Abstract
Purpose
Using real-world evidence, this study aimed to identify elderly nasopharyngeal carcinoma (NPC) patients who would benefit from chemotherapy.
Methods and materials
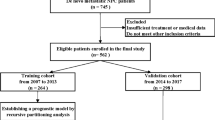
1714 elderly NPC patients between April 2007 and December 2017 were identified. Recursive partitioning analysis (RPA) was used to generate risk-stratified outcomes. Prognostic factors were performed for individual comparisons of different risk groups to assess chemotherapy benefits.
Results
The median follow-up was 59.3 (0.39–170.09) months. Epstein Barr virus (EBV) DNA and T stage were included in the RPA-generated risk stratification, categorizing patients into a good-prognosis group (EBV DNA ≤ 4000 copies/mL & T1–2), and a poor-prognosis group (EBV DNA ≤ 4000 copies/mL & T3–4 and EBV DNA > 4000 copies/mL & any T). Overall survival (OS) was significantly higher in the good-prognosis group compared with the training set (HR = 0.309, 95% CI 0.184–0.517; P < 0.001), and validated in the testing set (HR = 0.276, 95% CI 0.113–0.670; P = 0.002). In the poor-prognosis group, a significantly improved OS for chemoradiotherapy (CRT) compared with RT alone was observed (HR = 0.70, 95% CI 0.55–0.88; P = 0.003). Patients who received induction chemotherapy (IC) + concurrent chemoradiotherapy (CCRT) and CCRT had a significantly improved OS compared with RT alone (IC + CCRT vs. RT alone: P = 0.002; CCRT vs. RT alone: P = 0.008) but not in the IC + RT group (P = 0.306). The 5-year OS for CRT versus RT-alone with ACE-27 scores of 0, 1 and 2 were 76.0% versus 70.0% (P = 0.014), 80.5% versus 68.2% (P = 0.150) and 58.5% versus 62.2% (P = 0.490), respectively; for those aged 60–64, 65–70 and ≥ 70 years old they were 80.9% versus 75.9% (P = 0.068), 73.3% versus 63.4% (P = 0.270) and 64.8% versus 67.1% (P = 0.820), respectively.
Conclusions
For elderly NPC patients a simple screening cutoff for chemotherapy beneficiaries might be EBV DNA < 4000 copies/ml & T3–4 and EBV DNA ≥ 4000 copies/ml & any T, but not for those > 70 years old and with an ACE-27 score > 1. IC + CCRT and CCRT were effective forms of chemotherapy.
Similar content being viewed by others
Introduction
Nasopharyngeal carcinoma (NPC) is rare throughout the world, yet it is particularly prevalent in southern China, with the annual incidence nearly half of the global incidence [1, 2]. Radiotherapy (RT) is the primary curative treatment for NPC. Intensity-modulated radiotherapy (IMRT) is a relatively new RT technique that significantly improves local tumor control and reduces RT-related adverse events. It has been used in NPC patients for more than ten years [3,Follow-up Follow-up was measured from the day of the first-treatment up until to the last visit or death from any cause. All patients were followed up every 3 months during the first two years, every 6 months for first three years, and then annually thereafter. Each follow-up included a complete physical and an electronic nasopharyngoscopy examination. Biochemical profiles, plasma EBV DNA levels, chest X-ray/CT, abdominal ultrasound/CT, and MRI of the nasopharynx and neck were routinely performed. Additional tests were arranged when clinically indicated. PET-CT or a whole-body bone scan, or if possible, biopsy was recommended in patients with suspected clinical recurrence or metastasis. The main endpoint was OS, which was defined as the time from the first-treatment to death due to any-cause or the latest date that the patient was alive. The secondary endpoints were DSS (from the date of the first-treatment to tumor-cause mortality), DMFS (from the date of the first-treatment to the first distant relapse date), and LRRFS (from the date of the first-treatment to the first local/regional relapse). SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) and R version 4.0.4 (http://www.r-project.org/) were used to perform the statistical analyses and generate the figures. Continuous variables including hemoglobin (Hb), albumin, lactate dehydrogenase (LDH), and C-reactive protein (CRP) were transformed into categorical variables based on the clinical cut-off values. The pre-treatment plasma EBV DNA level was divided at the optimal cut-off determined by the receiver-operating characteristic (ROC) curve analysis. Categorical variables were described by frequency and percentage and compared using the Pearson chi-square test, while continuous variables were described by mean and standard deviation and compared using the Kruskal–Wallis rank sum test. Survival rates were