibiting the expression of target genes [10,1: S1).
ATF1 3′UTR sequence contained the binding sites for miR-589-3p (Fig. 6A), implying that ATF1 might be a downstream target of miR-589-3p.
miR-589-3p directly target to ATF1 3′UTR. A Putative binding sites between miR-589-3p and ATF1; B Luciferase reporter assay showed that miR-589-3p mimic transfection suppressed the relative luciferase activity of the ATF1-Wt reporter in PGLSCs; C Relative ATF1 expression in inhibitor NC, miR-589-3p inhibitor, mimic NC and miR-589-3p mimic groups. *P < 0.05
The luciferase activity assay revealed the miR-589-3p mimic suppressed ATF1 3′-UTR wild-type (WT) luciferase activity, whereas it had no effect on ATF1 3′-UTR mutant (Mut) luciferase activity compared with control in PDLSCs (Fig. 6B).
In contrast to miR-589-3p expression, Similar to BM-MSCs, the expression of ATF1 during the process of osteogenic differentiation of PDLSCs was reduced in a time-dependent manner (Fig. 6C).
Overexpression ATF1 reversed the effects of miR-589-3p on proliferation and osteogenic differentiation of PDLSCs
In order to validate whether miR-589-3p promoted osteogenic differentiation of PDLSCs by inhibiting ATF1, the rescue experiments were performed by co-transfection with miR-589-3p mimic and ATF1.
Compared with control group, better ALP activity and mineralized nodule formation was found in miR-589-3p group, but partially reversed by co-transfection with ATF1 overexpression plasmid. Meanwhile, ATF1 overexpression alone could also delay the osteogenic differentiation process (Fig. 7A). Real-time PCR and western blot assays further confirmed that the upregulation of RUNX2, OPN and OCN induced by miR-589-3p could be partially blockaded by overexpression of ATF1. Moreover, overexpression of ATF1 could also decrease the RUNX2, OPN and OCN expression (Fig. 7B, C).
Overexpression ATF1 partially reversed the effects of miR-589-3p-overexpression on PDLSCs. A Images of ALP and Alizarin Red S staining in control, miR-589-3p mimic, miR-589-3p mimic + ATF1 and ATF1 groups; B Relative RUNX2, OPN and OCN mRNA expression in control, miR-589-3p mimic, miR-589-3p mimic + ATF1 and ATF1 groups; C Relative RUNX2, OPN and OCN protein expression in control, miR-589-3p mimic, miR-589-3p mimic + ATF1 and ATF1 groups. *P < 0.05.
Discussion
In this study, we firstly identified the differentially expressed miRNAs between osteogenic induction and non-osteogenic induction PDLSCs through bioinformatic analysis. miR-153-3p mimic or ATF1 suppression promoted the osteogenic differentiation of PDLSCs, as demonstrated by increases in ALP activity and matrix mineralization. Further studies revealed that miR-589-3p promoted osteogenic differentiation of PDLSCs by sponging ATF1.
Among these altered miRNAs, miR-589-3p was chosen for further study for two reasons. First, miR-589-3p showed a significant fold change among the upregulated miRNAs. Also, this is the first report of altered miR-589-3p expression in osteogenic differentiation of PDLSCs. miR-589-3p upregulation promoted the osteogenic differentiation of PDLSCs, as demonstrated by increases in ALP activity, matrix mineralization, and ALP, Runx2, and OPN expression.
MicroRNA (miRNA) has been reported to become novel therapeutic targets for skeletal related diseases.
Some miRNAs are differentially expressed in stem cells, and have significant effects on the osteogenic differentiation of stem cells. Lu et al. [20] identified the role of miR-589-3p in human lumbar disc degeneration and its potential mechanism. They found that miR-589-3p was significantly upregulated in lumbar disc degeneration patients. Following dual-luciferase reporter assay, Smad was demonstrated to be a target gene for miR-589-3p.
Furthermore, ATF1, which is a target gene of miR-589-3p, decreased osteogenic differentiation of PDLSCs; however, these effects were partially reversed by miR-589-3p mimic.
ATF1 has been shown to play an important role in cell proliferation, differentiation and apoptosis [27]. ATF1, as a regulator, also promoting sexual differentiation and entry into the stationary phase in S. pombe [28]. AtfA and Atf1 are quite highly conserved and that they are involved in multiple cellular processes [28]. In this study, we found that overexpression of ATF1 partially reversed the promotion effects of miR-589-3p on osteogenic differentiation of PDLSCs. Moreover, luciferase activity verified that miR-589-3p could significantly inhibited the luciferase activity of wild-type ATF1, but it failed to suppress luciferase activity of mutated one.
