Abstract
Background
We aimed to evaluate the optimal management for elderly patients with nasopharyngeal carcinoma (NPC) with intensity-modulated radiotherapy (IMRT).
Methods
A total of 283 elderly patients with NPC diagnosed from 2015 to 2019 were enrolled in the study. Overall survival (OS) was the primary endpoint. Univariate and multivariate Cox regression analyses were preformed to identify potential prognostic factors. The recursive partitioning analysis (RPA) was used for risk stratification. Kaplan-Meier survival curves were applied to evaluate the survival endpoints, and log-rank test was utilized to assess differences between groups. The prognostic index (PI) was constructed to further predict patients’ prognosis displayed by nomogram model. The area under the receiver operating characteristic (ROC) curves (AUC) and the calibration curves were applied to assess the effectiveness of the model.
Results
Based on RPA-based risk stratification, we demonstrated that elderly NPC patients who were treated with IC followed by RT had similar OS as those with induction chemotherapy (IC) combined with concurrent chemoradiotherapy (CCRT) in the middle- (stage I-III and pre-treatment EBV > 1840 copies/ml) and high-risk groups (stage IVA). IMRT alone may be the optimal treatment option for the low-risk group (stage I-III with pre-treatment EBV ≤ 1840 copies/ml). We established an integrated PI which was indicted with stronger prognostic power than each of the factors alone for elderly NPC patients (The AUC of PI was 0.75, 0.80, and 0.82 for 1-, 3-, 5-year prediction of OS, respectively).
Conclusion
We present a robust model for clinical stratification which could guide individual therapy for elderly NPC patients.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC), a highly aggressive malignancy, is associated with unique epidemiological and geographical attributes, primarily prevalent in Southern China [24]. The final PI was selected based on the smallest AIC. A nomogram was presented using the “rms” R package to visualize the score of each parameter on the point scale. The area under the receiver operating characteristic (ROC) curves (AUC) and the calibration curves were depicted to validate the prediction accuracy of the PI for 1/3/5-year OS. The calibration curves close to ideal line was considered as the best prediction, and an AUC value more than 0.7 was seen to be significant predictive performance.
Statistics
Survival outcomes were assessed using Kaplan-Meier survival analysis, and differences between groups were compared using the log-rank test. Pairwise comparisons between groups were carried out using the Bonferroni test. R software (https://www.r-project.org, v3.6.2) and SPSS Statistics v25.0 were conducted for all statistical analysis. A P-value < 0.05 was deemed statistically significant, unless otherwise indicated.
Results
Patient characteristics and survival
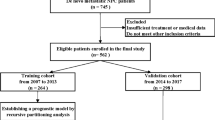
Totally 354 elderly patients were diagnosed with NPC in our center from January 2015 to December 2019. Among them, 7 cases received previous anti-tumor treatment, 12 cases received palliative treatment, 28 cases abandoned or interrupted treatment. thus excluding them from this study. Then 307 patients completed radical treatment based on IMRT in our center. After further exclusion of 6 patients who were lost to follow-up, 16 with confirmed distant metastasis, and 2 with controversial metastasis, we were left with a cohort of 283 patients. These patients were between the ages of 65 and 87, and included 203 males and 80 females. The median NLR was 2.16 (range 0.61–7.84). The range of ALBI for the whole cohort was − 3.62 to -1.60 (median = −2.70). The median pre-treatment plasma EBV DNA level was 2310 copies/mL with a range of 0-9.85×105 copies/mL. Treatment regimens for the cohort were as follows: 57 (20.14%) patients were treated with RT alone, 82 (28.98%) patients received IC followed by RT, 29 (10.25%) patients received CCRT, and 115 (40.64%) patients received IC combined with CCRT. Table 1 showed the baseline characteristics of the cohort.
Median follow-up time was 35.7 months (range 2–84 months). In total, 54 (19.08%) patients died, 18 (6.36%) patients experienced local or regional recurrence, 19 (6.71%) developed distant failure, 70 (24.73%) suffered disease progression at their last follow-up. Estimated 3-year OS, PFS, LRFS and DMFS rates were 82.35%, 76.47%, 79.61% and 80.58%, respectively (Supplementary Fig. S2).