calculated and compared using the Kaplan–Meier curve and log-rank tests, respectively. Univariate and multivariate Cox regression were adopted to estimate the effect of the variables on OS, DSS, DMFS and LRRFS. Predictors with statistical significance in the univariate analysis were entered into the multivariate analysis. Statistically significant predictors for 5-year OS in the multivariate Cox regression were included in the RPA analysis using the rpart package in R to stratify patients into risk groups with significantly different prognoses [24]. Excessive branches of RPA-generated risk stratification were removed using the prune function in the rpart package for realistic clinical application [24]. The survival of patients treated with CRT or RT alone in each risk group was compared to investigate chemotherapy benefits among patients in different risk groups. Subgroup analyses stratified by age, ACE-27, and smoking were performed to explore differences in chemotherapy benefits according to these variables in different risk groups. All tests were two-sided; a P < 0.05 was significant.Statistical analysis
Results
From April 2007 to December 2017, 1,714 eligible patients were included in this study. The median age was 64 years old (range 60–85 years), with a sex (M/F) ratio of 3.16:1.00. Non-keratinizing undifferentiated NPC (WHO type III) accounted for the majority of cases (97.8%). 38.6% of patients had pre-treatment plasma EBV DNA levels greater than 4000 copies/mL. The proportion of patients classified as stage I, II, III and IV was 4.5%, 14.1%, 46.9% and 34.5%, respectively. There were 835 patients with an ACE-27 score of 0, 741 patients with a score of 1 and 138 patients a score of 2–3. Most patients underwent IC + CCRT (26.8%) or CCRT (36.9%), 10.0% underwent IC + RT, and 26.3% were treated with RT alone. The baseline characteristics are shown in Table 1.
The median follow-up time was 59.3 months (range 0.39–170.09 months), 118 (6.9%) patients developed locoregional recurrence, 189 (11.0%) had distant metastasis and 33 (1.9%) had both. 463 (27.0%) patients died, the causes of death were tumor recurrence in 287 (16.7%) patients, complications after radiotherapy in 22 (1.3%) patients, non-NPC-related causes in 149 (8.7%) patients, and unknown cause in 5 (0.3%) patients. The non-NPC-related causes included 19 (1.1%) patients who died from other cancers, 57 (3.3%) patients from other chronic diseases, 6 (0.4%) patients from accidents, and 67 (3.9%) patients from natural death. The 5-year OS, DSS, DMFS and LRRFS for the whole cohort were 77.9%, 83.6%, 86.7% and 91.3%, respectively.
RPA-generated risk stratification
The RT alone dataset (n = 451) was randomly divided into a training set (60%) and a testing set (40%) for the explorative construction and validation of the RPA models for risk stratification. Table 2 shows the detailed clinicopathological characteristics for the RT alone training set among elderly NPC patients. The ROC curve analysis for pre-treatment plasma EBV DNA indicated that the TNM staging for elderly NPC patients could be redefined with a cut-off value of 4000 copies/mL (Additional file 1: Fig. S1). After adjustment in the multivariate analysis, plasma EBV DNA, LDH, and T stage were verified to have significant effects on OS (P < 0.001, P = 0.001, P = 0.007, respectively) (Table 2). Risk stratification was performed using all validated predictors in the RPA. Automaticrpart algorithms were run to realize the modification of branches. Plasma EBV DNA and T stage remained in the final model while non-essential factors were removed.
310 elderly NPC patients treated with RT alone were classified into low-risk (n = 131; pre-treatment plasma EBV DNA ≤ 4000 copies/mL & T1–2), intermediate-risk (n = 108; pre-treatment plasma EBV DNA ≤ 4000 copies/mL & T3–4) and high-risk groups (n = 71; pre-treatment plasma EBV DNA > 4000 copies/mL & any T) (Fig. 1A). Survival curves showed significant discrimination in OS among the groups. The corresponding 5-year OS rates were 88.39%, 75.17% and 57.81%, respectively (P < 0.001; Additional file 1: Fig. S2). As such, the low-risk group was considered the good-prognosis group, while the intermediate-risk and high-risk group were considered the poor-prognosis group. OS in the good-prognosis group was significantly higher than in the poor-prognosis group in the training set (HR = 0.309, 95% CI 0.184–0.517; P < 0.001; Fig. 1B). A similar situation was observed in the testing set (HR = 0.276, 95% CI 0.113–0.670; P = 0.002; Fig. 1C).