Limitation of this study can be listed as follows: (1) in vivo study was lack and thus should be further study in future studies; (2) following signaling pathway should be further explored the mechanism of miR-589-3p.
Conclusion
In conclusion, this study suggested that miR-589-3p promotes osteogenic differentiation of PDLSCs by targeting ATF1. The results of the present study demonstrated that the miR-589-3p/ATF1 interaction network may serve as a potential regulatory mechanism underlying PDLSCs osteogenesis.
Availability of data and materials
All the data will be available upon motivated request to the corresponding author of the present paper.
Abbreviations
- PDLSCs:
-
Periodontal ligament stem cells
- GEO:
-
Gene Expression Omibus
- MSCs:
-
Mesenchymal stem cells
- 3′-UTR:
-
3′-Untranslated region
- GO:
-
Gene ontology
- DAVID:
-
Database for Annotation, Visualization and Integrated Discovery
- BP:
-
Biological process
- CC:
-
Cellular component
- MF:
-
Molecular function
- DMEM:
-
Dulbecco’s modified Eagle medium
- FBS:
-
Fetal bovine serum
- PVDF:
-
Polyvinylidene fluoride
- TBST:
-
Tris buffered saline-Tween
References
Venkataiah VS, Handa K, Njuguna MM, et al. Periodontal regeneration by allogeneic transplantation of adipose tissue derived multi-lineage progenitor stem cells in vivo. Sci Rep. 2019;9:921. https://doi.org/10.1038/s41598-018-37528-0.
Fu X, ** L, Ma P, et al. Allogeneic stem cells from deciduous teeth in treatment for periodontitis in miniature swine. J Periodontol. 2014;85:845–51. https://doi.org/10.1902/jop.2013.130254.
Liu L, Wei X, Huang R, et al. Effect of bone morphogenetic protein-4 on the expression of Sox2, Oct-4, and c-Myc in human periodontal ligament cells during long-term culture. Stem Cells Dev. 2013;22:1670–7. https://doi.org/10.1089/scd.2012.0548.
Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–62. https://doi.org/10.1111/j.1365-2591.2009.01622.x.
Shirai K, Ishisaki A, Kaku T, et al. Multipotency of clonal cells derived from swine periodontal ligament and differential regulation by fibroblast growth factor and bone morphogenetic protein. J Periodontal Res. 2009;44:238–47. https://doi.org/10.1111/j.1600-0765.2008.01140.x.
Trubiani O, Scarano A, Orsini G, et al. The performance of human periodontal ligament mesenchymal stem cells on xenogenic biomaterials. Int J Immunopathol Pharmacol. 2007;20:87–91. https://doi.org/10.1177/039463200702001s17.
Yang P, Li C, Kou Y, et al. Notum suppresses the osteogenic differentiation of periodontal ligament stem cells through the Wnt/Beta catenin signaling pathway. Arch Oral Biol. 2021;130: 105211. https://doi.org/10.1016/j.archoralbio.2021.105211.
Yu M, Sun L, Ba P, et al. Progranulin promotes osteogenic differentiation of periodontal membrane stem cells in both inflammatory and non-inflammatory conditions. J Int Med Res. 2021;49:3000605211032508. https://doi.org/10.1177/03000605211032508.
Sanz JL, Guerrero-Gironés J, Pecci-Lloret MP, et al. Biological interactions between calcium silicate-based endodontic biomaterials and periodontal ligament stem cells: a systematic review of in vitro studies. Int Endod J. 2021;54:2025–43. https://doi.org/10.1111/iej.13600.
Li G, Shao Y, Guo HC, et al. MicroRNA-27b-3p downregulates FGF1 and aggravates pathological cardiac remodelling. Cardiovasc Res. 2021. https://doi.org/10.1093/cvr/cvab248.
**e F, Liu YL, Chen XY, et al. Role of microRNA, LncRNA, and exosomes in the progression of osteoarthritis: a review of recent literature. Orthop Surg. 2020;12:708–16. https://doi.org/10.1111/os.12690.
Wang J, Liu S, Li J, et al. Roles for miRNAs in osteogenic differentiation of bone marrow mesenchymal stem cells. Stem Cell Res Ther. 2019;10:197. https://doi.org/10.1186/s13287-019-1309-7.
