Abstract
Background
Early-stage lung cancer, primarily treated with surgery, often occur in poor surgical candidates (impaired respiratory function, prior thoracic surgery, severe comorbidities). Stereotactic ablative radiotherapy (SABR) is a non-invasive alternative that provides comparable local control. This technique is particularly relevant for surgically resectable metachronous lung cancer, in patients unable to undergo surgery.. The objective of this study is to evaluate the clinical outcome of patients treated with SABR for stage I metachronous lung cancer (MLC) versus stage I primary lung cancer (PLC).
Patients and methods
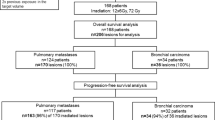
137 patients treated with SABR for stage I non-small cell lung cancer were retrospectively reviewed, of which 28 (20.4%) were MLC and 109 (79.6%) were PLC. Cohorts were evaluated for differences in overall survival (OS), progression-free survival (PFS), metastasis-free survival, local control (LC), and toxicity.
Results
After SABR, patients treated for MLC have comparable median age (76.6 vs 78.6, p = 0.2), 3-year LC (83.6% vs. 72.6%, p = 0.2), PFS (68.7% vs. 50.9%, p = 0.9), and OS (78.6% vs. 52.1%, p = 0.9) as PLC, along with similar rates of total (54.1% vs. 42.9%, p = 0.6) and grade 3 + toxicity (3.7% vs. 3.6%, p = 0.9). Previous treatment of MLC patients was either surgery (21/28, 75%) or SABR (7/28, 25%). The median follow-up was 53 months.
Conclusion
SABR is a safe and effective approach for localized metachronous lung cancer.
Similar content being viewed by others
Background
Lung cancer is the leading cause of cancer death. Despite major progress in the understanding and management of this cancer, it is responsible for more than 33,000 deaths per year in France and 1.8 million deaths per year worldwide [1].
The standard treatment for localized non-small cell lung cancer (NSCLC) is parenchymal resection with lymph node dissection and there is increasing evidence in favor of minimally invasive surgical techniques to reduce operative risks and optimize functional recovery [2].
However, with higher average life expectancies and improved screening techniques (e.g. low dose thoracic computed tomography (CT)), there is an increased incidence of early stage I lung cancer diagnosed in the elderly or higher surgical risk patients [3]. Depending on the series, up to 25% of potentially resectable patients are not operable or refuse surgery [4, 5]. Notably, some patients will present with a second primary localized lung cancer after resection of their prior lung cancer, where operability can be challenging. These second cancers are defined as metachronous when they appear after a cancer free interval from the initial primary, as opposed to synchronous cancers, discovered concomitantly with the first. The cumulative incidence of metachronous lung cancer (MLC) is estimated at 8.3% at 5 years [6]. Although it is clearly accepted that MLC requires an ablative treatment (e.g. surgery, SABR, radiofrequency ablation), the choice of the therapeutic approach is debatable [7,
SABR treatment
The majority of patients were treated with 60 Gy in 3 fractions (n = 116, 84.7%), followed by five fractions regimens of 10 to 15 Gy (n = 21, 15.3%) (Table 2). The median radiation therapy duration was three days and only exceeded one week in 12 (8.8%) patients. The median interval between primary lung cancer to the second metachronous cancer was 39.4 months (13.9–121.4), which were initially treated with resection (n = 21, 75%) or SABR (n = 7, 25%) for their primary lung cancer. GTV and PTV values were significantly smaller (p = 0.007 and p = 0.001 respectively) for MLC when compared to the PLC cohort.
Response and survival data
Overall, 8 (5.8%) patients had a complete response after SABR, 35 (25.5%) had a partial response, 55 (40.1%) had stable disease, 28 (20.4%) had progressive disease, and 11 (8%) patients could not be evaluated due to pneumonitis. Approximately half (n = 66, 48.2%) of patients recurred after SABR, with 30 (21.9%) having local recurrence, 56 (40.9%) having regional recurrence, and 54 (39.4%) having distant recurrence.
The 1-year/3-year LC and RC rate were 83.6%/72.6%, and 73.8%/58.2%, respectively. The 1-year/3-year MFS, PFS, and OS were 76.1%/58.9%, 68.7%/50.9%, and 78.6%/52.1%, respectively.
A total of 85 died (62%), with 7 (5.1%) from unknown cause, 51 (37.2%) experienced cancer specific mortality, 3 (2.2%) attributed to SABR toxicity, and 24 (17.5%) unrelated, of which consisted of 12 (50%) of respiratory failure, 8 (33.3%) of cardiac failure, 3 (12.5%) of cachexia, and 1 (4.2%) of fall trauma.