RPA-generated risk stratification for the RT alone in training set (A) and comparisons between the good-prognosis and poor-prognosis groups in the training set (B) and the testing set (C). RPA = recursive partitioning analysis; NPC = nasopharyngeal carcinoma; EBV = Epstein–Barr virus; HR = hazard ratio; CI = confidence interval; OS = overall survival
Selection of chemotherapy and chemotherapy regimen beneficiaries
In the whole elderly NPC patient dataset, chemotherapy was administered to 73.7% (1263/1714) of the patients. In the good-prognosis group, 180 patients received radiation therapy alone and 136 patients received chemoradiotherapy (CRT). The 5-year OS, DSS, DMFS and LRRFS of the CRT group versus RT alone group were 88.1% versus 88.3% (P = 0.570), 91.2% versus 92.0% (P = 0.380), 90.9% versus 95.3% (P = 0.047) and 96.0% versus 94.9% (P = 0.580) (survival curves are shown in Additional file 1: Fig. S3).
In the poor-prognosis group, chemotherapy was administered to 80.6% (1127/1398) of the patients. The basic information is shown in Additional file 1: Table E1. Patients who received RCT in the poor-prognosis group had a significantly improved OS than those who received RT alone, (HR = 0.70, 95% CI 0.55–0.88; P = 0.003) and the high-risk subgroup (HR = 0.58, 95% CI 0.43–0.78; P < 0.001) but not in the intermediate-risk subgroup (HR = 0.80, 95% CI 0.54–1.17; P = 0.252). In the high-risk subgroup, CRT was validated to have significant survival benefits compared with RT alone for OS (P < 0.001), DSS (P = 0.012) and DMFS (P = 0.019), but not for LRRFS (P = 0.74) (see Additional file 1: Fig. S4).
For chemotherapy regimens, 38.1% (429/1127) of patients received IC + CCRT, 48.1% (542/1127) received CCRT and 13.8% (156/1127) received IC + RT in the poor-prognosis group. Patients who received IC + CCRT and CCRT had significantly improved OS than those who received RT alone (IC + CCRT vs. RT alone: HR = 0.64, 95% CI 0.48–0.85; P = 0.002; CCRT vs. RT alone: HR = 0.70, 95% CI 0.54–0.91; P = 0.008) but not in the IC + RT group (IC + RT vs. RT alone: HR = 0.83, 95% CI 0.59–1.17; P = 0.306) (survival curves are shown in Fig. 2A). Discrimination of survival benefits were not evident compared with RT alone for DSS (IC + CCRT vs. RT alone: P = 0.148; CCRT vs. RT alone: P = 0.184; IC + RT vs. RT alone: P = 0.574), DMFS (IC + CCRT vs. RT alone: P = 0.389; CCRT vs. RT alone: P = 0.355; IC + RT vs. RT alone: P = 0.706) or LRRFS (IC + CCRT vs. RT alone: P = 0.910; CCRT vs. RT alone: P = 0.736; IC + RT vs. RT alone: P = 0.515) (survival curves are shown in Fig. 2B–D). More detailed information of patient chemotherapy regimens in the poor-prognosis group are listed in Additional file 1: Table E2.
Kaplan–Meier OS (A), DSS, DMFS and LRRFS (B–D) curves for the poor-prognosis group between IC + CCRT, CCRT, IC + RT and RT alone. Poor-prognosis group = intermediate-risk group + high-risk group (intermediate-risk group: plasma EBV DNA titer ≤ 4000 copies/mL & T3–4; high-risk group: plasma EBV DNA titer > 4000 copies/mL & any T). EBV = Epstein–Barr virus; OS = overall survival; DSS = disease-specific survival; DMFS = distant metastasis-free survival; LRRFS = locoregional recurrence-free survival; CCRT = concurrent chemoradiotherapy; IC = induction chemotherapy; RT = radiotherapy
Subgroup analysis in the poor-prognosis group
To clarify whether other clinical factors impacted the prognosis of elderly NPC patients in the poor-prognosis group, univariate and multivariate analysis was performed with OS, DSS, DMFS and LRRFS (Table 3). Only the ACE-27 score was significant in predicting OS, DSS, DMFS and LRRFS (all P < 0.05). However, age and smoking status were significant in predicting OS (age ≥ 70 years vs. 60–64 years, P < 0.001; age 65–69 years vs. 60–64 years, P = 0.001; smoking vs. non-smoking: P = 0.023), as shown in Table 3.