Chen L, Heikkinen L, Wang C, et al. Trends in the development of miRNA bioinformatics tools. Brief Bioinform. 2019;20:1836–52. https://doi.org/10.1093/bib/bby054.
Tiwari A, Mukherjee B, Dixit M. MicroRNA key to angiogenesis regulation: MiRNA biology and therapy. Curr Cancer Drug Targets. 2018;18:266–77. https://doi.org/10.2174/1568009617666170630142725.
Cao T, Zhen XC. Dysregulation of miRNA and its potential therapeutic application in schizophrenia. CNS Neurosci Ther. 2018;24:586–97. https://doi.org/10.1111/cns.12840.
Yan GQ, Wang X, Yang F, et al. MicroRNA-22 promoted osteogenic differentiation of human periodontal ligament stem cells by targeting HDAC6. J Cell Biochem. 2017;118:1653–8. https://doi.org/10.1002/jcb.25931.
Li Z, Sun Y, Cao S, et al. Downregulation of miR-24-3p promotes osteogenic differentiation of human periodontal ligament stem cells by targeting SMAD family member 5. J Cell Physiol. 2019;234:7411–9. https://doi.org/10.1002/jcp.27499.
Xu Y, Ren C, Zhao X, et al. microRNA-132 inhibits osteogenic differentiation of periodontal ligament stem cells via GDF5 and the NF-κB signaling pathway. Pathol Res Pract. 2019;215: 152722. https://doi.org/10.1016/j.prp.2019.152722.
Guo F, Zhu X, Zhao Q, et al. miR-589-3p sponged by the lncRNA TINCR inhibits the proliferation, migration and invasion and promotes the apoptosis of breast cancer cells by suppressing the Akt pathway via IGF1R. Int J Mol Med. 2020;46:989–1002. https://doi.org/10.3892/ijmm.2020.4666.
Lu A, Wang Z, Wang S. Role of miR-589-3p in human lumbar disc degeneration and its potential mechanism. Exp Ther Med. 2018;15:1616–21. https://doi.org/10.3892/etm.2017.5593.
Cesarini V, Silvestris DA, Tassinari V, et al. ADAR2/miR-589-3p axis controls glioblastoma cell migration/invasion. Nucleic Acids Res. 2018;46:2045–59. https://doi.org/10.1093/nar/gkx1257.
Liu K, Cho YY, Yao K, et al. Eriodictyol inhibits RSK2-ATF1 signaling and suppresses EGF-induced neoplastic cell transformation. J Biol Chem. 2011;286:2057–66. https://doi.org/10.1074/jbc.M110.147306.
Rodríguez-Gabriel MA, Burns G, McDonald WH, et al. RNA-binding protein Csx1 mediates global control of gene expression in response to oxidative stress. EMBO J. 2003;22:6256–66. https://doi.org/10.1093/emboj/cdg597.
Wang M, Zhong B, Li M, et al. Identification of potential core genes and pathways predicting pathogenesis in head and neck squamous cell carcinoma. 2021. Biosci Rep. https://doi.org/10.1042/bsr20204148.
Gene Ontology Consortium. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43:D1049-1056. https://doi.org/10.1093/nar/gku1179.
Ye Y, Ke Y. CircRNA FAT1 regulates osteoblastic differentiation of periodontal ligament stem cells via miR-4781-3p/SMAD5 pathway. Stem Cells Int. 2021;2021:5177488. https://doi.org/10.1155/2021/5177488.
Han L, Sheng B, Zeng Q, et al. Correlation between MMP2 expression in lung cancer tissues and clinical parameters: a retrospective clinical analysis. BMC Pulm Med. 2020;20:283. https://doi.org/10.1186/s12890-020-01317-1.
Zhao S, Liao XZ, Wang JX, et al. Transcription factor Atf1 regulates expression of cellulase and xylanase genes during solid-state fermentation of ascomycetes. Appl Environ Microbiol. 2019. https://doi.org/10.1128/aem.01226-19.
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FS and RH was responsible for the cell culture and osteogenic induction. JHZ and TL conducted the PCR and Western blot assay. LJZ and CFS performed statistically analysis. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by The Affiliated Hospital of Hangzhou Normal University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: S1.
Venn diagram revealed the overlapped miRNAs in Targetscan, miRanda and miRDB databases.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, F., He, R., Zhu, J. et al. miR-589-3p promoted osteogenic differentiation of periodontal ligament stem cells through targeting ATF1. J Orthop Surg Res 17, 221 (2022). https://doi.org/10.1186/s13018-022-03000-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-022-03000-z