Identification of prognostic factors
We evaluated several parameters to identify potential prognostic factors. Among these variables, it revealed that age (HR: 1.098, 95%CI: 1.051–1.147, P < 0.001), NLR (HR: 1.566, 95%CI: 1.266–1.937, P < 0.001), ACCI (HR: 1.321, 95%CI: 1.025–1.704, P = 0.032), T classification (HR: 1.699, 95%CI: 1.235–2.335, P = 0.001), clinical stage (HR: 1.356, 95%CI: 1.522–3.647, P < 0.001), IC (HR: 0.495, 95%CI: 0.289–0.847, P = 0.010), and pre-treatment plasma EBV DNA levels (HR: 1.000, 95%CI: 1.000–1.000, P < 0.001) significantly affected OS in univariate analysis (Fig. 1). Multivariate analysis further indicated that a higher NLR (HR: 1.431, 95%CI: 1.135–1.803, P = 0.002), stage IVA (HR: 2.876, 95%CI: 1.453–5.696, P = 0.002), and higher pre-treatment plasma EBV DNA levels (HR: 1.000, 95%CI: 1.000–1.000, P < 0.001) were poorer independent risk factors for OS. Conversely, IC was an independent protective factor for OS (HR: 0.275, 95%CI: 0.132–0.577, P = 0.001) .
Univariate and multivariate analysis of prognostic factors for overall survival. HR, hazard ratio; CI, confidence interval; ALBI, albumin-bilirubin grade; NLR, neutrophil-to-lymphocyte ratio; ACCI, age-adjusted Charlson comorbidity index; EBV, Epstein-barr virus; EBV DNApre, pre-treatment EBV DNA level. a The HR was > 1.000. The red dot represents the significant prognostic factor (P < 0.05); Blue dot represents non-significant factor
RPA risk stratification and subgroup analysis
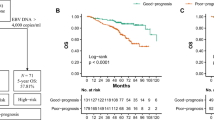
We selected 3 pre-treatment independent prognostic predictors including NLR, clinical stage, and pre-treatment plasma EBV DNA levels for risk stratification based on OS. Finally, we established a risk model by the RPA algorithm which consisted of 3 groups, with 87 (30.74%), 88 (31.10%), and 108 (38.16%) patients assigned to low-, middle- and high-risk groups, respectively (Fig. 2). The low-risk group refers to patients in stage I-III with pre-treatment EBV DNA levels ≤ 1840 copies/mL. The middle-risk group refers to patients in stage I-III with pre-treatment EBV DNA levels > 1840 copies/mL. The high-risk group refers to patients in stage IVA. The RPA-based risk model showed satisfactory prognostic value for OS of elderly NPC patients as shown in Fig. 3A (high-risk group vs. low-risk group: HR: 8.847, 95%CI: 4.760-16.442, P < 0.001; high-risk group vs. middle-risk group: HR: 2.442, 95%CI: 1.402–4.251, P = 0.010; middle-risk group vs. low-risk group: HR: 3.577, 95%CI: 1.420–9.012, P = 0.047). There was also a significant difference in LRFS among the three risk groups (high-risk group vs. low-risk group: HR: 6.609, 95%CI: 3.708–11.781, P < 0.001; high-risk group vs. middle-risk group: HR: 3.139, 95%CI: 1.410–6.989, P = 0.030; middle-risk group vs. low-risk group: HR: 2.112, 95%CI: 1.262–3.535, P = 0.021; Fig. 3C). In addition, patients in the high-risk group had poorer PFS (high-risk group vs. low-risk group: HR: 5.195, 95%CI: 3.000-8.995, P < 0.001; high-risk group vs. middle-risk group: HR: 2.158, 95%CI: 1.311–3.550, P = 0.013, Fig. 3B) and DMFS (high-risk group vs. low-risk group: HR: 6.304, 95%CI: 3.514–11.309, P < 0.001; high-risk group vs. middle-risk group: HR: 2.479, 95%CI: 1.454–4.227, P = 0.006, Fig. 3D) than those in the low- and middle-risk groups.