In the poor-prognosis group, there were 591 patients with an ACE-27 score of 0, 482 patients with a score of 1 and 54 patients with a score of 2–3 in the CRT group and 117 patients with a score of 0, 101 patients with a score of 1 and 53 patients a score of 2–3 score in the RT alone group, respectively. The 5-year OS for CRT versus RT-alone with ACE-27 scores of 0, 1 and 2 were 76.0% versus 70.0% (P = 0.014), 80.5% versus 68.2% (P = 0.150) and 58.5% versus 62.2% (P = 0.490), respectively (survival curves are shown in Fig. 3). In the intermediate-risk group, the 5-year OS for CRT versus RT-alone with ACE-27 scores of 0, 1 and 2 were 82.9% versus 74.7% (P = 0.058), 86.2% versus 81.7% (P = 0.580) and 66.1% vs. 73.1% (P = 0.240), respectively (survival curves are shown in Additional file 1: Fig. S5). In the high-risk group, the 5-year OS for CRT versus RT-alone with ACE-27 scores of 0, 1 and 2 were 70.0% versus 59.6% (P = 0.014), 73.3% versus 54.2% (P = 0.037) and 48.5% versus 47.7% (P = 0.660), respectively (survival curves are shown in Additional file 1: Fig. S6).
Kaplan–Meier OS, DSS, DMFS and LRRFS curves for the poor-prognosis group between CRT and RT alone with an ACE-27 score of 0 (A), an ACE-27 score of 1 (B) and ACE-27 scores of 2–3 (C). Poor-prognosis grou P = intermediate-risk group + high-risk group (intermediate-risk group: plasma EBV DNA titer ≤ 4000 copies/mL & T3–4; high-risk group: EBV DNA titer > 4000 copies/mL & any T). EBV = Epstein–Barr virus; ACE-27 = adult comorbidity evaluation 27; OS = overall survival; DSS = disease-specific survival; DMFS = distant metastasis-free survival; LRRFS = locoregional recurrence-free survival; CRT = chemoradiotherapy; RT = radiotherapy
In the poor-prognosis group, there were 648 patients aged 60–64 years old, 358 patients were 65–70 years old and 121 patients aged ≥ 70 years old in the CRT group and 58 patients aged 60–64 years old, 96 patients aged 65–70 years old and 117 patients aged ≥ 70 years old in the RT alone group, respectively. The 5-year OS for CRT versus RT-alone with 60–64, 65–70 and ≥ 70 years old were 80.9% versus 75.9% (P = 0.068), 73.3% versus 63.4% (P = 0.270) and 64.8% versus 67.1% (P = 0.820), respectively (survival curves are shown in Fig. 4). In the intermediate-risk group, the 5-year OS for CRT versus the RT-alone with 60–64, 65–70 and ≥ 70 years old were 85.9% versus 89.7% (P = 0.840), 79.7% versus 75.1% (P = 0.630) and 81.5% versus 70.5% (P = 0.088), respectively (survival curves are shown in Additional file 1: Fig. S7). In the high-risk group, the 5-year OS for CRT versus RT-alone with 60–64, 65–70 and ≥ 70 years olds were 75.9% versus 47.4% (P < 0.001), 66.1% versus 51.5% (P = 0.130) and 46.6% versus 62.2% (P = 0.120), respectively (survival curves are shown in Additional file 1: Fig. S8).
Kaplan–Meier OS, DSS, DMFS and LRRFS curves for the poor-prognosis group between CRT and RT alone for 60–64 years old (A), 65–70 years old (B) and ≥ 70 years old (C). Poor-prognosis grou P = intermediate-risk group + high-risk group (intermediate-risk group: plasma EBV DNA titer ≤ 4000 copies/mL & T3–4; high-risk group: plasma EBV DNA titer > 4000 copies/mL & any T). EBV = Epstein–Barr virus; ACE-27 = adult comorbidity evaluation 27; OS = overall survival; DSS = disease-specific survival; DMFS = distant metastasis-free survival; LRRFS = locoregional recurrence-free survival; CRT = chemoradiotherapy; RT = radiotherapy
In the poor-prognosis group, there were 487 smokers and 640 non-smokers in the CRT group and 86 smokers and 185 non-smokers in the RT alone group, respectively. The 5-year OS for CRT versus RT-alone with smokers and non-smokers were 73.2% versus 67.0% (P = 0.091) and 80.1% versus 68.4% (P = 0.004), respectively (survival curves are shown in Additional file 1: Fig. S9). In the intermediate-risk group, the 5-year OS for CRT versus RT-alone with smoking and non-smoking were 79.1% versus 77.9% (P = 0.840) and 87.2% versus 76.4% (P = 0.099), respectively (survival curves are shown in Additional file 1: Fig. S10.). In the high-risk group, the 5-year OS for CRT versus RT-alone with smokers and non-smokers were 67.0% versus 50.5% (P = 0.009) and 72.8% versus 57.5% (P = 0.004), respectively (survival curves are shown in Supplementary Figure S11.)