The median OS was 38 months in the PLC group versus 34 months for MLC (p = 0.9). There was a non-significant increase in cancer specific mortality in the PLC group (40.4% vs. 25%, p = 0.4), when compared to MLC. There was also no significant difference between PLC and MLC, in the 3-year PFS (68.7% vs. 50.9%, p = 0.9), MFS (76.1% vs. 58.9%, p = 0.3), LC (83.6% vs. 72.6%, p = 0.2) and RC (73.8% vs. 58.2%, p = 0.8) (Fig. 1).
Survival analyses (Kaplan–Meier). A Overall survival, B recurrence-free survival, C metastasis-free survival, D local control, E regional control. Solid lines correspond to the stage I primary lung tumor group. Dashed lines correspond to the metachronous tumor group. Cross marks correspond to censored data
Tumor volume is significantly different between the 2 cohorts and could consequently influence tumor control. With a cut-off of 8 mL (median), GTV was not found to be correlated with local control, locoregional control, metastasis-free-survival or progression-free survival (p > 0.05).
We divided the whole group in a group with small GTV (≤ 8 mL) and another one with larger GTV (> 8 mL). The prognostic impact of the cohort (primary versus metachronous) on local control was then analyzed in these 2 groups. No significant correlation was found (p = 0.5 if GTV ≤ 8 mL; p = 0.7 if GTV > 8 mL).
Toxicities data
In the overall cohort, 71/137 (51.8%) patients experienced at least one toxicity attributed to SABR, with 66 (48.2%) grade 1–2 toxicity and 5 (3.6%) grade 3 + toxicity (Table 3).
There were 3 (2.2%) grade 5 toxicities, 2 in the PLC cohort (1 radiation pneumonitis, 1 acute respiratory failure) and 1in the MLC cohort (acute respiratory distress).
There was no significant difference between the PLC and MLC groups in terms of total (54.1% vs. 42.9%, p = 0.6), grade 1 to 2 (50.5% vs. 39.3%, p = 0.6) and grade 3 + toxicities (3.7% vs. 3.6%, p = 0.9).
Discussion
This study showed that SABR used in patients with unresected metachronous stage I lung cancer do not achieve different disease control and survival, when compared to stage I primary lung cancer, and do not lead to significant increase in toxicity. Matthiesen et al. [17] had also highlighted the value of this therapeutic approach in patients with a second lung cancer, but only one patient had a metachronous cancer in this cohort. There is also evidence that SABR treatment for metachronous cancer was associated with greater OS than patients treated for a synchronous cancer, but they did not compare survival data to a control population with stage I primary lung cancer [13].
Our data is consistent with prior prospective data, [18] which showed that patients treated with SABR for metachronous cancer had equivalent or even higher OS than those treated for stage I primary lung cancer. However, it should be noted that the OS of these patients were calculated from the primary cancer and not the diagnosis of their metachronous cancer, which causes a survivor bias. Since this bias is closely related to the stage of diagnosis, we chose to include only patients with stage I lung cancer. To our knowledge, only four studies evaluating SABR treatment of a second lung cancer analyzed survival from the second cancer rather than the first [14, 17,18,19], of which only 2 studies [17, 18] included patients with metachronous lung cancer. Studying this particular population is of major importance, indeed the treatment planning of previously irradiated patients represents a challenge regarding dosimetry constraints and patients’ therapeutic adhesion.
Of note, we underline an important limitation of this work related to the small number of patients in the MLC cohort which would require a larger cohort to strengthen these results and ensure the absence of statistical difference in outcomes of patients. Indeed, the local control curve in the MLC cohort appears to shift to the advantage of PLC cohort; an increase in study power could potentially reveal a statistical signal. In our study, local control of MLC was 72.6%, which is lower than a previous work [18] reporting at least 90.3% of local control in these patients, however the definition of metachronous patients included in this study is not as we defined in our study; indeed, we used a restrictive definition of metachronous patients.
The discovery of a lung nodule or mass in a patient previously treated for lung cancer is a situation that is not uncommon, with an incidence of a second lung cancer estimated to be between 1.5 and 3% per year per patient [26], especially in active smokers. If intra-lobar resection confers excellent results and is both therapeutic and diagnostic for operable patients [35].
Conclusion
In conclusion, our results show that SABR is safe and effective treatment for stage I metachronous lung cancer, with outcomes not significatively different to primary stage I lung cancer, with the proviso of a limited power in this present study. With over 4 years of follow-up, utilizing the follow-up from the diagnosis of the second metachronous cancer to avoid survivor bias, this study allows a fair representation between the long-term outcomes of primary versus metachronous NSCLC. However, these findings must be handled with the limitations related to the retrospective nature of our study, the bias of classification of oligometastatic versus metachronous lung cancer, and limited power due to a small number of patients with metachronous lung cancer.
Larger scale studies are required to confirm these results.