We investigated the survival benefits of different treatment strategies for elderly NPC patients. It suggested that patients who received with IC + CCRT achieved higher OS as compared to those treated with RT alone (IC + CCRT vs. RT, HR: 3.143, 95%CI: 1.550–6.373, P = 0.002). However, the survival benefits of IC + RT and CCRT were not noted than RT alone (IC + RT vs. RT, HR: 2.393, 95%CI: 1.211–4.726, P = 0.054; CCRT vs. RT, HR: 3.787, 95%CI: 1.638–8.757, P = 0.121; Fig. 4A). No significant differences were found between IC + RT, CCRT and IC + CCRT group, with P > 0.05 for each of the two groups. Further analysis was performed to explore the survival benefits of treatment within three subgroups based on RPA risk model. Given the limited number of patients treated with CCRT, they weren’t included in further analysis. In the low-risk group, no significant difference in OS was found between patients with RT, IC + RT or IC + CCRT (Ps > 0.05, Fig. 4B), indicating that elderly NPC patients in this subgroup could achieve sufficient survival benefits from RT alone. In the middle-risk group, patients treated with IC + RT and IC + CCRT achieved higher OS compared to those with RT alone, which was similar to the result observed in the high-risk group (Ps < 0.05). However, no significant difference was found between patients treated with IC + RT and IC + CCRT in these two groups (Ps > 0.05, Figs. 4C and 4D).
Construction of nomogram model for OS in elderly NPC patients
To further predict patient outcomes, we enrolled all relevant prognosis factors, including age, gender, NLR, ACCI, ALBI, T stage, N stage and RPA grou** to construct a prognostic model. Finally, the PI was developed utilizing age, gender, NLR and RPA grou** for robust clinical stratification. A nomogram showed the individual assessment based on the PI that is tailored to each elderly NPC patient (Fig. 5A). The PI score was calculated based on the score of each prognostic variable, which also enabled estimation of individual probabilities for 1-, 3-, and 5-year OS. The calibration curves showed the good performance of the nomogram (Fig. 5B). Additionally, a comparative analysis was performed on the prognostic performance of the PI in comparison to other factors, which showed that when combined with RPA grou**, our PI presented a superior prognostic discriminatory power when compared to other individual indicators in terms of predicting the OS of elderly NPC. The AUC of PI was determined to be 0.75, 0.80, and 0.82 for 1-, 3-, and 5‐year OS prediction, respectively, thus significantly enhancing the predictive performance when compared to RPA grou**, age, gender, NLR, stage, and pre-treatment EBV DNA levels (Figs. 5C-5E).
Construction and validation of the predictive model in elderly NPC patients. (A) Nomogram model established by prognostic index to estimate the 1-, 3- and 5-year survival possibility. (B) The calibration curves for validation of the nomogram. (C-E) The ROC curves comparing the accuracy of PI, RPA grou** and the other prognosis factors for predicting 1-, 3- and 5-year survival rate. NLR, neutrophil-to-lymphocyte ratio; RPA, recursive partitioning analysis; AUC, the area under the receiver operating characteristic curves; CI, confidence interval; PI, prognostic index; EBV, Epstein-barr virus; EBV DNApre, pre-treatment EBV DNA level. TPR, true positive rate; FPR, false positive rate
Discussion
In current study, we developed an RPA-based risk stratification which integrated clinical stage and pre-treatment EBV DNA level for elderly NPC patients who underwent IMRT. It was shown that patients in the high-risk group exhibit inferior OS, PFS, LRFS and DMFS compared to those in the low- and middle-risk groups. Our findings revealed that patients in the low-risk group do not benefit from additional IC or concurrent chemotherapy compared to RT alone, whereas IC followed by RT or IC combined with CCRT may be the optimal treatment opinions for patients in the middle- and high-risk groups. In addition, we established an integrated RPA-based PI for elderly NPC, which exhibits superior prognostic performance compared to other single factor.