Discussion
To our knowledge, this retrospective study is the first, largest real-world dataset to use a combination of pre-treatment plasma EBV DNA and T stage to identify chemotherapy beneficiaries in elderly NPC patients. Our results revealed that patients with a plasma EBV DNA level < 4000 copies/ml & stage T3–4, and those with a plasma EBV DNA level ≥ 4000 copies/ml & any T stage are indicators for identifying elderly NPC chemotherapy beneficiaries, which is a simple and reasonable screening method. However, these indicators were not useful for those aged > 70 years old and with an ACE-27 score > 1. IC + CCRT and CCRT were effective forms of chemotherapy.
Cancer is a heterogeneous disease and the TNM staging system is the only tool to guide patient selection for chemotherapy beneficiaries in NPC. However, patients who are in the same TNM stage and undergo similar treatments, can have differing responses with more than 20% of patients having a poor effect [25,26,27,28]. These differences in prognosis might be due to biological heterogeneity, in which molecular investigations may yield biomarkers for guiding better treatment decisions for patients in different risk groups. A number of noteworthy projects have examined molecular biomarkers for NPC, such as EBV DNA, LDH, and miRNAs [29,30,31,32]. So far, EBV DNA was reported to have an independent correlation with treatment outcomes [33,34,35,36,37]. A previous retrospective study reported that elderly NPC patients (aged > 60 years old) receiving IMRT were not CCRT beneficiaries, which may be related to the fact that pre-treatment plasma EBV DNA levels were not included in the screening method [15]. Similarly, in this study, no differences in OS between IC + CCRT, CCRT, IC + RT and RT alone were significant in elderly NPC patients when they were not stratified (Additional file 1: Fig. S12). Two recent studies have attempted to incorporate-treatment plasma EBV DNA levels within the 8th Edition of the TNM staging system [38, 39]. Therefore, elderly NPC patients with high EBV DNA levels may be chemotherapy beneficiaries.
To select chemotherapy beneficiaries among elderly NPC patients treated by IMRT, we built a RPA model with validated predictors including plasma EBV DNA levels, TNM staging system, hematology and biochemistry (e.g. LDH), and clinical risk factors. Prognostic factors including plasma EBV DNA and T stage were predictive of treatment outcomes and categorized patients into good and poor-prognosis groups. Our results showed that chemotherapy for the poor-prognosis group was favorable to improve OS but not in the good-prognosis group (Additional file 1: Figs. S3 and S4). In our study, the most likely reason why chemotherapy benefits the poor-prognosis group patients may be the bulky or extensive tumor load, which can result in an increased risk of distant metastasis [40]. Hence, a plasma EBV DNA < 4000 copies/ml & T3–4 stage, and plasma EBV DNA ≥ 4000 copies/ml & any T stage may be able to provide a simple and reasonable screening method for chemotherapy beneficiaries in elderly NPC patients. Further prospective clinical trials are warranted.
Since IC is better tolerated, it allows for more follow-up chemotherapy, thus increasing the effectiveness in eradicating micro-metastasis [41]. The survival benefits of concurrent chemotherapy are mainly achieved by control of the locoregional tumor instead of distant metastases, due to the nature of its additive and synergistic effect with radiotherapy [42]. Our study showed that the OS benefit for the poor-prognosis group was mainly reflected in the IC + CCRT and CCRT regimens but not in the IC + RT regimens (Fig. 2). Intensive management is possible for patients receiving IC + CCRT regimens including stronger intensity treatment, longer hospital duration, better nursing care, and more supportive therapies, while intensive management itself may bring about better prognosis [43, 44]. The intermediate-risk group may prefer to avoid IC, considering IC-related expenses and toxicity, prolonged waiting times before definitive IMRT, as well as, the relatively small expected benefits (Additional file 1: Fig. S4).
Univariate and multivariate analysis with the OS, DSS, LRRFS and DMFS in the poor-prognosis group showed that ACE-27, age and smoking were significant OS predictors (Table 3). The analysis based on ACE-27 scores and age showed that the CRT group had a lower OS than the RT alone group with an ACE-27 score > 1 (58.5% vs. 62.2%) (Fig. 3) or those aged > 70 years old (64.8% vs. 67.1%) (Fig. 4). However, this result should be considered with caution, since an ACE-27 score of > 1 and the > 70 years old subgroups already had worse physiological function and lower compensative capacity. When treated with chemotherapy, the benefits might be offset by substantial effects on normal functioning with a number adverse effects [9, 10, 45]. Patients with head and neck cancer can have significant comorbidities owing to the high incidence of smoking. Patients’ baseline physical health and functioning should also be assessed when considering chemotherapy, and the related risks and benefits carefully analyzed [46].