Given the aging population in China, the burden of NPC is growing [25]. Some characteristics of NPC display regional variations [2]. Over he age of 65 represents the second peak of the increasing incidence of NPC in low-incidence regions [26]. Epidemiological studies have revealed that the proportion of elderly NPC patients (≥ 60 years old) is 35.0% in non-endemic areas, which is higher than that in endemic areas (13.8%) [27, 28]. Management of IMRT for elderly NPC patients remains challenges. Some retrospective studies have suggested that RT combined with chemotherapy could improve survival rates in elderly NPC patients receiving conventional RT [27]. Conversely, others have argued that chemotherapy may not provide additional more survival benefits and may even bring more therapeutic toxicity in elderly NPC patients in the IMRT era [29]. In a comparison between CCRT and RT alone, grade 3–4 severe mucositis and dermatitis were observed more frequently in the former, and comorbidities were found to increase the likelihood of severe toxic reactions [4]. A study conducted by Wen et al. also demonstrated that CCRT was associated with higher rates of hematological adverse reactions, such as leukopenia, neutropenia, and thrombocytopenia [30]. Additionally, Ou et al. observed that patients receiving IC combined with CCRT had lower rates of grade 3–4 late toxicities compared to those receiving CCRT alone in non-endemic areas [31]. However, in an analysis of NPC patients over 60 years old, the addition of IC did not significantly affect survival CCRT, but instead increased grade 3 to 4 acute toxicities [9]. The differences in the pharmacokinetics and pharmacodynamics of chemotherapeutic agents in patients of different ages, with the same drugs tending to have a higher toxicity profile in the elderly [32]. Thus, reducing treatment-related toxicity is a focus of concern in treatment decisions [33, 34]. However, we lacked the data on treatment-related toxicities in this study. In terms of efficacy, our study showed that the survival benefits of IC prior to RT was comparable to that of IC combined with CCRT as a first-line treatment in selected middle- and high-risk patients. A less intensive treatment regimen was warranted for low-risk patients. However, we didn’t find the benefits of CCRT in elderly patients with NPC, which is consistent with previous reports [35]. Furthermore, multivariate analyses indicated that IC was an independent protective factor for OS, while concurrent chemotherapy was not. This outcome may be attributed to the greatly enhanced curative effect of IMRT on NPC [36]. In addition, elderly patients may have lower tolerance owing to multiple comorbidities, poor organ functional status and performance status [11]. Thus, the magnitude of the impact of concurrent chemotherapy may be limited.
Up to now, only a limited number of studies have evaluated clinical endpoints in elderly NPC patients, and none have taken into account risk stratification [9, 12, 35]. The RPA model has been widely used in many malignancies for prognosis stratification according to homogeneous survival performance [37]. Notably, we considered both anatomical prognostic factors (i.e. AJCC stage) and some non-anatomic one, such as plasma EBV DNA level and parameters about geriatric assessment, which were closely associated with survival outcomes of elderly NPC patients. Therefore, taking prognostic factors before treatment into consideration, our RPA model considered clinical stage as the first split and pre-treatment EBV DNA level as the second split. It has been demonstrated the value of EBV DNA in NPC prognostication and as an important complement to the AJCC staging, which contributing to stratify patients with risk subgroups [38,39,40]. However, the optimal cut-off value of pre-treatment EBV DNA remains controversial. Some studies suggest that levels above 4000 copies/mL or 8000 copies/mL are predictive of poor prognosis, others report levels below 1500 copies/mL as prognostically significant in elderly NPC patients [41,42,43]. In our study, we found that a pre-treatment EBV DNA cut-off value of 1840 copies/mL was a good predictor of prognostic stratification for elderly NPC patients with clinical stage I to III, close to the results of Guo et al., who obtained 2000 copies/mL of EBV DNA level before treatment by using RPA analysis [38].
In terms of heterogeneity in this population, the assessment of prognosis remains challenging in elderly NPC patients. Age is known to affect various aspects of tumor, including its growth mode, genomic stability, protein homeostasis, tissue repair ability, metabolism, intercellular communication, etc. [44]. Prior research has shown that old age is an adverse factor for survival and an independent risk factor for lethal nasopharyngeal necrosis after NPC re-irradiation [45, 46]. However, our study findings suggest that age may not be a strong prognostic factor in the elderly population, which is consistent with the findings of Chan et al. and Li et al. [47, 48]. Age alone should not be the sole basis for treatment decisions in elderly NPC patients, as other factors such as functional status, comorbidity, nutrition, and chronic inflammation may also impact prognosis [49]. Comorbidity is an important prognostic factor for elderly patients, and it may contribute to patient weakness, delay in treatment completion, and worsening of treatment-related toxicity [13]. The ACCI has been shown to predict survival and influence clinical manifestation, therapeutic interventions, and outcomes of NPC patients after RT [19]. The prognostic value of inflammatory markers have also been identified in various cancers [21]. A high NLR has been found to be associated with frailty in the elderly with cancer, indicating a decrease in physiologic reserve and an increase in vulnerability to multiple organ systems, which leads to poor health outcomes [50]. Although the predictive model based on clinical parameters has been shown to have favorable discriminative performance, it is suggested that more biomarkers should be taken into account in elderly patients. Future studies should consider the comprehensive and professional geriatric assessment, which could better strengthen the management of elderly patients and inform clinical decision making for this patient population. Our results demonstrated that the newly integrated PI, which combined the RPA model and additional biomarkers, had greater predictive power than using each biomarker separately. These findings suggest that the PI could serve as a satisfactory predictive indicator in elderly NPC patients.