The main strength of this study is the large-scale data from our hospital’s medical records, the actual treatment regimens and patient’s real health status. In order to be of more clinical utility, all patients in this study were restaged according the latest 8th AJCC/UICC staging system. Nevertheless, this study has several limitations. Firstly, limited by the nature of a retrospective study, there was inevitable heterogeneity in patients’ chemotherapy regimens and dosages. Secondly, as other hospitals did not provide enough patient information, external validation was not performed. Finally, the sensitivity of PCR-based EBV DNA detection is 53 to 96% accurate [47], thus the present heterogeneity is an important issue. Therefore, a large prospective multicenter clinical trial is necessary to validate our results. Nonetheless, this study provides essential information to clinicians which may inform better decision making and enable improvements in patient outcomes.
Conclusion
Our RPA combined plasma EBV DNA and T stage, and classified elderly NPC patients into two groups with appropriate recommendations for individual therapies in real-world practice as follows: (1) plasma EBV DNA titer ≤ 4000 copies/mL & T1–2 stage: RT alone, (2) plasma EBV DNA titer ≤ 4000 copies/mL & T3–4 stage and plasma EBV DNA titer > 4000 copies/mL & any T: CRT. Patients aged > 70 years old with an ACE-27 score of > 1 should choose chemotherapy carefully. IC + CCRT and CCRT were effective forms of chemotherapy. Further validation in a larger population is still required for more specific treatment recommendations.
Availability of data and material
Research data are stored in the Research Data Deposit public platform (www.researchdata.org.cn) (RDDA2022454508).
Abbreviations
- NPC:
-
Nasopharyngeal carcinoma
- IMRT:
-
Intensity-modulated radiation therapy
- RPA:
-
Recursive partitioning analysis
- EBV DNA:
-
Epstein Barr virus DNA
- CRT:
-
Chemoradiotherapy
- IC:
-
Induction chemotherapy
- CCRT:
-
Concurrent chemoradiotherapy
- RT:
-
Radiotherapy
- NCCN:
-
National Comprehensive Cancer Network
- RCTs:
-
Randomized controlled trials
- LRRFS:
-
Locoregional relapse-free survival
- DMFS:
-
Distant metastasis-free survival
- DSS:
-
Disease-specific survival
- OS:
-
Overall survival
- TNM:
-
Tumor-node-metastases
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- PET:
-
Positron emission tomography
- PCR:
-
Polymerase chain-reaction
- AJCC/UICC:
-
American Joint Committee on Cancer/Union for International Cancer Control
- ACE-27:
-
Adult Comorbidity Evaluation 27
- Hb:
-
Hemoglobin
- LDH:
-
Lactate dehydrogenase
- CRP:
-
C-reactive protein
- ROC:
-
Receiver-operating characteristic
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. https://doi.org/10.1016/S0140-6736(19)30956-0.
Lin S, Pan J, Han L, et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys. 2009;75(4):1071–8. https://doi.org/10.1016/j.ijrobp.2008.12.015.
**ao WW, Huang SM, Han F, et al. Local control, survival, and late toxicities of locally advanced nasopharyngeal carcinoma treated by simultaneous modulated accelerated radiotherapy combined with cisplatin concurrent chemotherapy: long-term results of a phase 2 study. Cancer. 2011;117(9):1874–83. https://doi.org/10.1002/cncr.25754.
Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer–success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–84. https://doi.org/10.1016/j.radonc.2014.02.003.
Miao J, **ao W, Wang L, et al. The value of the Prognostic Nutritional Index (PNI) in predicting outcomes and guiding the treatment strategy of nasopharyngeal carcinoma (NPC) patients receiving intensity-modulated radiotherapy (IMRT) with or without chemotherapy. J Cancer Res Clin Oncol. 2017;143(7):1263–73. https://doi.org/10.1007/s00432-017-2360-3.
Tang LL, Chen YP, Mao YP, et al. Validation of the 8th edition of the UICC/AJCC staging system for nasopharyngeal carcinoma from endemic areas in the intensity-modulated radiotherapy era. J Natl Compr Canc Netw. 2017;15(7):913–9. https://doi.org/10.6004/jnccn.2017.0121.
Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol. 2013;31(31):3877–82. https://doi.org/10.1200/JCO.2012.47.7430.
Huang WY, Lin CL, Lin CY, et al. Survival outcome of patients with nasopharyngeal carcinoma: a nationwide analysis of 13 407 patients in Taiwan. Clin Otolaryngol. 2015;40(4):327–34. https://doi.org/10.1111/coa.12371.
Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–7. https://doi.org/10.1001/jama.291.20.2441.
Zhang Y, Yi JL, Huang XD, et al. Inherently poor survival of elderly patients with nasopharyngeal carcinoma. Head Neck. 2015;37(6):771–6. https://doi.org/10.1002/hed.23497.
Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284(1):E7-12. https://doi.org/10.1152/ajpendo.00366.2002.
Staessen JA, Wang J, Bianchi G, et al. Essential hypertension. Lancet. 2003;361(9369):1629–41. https://doi.org/10.1016/S0140-6736(03)13302-8.
Yancik R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer. 1997;80(7):1273–83.
Miao J, Wang L, Zhu M, et al. Reprint of Long-term survival and late toxicities of elderly nasopharyngeal carcinoma (NPC) patients treated by high-total- and fractionated-dose simultaneous modulated accelerated radiotherapy with or without chemotherapy. Oral Oncol. 2019;90:126–33. https://doi.org/10.1016/j.oraloncology.2019.01.005.
Bray F, Haugen M, Moger TA, et al. Age-incidence curves of nasopharyngeal carcinoma worldwide: bimodality in low-risk populations and aetiologic implications. Cancer Epidemiol Biomark Prev. 2008;17(9):2356–65. https://doi.org/10.1158/1055-9965.EPI-08-0461.
Xu C, Chen YP, Liu X, et al. Establishing and applying nomograms based on the 8th edition of the UICC/AJCC staging system to select patients with nasopharyngeal carcinoma who benefit from induction chemotherapy plus concurrent chemoradiotherapy. Oral Oncol. 2017;69:99–107. https://doi.org/10.1016/j.oraloncology.2017.04.015.
Lv JW, Chen YP, Huang XD, et al. Hepatitis B virus screening and reactivation and management of patients with nasopharyngeal carcinoma: a large-scale, big-data intelligence platform-based analysis from an endemic area. Cancer. 2017;123(18):3540–9. https://doi.org/10.1002/cncr.30775.
Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;13(2):163–71. https://doi.org/10.1016/S1470-2045(11)70320-5.
Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein–Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol. 2013;31(23):2861–9. https://doi.org/10.1200/JCO.2012.46.0816.
Shao JY, Zhang Y, Li YH, et al. Comparison of Epstein–Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer Res. 2004;24(6):4059–66.
Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. https://doi.org/10.3322/caac.21388.
Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110(4):593–602. https://doi.org/10.1097/00005537-200004000-00011.
Atkinson EJ, Therneau TM. An introduction to recursive partitioning using the RPART routines. Rochester: Rochester Mayo Foundation; 2000.
Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–92. https://doi.org/10.1016/S0140-6736(16)31388-5.
OuYang PY, Su Z, Ma XH, et al. Comparison of TNM staging systems for nasopharyngeal carcinoma, and proposal of a new staging system. Br J Cancer. 2013;109(12):2987–97. https://doi.org/10.1038/bjc.2013.659.
OuYang PY, **ao Y, You KY, et al. Validation and comparison of the 7th and 8th edition of AJCC staging systems for non-metastatic nasopharyngeal carcinoma, and proposed staging systems from Hong Kong, Guangzhou, and Guangxi. Oral Oncol. 2017;72:65–72. https://doi.org/10.1016/j.oraloncology.2017.07.011.
Yang XL, Wang Y, Liang SB, et al. Comparison of the seventh and eighth editions of the UICC/AJCC staging system for nasopharyngeal carcinoma: analysis of 1317 patients treated with intensity-modulated radiotherapy at two centers. BMC Cancer. 2018;18(1):606. https://doi.org/10.1186/s12885-018-4419-1.
Lin JC, Chen KY, Wang WY, et al. Detection of Epstein–Barr virus DNA in the peripheral-blood cells of patients with nasopharyngeal carcinoma: relationship to distant metastasis and survival. J Clin Oncol. 2001;19(10):2607–15. https://doi.org/10.1200/JCO.2001.19.10.2607.
Zhou GQ, Tang LL, Mao YP, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys. 2012;82(3):e359–65. https://doi.org/10.1016/j.ijrobp.2011.06.1967.
Liu N, Chen NY, Cui RX, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol. 2012;13(6):633–41. https://doi.org/10.1016/S1470-2045(12)70102-X.
Kim KY, Le QT, Yom SS, et al. Clinical utility of Epstein–Barr virus DNA testing in the treatment of nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2017;98(5):996–1001. https://doi.org/10.1016/j.ijrobp.2017.03.018.