In this study, we construct a risk stratification model to explore the optimal treatment strategy for elderly NPC patients. The inclusion of a large number of recently treated patients within a relatively short timeframe may be one of the main strengths of our study, which can provide valuable insights into current treatment practices and outcomes in clinical practice. Nonetheless, we acknowledge that there are several limitations to our study. Firstly, it is a retrospective analysis, we only chose OS as the primary clinical outcome to avoid the possible bias from changes in the follow-up programme. Secondly, comprehensive geriatric assessments by geriatricians were not available for our patients, but we attempted to consider several aspects that could impact the elderly. Thirdly, we recognize that biases related to heterogeneity in chemotherapy prescription, including the diversity of treatment regimens and chemotherapy cycles, are likely to persist. Furthermore, due to the limited number of cases with CCRT alone, we didn’t analyze these patients separately, and a larger sample size is required to explore the long-term outcomes of this patient population. Finally, limited to the retrospective nature, this study lacked information on treatment-related adverse effects for different treatment methods.
Conclusions
In conclusion, we developed an RPA model for risk stratification and our constructed PI is robust in predicting survival in elderly patients with NPC which could serve as a tool in treatment decision-making for physicians.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NPC:
-
Nasopharyngeal carcinoma
- RT:
-
Radiotherapy
- IMRT:
-
Intensity-modulated radiotherapy
- IC:
-
Induction chemotherapy
- CCRT:
-
Concurrent chemoradiotherapy
- EBV:
-
Epstein-barr virus
- RPA:
-
Recursive partitioning analysis
- PI:
-
Prognostic index
- OS:
-
Overall survival
- KPS:
-
Karnofsky performance score
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- ECT:
-
Emission computed tomography
- qPCR:
-
Quantitative polymerase chain reaction
- SIB:
-
Simultaneous integrated boost
- OARs:
-
Organs at risk
- GTV:
-
Gross tumor volume
- CTV:
-
Clinical target volume
- PTV:
-
Planning target volume
- ACCI:
-
Age-adjusted Charlson comorbidity index
- NLR:
-
Neutrophil-lymphocyte ratio
- ALBI:
-
Albumin-bilirubin
- PFS:
-
Progression-free survival
- LRFS:
-
Locoregional relapse-free survival
- DMFS:
-
Distant metastasis-free survival
- CART:
-
Classification and regression tree
- AIC:
-
Akaike information criterion
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the ROC curves
References
Zhang LF, Li YH, **e SH, et al. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period-cohort analysis. Chin J Cancer. 2015;34(8):350–7.
Bossi P, Chan AT, Licitra L, et al. Nasopharyngeal carcinoma: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(4):452–65.
Oubaya N, Soubeyran P, Reinald N, et al. Prognostic value of routinely measured inflammatory biomarkers in older cancer patients: Pooled analysis of three cohorts. Cancers. 2021;13(24):6154.
Lyu Y, Ni M, Zhai R, et al. Clinical characteristics and prognosis of elderly nasopharyngeal carcinoma patients receiving intensity-modulated radiotherapy. Eur Arch Otorhinolaryngol. 2021;278(7):2549–57.
Wong KCW, Hui EP, Lo KW, et al. Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol. 2021;18(11):679–95.
Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–55.
VanderWalde NA, Fleming M, Weiss J, Chera BS. Treatment of older patients with head and neck cancer: a review. Oncologist. 2013;18(5):568–78.
Chan AT, Grégoire V, Lefebvre JL, et al. Nasopharyngeal cancer: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii83–5.
Cao C, Hu Q, Chen X. Intensity-modulated radiotherapy for elderly patients with nasopharyngeal carcinoma. Head Neck. 2018;40(3):590–5.
Wang C, Tang X, Wang J, et al. Induction chemotherapy plus concurrent chemoradiotherapy vs concurrent chemoradiotherapy in elderly patients with advanced nasopharyngeal carcinoma. Otolaryngol Head Neck Surg. 2017;157(2):233–8.