Xu M, Yao Y, Chen H, et al. Genome sequencing analysis identifies Epstein–Barr virus subtypes associated with high risk of nasopharyngeal carcinoma. Nat Genet. 2019;51(7):1131–6. https://doi.org/10.1038/s41588-019-0436-5.
Twu CW, Wang WY, Liang WM, et al. Comparison of the prognostic impact of serum anti-EBV antibody and plasma EBV DNA assays in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2007;67(1):130–7. https://doi.org/10.1016/j.ijrobp.2006.07.012.
Wang WY, Twu CW, Chen HH, et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein–Barr virus DNA levels. Cancer. 2013;119(5):963–70. https://doi.org/10.1002/cncr.27853.
Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004;350(24):2461–70. https://doi.org/10.1056/NEJMoa032260.
Young LS, Rickinson AB. Epstein–Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757–68. https://doi.org/10.1038/nrc1452.
Guo R, Tang LL, Mao YP, et al. Proposed modifications and incorporation of plasma Epstein–Barr virus DNA improve the TNM staging system for Epstein–Barr virus-related nasopharyngeal carcinoma. Cancer. 2019;125(1):79–89. https://doi.org/10.1002/cncr.31741.
Lee VH, Kwong DL, Leung TW, et al. The addition of pretreatment plasma Epstein–Barr virus DNA into the eighth edition of nasopharyngeal cancer TNM stage classification. Int J Cancer. 2019;144(7):1713–22. https://doi.org/10.1002/ijc.31856.
Lin JC, Liang WM, Jan JS, et al. Another way to estimate outcome of advanced nasopharyngeal carcinoma—is concurrent chemoradiotherapy adequate? Int J Radiat Oncol Biol Phys. 2004;60(1):156–64. https://doi.org/10.1016/j.ijrobp.2004.03.002.
Chua DT, Ma J, Sham JS, et al. Long-term survival after cisplatin-based induction chemotherapy and radiotherapy for nasopharyngeal carcinoma: a pooled data analysis of two phase III trials. J Clin Oncol. 2005;23(6):1118–24. https://doi.org/10.1200/JCO.2005.12.081.
Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. 2003;21(4):631–7. https://doi.org/10.1200/JCO.2003.06.158.
Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–20. https://doi.org/10.1016/S1470-2045(16)30410-7.
Frikha M, Auperin A, Tao Y, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006–02). Ann Oncol. 2018;29(3):731–6. https://doi.org/10.1093/annonc/mdx770.
Lee AW, Ng WT, Chan LL, et al. Evolution of treatment for nasopharyngeal cancer—success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110(3):377–84. https://doi.org/10.1016/j.radonc.2014.02.003.
VanderWalde NA, Meyer AM, Deal AM, et al. Effectiveness of chemoradiation for head and neck cancer in an older patient population. Int J Radiat Oncol Biol Phys. 2014;89(1):30–7. https://doi.org/10.1016/j.ijrobp.2014.01.053.
Kim KY, Le QT, Yom SS, et al. Current state of PCR-based Epstein–Barr virus DNA testing for nasopharyngeal cancer. J Natl Cancer Inst. 2017;109(4):djx007. https://doi.org/10.1093/jnci/djx007.
Acknowledgements
We thank Yiducloud (Bei**g) Technology Ltd for supporting part of the data extraction and processing.
Funding
This work was partially supported by the National Natural Science Foundation of China (82002428) and (81902762), the Sun Yat-sen University Clinical Research 5010 Program (2019014), and the Natural Science Foundation of Guangdong Province of China (2021A1515010853). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Study concepts: YW, YH, LT; Study design: YW, LC, LT, JM; Data acquisition: XH, LL, QH; Quality control of data and algorithms: WL, YH, QH; Data analysis and interpretation: YC, LT; Statistical analysis: KY, JS; Manuscript preparation: YW, KY; Manuscript editing: YW, WL, LT; Manuscript review: WL, YM, JM. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This project was approved by Sun Yat-sen University Cancer Center’s institutional review committee (B2020-148-01). The need for informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
**g-Rong Shi: author responsible for statistical analysis
Supplementary Information
Additional file 1:
Supplementary Methods, Figures and Tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, YL., Yang, KB., Huang, Y. et al. Selection and validation of chemotherapy beneficiaries among elderly nasopharyngeal carcinoma (NPC) patients treated with intensity-modulated radiation therapy (IMRT): a large real-world study. Radiat Oncol 17, 138 (2022). https://doi.org/10.1186/s13014-022-02095-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-022-02095-2