Amini A, Morris L, Ludmir EB, et al. Radiation therapy in older adults with cancer: A critical modality in geriatric oncology. J Clin Oncol. 2022;40(16):1806–11.
Zhang Y, Yi JL, Huang XD, et al. Inherently poor survival of elderly patients with nasopharyngeal carcinoma. Head Neck. 2015;37(6):771–6.
Huang Y, Chen W, Haque W, et al. The impact of comorbidity on overall survival in elderly nasopharyngeal carcinoma patients: a National Cancer Data Base analysis. Cancer Med. 2018;7(4):1093–101.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10(3):319–29.
Soto-Perez-de-Celis E, Li D, Yuan Y, et al. Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol. 2018;19(6):e305–16.
Shao JY, Li YH, Gao HY, et al. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004;100(6):1162–70.
Radiation therapy oncology group protocol 02–25. Available at: http://www.rtog.org/members/protocols/0225/0225.pdf [accessed 08.26.08].
Yang CC, Chen PC, Hsu CW, et al. Validity of the age-adjusted charlson comorbidity index on clinical outcomes for patients with nasopharyngeal cancer post radiation treatment: a 5-year nationwide cohort study. PLoS ONE. 2015;10(1):e0117323.
Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–51.
Nishijima TF, Deal AM, Lund JL, et al. Inflammatory markers and overall survival in older adults with cancer. J Geriatr Oncol. 2019;10(2):279–84.
Takada K, Takamori S, Shimokawa M, et al. Assessment of the albumin-bilirubin grade as a prognostic factor in patients with non-small-cell lung cancer receiving anti-PD-1-based therapy. ESMO open. 2022;7(1):100348.
Sun XS, Zhu MY, Wen DX, et al. Establishment and validation of a recursive partitioning analysis based prognostic model for guiding re-radiotherapy in locally recurrent nasopharyngeal carcinoma patients. Radiother Oncol. 2022;168:61–8.
Royston P, Moons KG, Altman DG, et al. Prognosis and prognostic research: Develo** a prognostic model. BMJ. 2009;338:b604.
Leu YS, Chang YF, Lee JC, et al. Prognosis of nasopharyngeal carcinoma in the elderly is worse than in younger individuals-experience of a medical institute. Int J Gerontol. 2014;8(2):81–4.
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Zeng Q, **ang YQ, Wu PH, et al. A matched cohort study of standard chemo-radiotherapy versus radiotherapy alone in elderly nasopharyngeal carcinoma patients. PLoS ONE. 2015;10(3):e0119593.
Wu SG, Liao XL, He ZY, et al. Demographic and clinicopathological characteristics of nasopharyngeal carcinoma and survival outcomes according to age at diagnosis: A population-based analysis. Oral Oncol. 2017;73:83–7.
Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403.
Wen YF, Sun XS, Yuan L, et al. The impact of Adult Comorbidity Evaluation-27 on the clinical outcome of elderly nasopharyngeal carcinoma patients treated with chemoradiotherapy or radiotherapy: A matched cohort analysis. J Cancer. 2019;10(23):5614–21.
Ou D, Blanchard P, El Khoury C, et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016;62:114–21.
Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology summary. J Oncol Pract. 2018;14(7):442–6.
DE Sanctis V, Belgioia L, Cante D, et al. Lactobacillus brevis CD2 for prevention of oral mucositis in patients with head and neck tumors: A multicentric randomized study. Anticancer Res. 2019;39(4):1935–42.
Orlandi E, Iacovelli NA, Tombolini V, et al. Potential role of microbiome in oncogenesis, outcome prediction and therapeutic targeting for head and neck cancer. Oral Oncol. 2019;99:104453.
Mi JL, Meng YL, Wu HL, et al. Comparison of intensity-modulated radiation therapy alone vs. intensity-modulated radiation therapy combined with chemotherapy in elderly nasopharyngeal carcinoma patients (aged > 65 years). Strahlenther Onkol. 2020;196(3):270–9.
Lai SZ, Li WF, Chen L, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80(3):661–8.
** M, Liao Z, Deng W, et al. Recursive partitioning analysis identifies pretreatment risk groups for the utility of induction chemotherapy before definitive chemoradiation therapy in esophageal cancer. Int J Radiat Oncol Biol Phys. 2017;99(2):407–16.
Guo R, Tang LL, Mao YP, et al. Proposed modifications and incorporation of plasma Epstein-Barr virus DNA improve the TNM staging system for Epstein-Barr virus-related nasopharyngeal carcinoma. Cancer. 2019;125(1):79–89.
Huang CL, Sun ZQ, Guo R, et al. Plasma Epstein-Barr virus DNA load after induction chemotherapy predicts outcome in locoregionally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2019;104(2):355–61.
Liu LT, Tang LQ, Chen QY, et al. The prognostic value of plasma Epstein-Barr viral DNA and tumor response to neoadjuvant chemotherapy in advanced-stage nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2015;93(4):862–9.
Wu Y, Yang K, Huang Y, et al. Selection and validation of chemotherapy beneficiaries among elderly nasopharyngeal carcinoma (NPC) patients treated with intensity-modulated radiation therapy (IMRT): A large real-world study. Radiat Oncol. 2022;17(1):138.
Chai SJ, Pua KC, Saleh A, et al. Clinical significance of plasma Epstein-Barr Virus DNA loads in a large cohort of Malaysian patients with nasopharyngeal carcinoma. J Clin Virol. 2012;55:34–9.
Wang WY, Twu CW, Chen HH, et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer. 2013;119:963–70.
Havas A, Yin S, Adams PD. The role of aging in cancer. Mol Oncol. 2022;16(18):3213–9.
Li YQ, Tian YM, Tan SH, et al. Prognostic model for stratification of radioresistant nasopharynx carcinoma to curative salvage radiotherapy. J Clin Oncol. 2018;36(9):891–9.
Ng WT, Wong ECY, Cheung AKW, et al. Patterns of care and treatment outcomes for local recurrence of NPC after definite IMRT-A study by the HKNPCSG. Head Neck. 2019;41(10):3661–9.
Chan JY, Wong ST, Kwan WH, et al. Prognostic factors for survival and electroglottographic changes in elderly nasopharyngeal carcinoma patients: a retrospective cohort study. Ann Oncol. 2018;29(6):1431–7.
Li X, Wu X, Li S, et al. Age is not an independent prognostic factor in nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2019;145(4):1015–24.
Devons CA. Comprehensive geriatric assessment: making the most of the aging years. Curr Opin Clin Nutr Metab Care. 2002;5(1):19–24.
Nishijima TF, Deal AM, Williams GR, et al. Frailty and inflammatory markers in older adults with cancer. Aging. 2017;9(3):650–64.
Acknowledgements
Not applicable.
Funding
This work was supported by the grants of Science and Technology Program of Fujian Province, China (2018Y2003); Fujian Provincial Clinical Research Center for Cancer Radiotherapy and Immunotherapy (2020Y2012); Supported by the National Clinical Key Specialty Construction Program (2021); Fujian Clinical Research Center for Radiation and Therapy of Digestive, Respiratory and Genitourinary Malignancies; National Natural Science Foundation of China (82072986); Major Research Projects for Young and Middle-aged Health Researchers of Fujian Province, China (2021ZQNZD010); Joint Funds for the Innovation of Science and Technology, Fujian Province (2021Y9196); Innovative Medicine Subject of Fujian Provincial Health Commission, China (2021CXA029); Science and Technology Pilot Program of Fujian Province, China (2021Y0053); and High-level Talent Training Program of Fujian Cancer Hospital (2022YNG07).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Lu J, Hu D, and Qiu S contributed to design of the research. Material preparation was performed by Chen X. Data collection was performed by Cai S and Ding Q. Pan Y and Wu Z contributed to data analysis. The first draft of the manuscript was written by Li Y, Weng Y, and Huang Z and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Retrospective ethical approval was obtained from the Ethics Committee (K2022-203-01) and, wherever applicable, patients consented to retrospective data collection including use of encoded data for publication.
Consent for publication
Informed consent was obtained from the patients for publication of this report.
Competing interests
The author reports no competing interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Weng, Y., Huang, Z. et al. Prognostic model on overall survival in elderly nasopharyngeal carcinoma patients: a recursive partitioning analysis identifying pre-treatment risk stratification. Radiat Oncol 18, 104 (2023). https://doi.org/10.1186/s13014-023-02272-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02272-x