Abstract
Background
Levels of the biomarkers amyloid-β 1–42 (Aβ42), tau and phosphorylated tau (p-tau) are decreased in the cerebrospinal fluid (CSF) of patients with idiopathic normal pressure hydrocephalus (iNPH). The mechanism behind this is unknown, but one potential explanation is dilution by excessive CSF volumes. The aim of this study was to investigate the presence of a dilution effect, by studying the relationship between ventricular volume (VV) and the levels of the CSF biomarkers.
Methods
In this cross-sectional observational study, preoperative magnetic resonance imaging (MRI) and lumbar CSF was acquired from 136 patients with a median age of 76 years, 89 men and 47 females, selected for surgical treatment for iNPH. The CSF volume of the lateral and third ventricles was segmented on MRI and related to preoperative concentrations of Aβ42, tau and p-tau.
Results
In the total sample VV (Median 140.7 mL) correlated weakly (rs = − 0.17) with Aβ42 (Median 534 pg/mL), but not with tau (Median 216 pg/mL) nor p-tau (Median 31 pg/mL). In a subgroup analysis, the correlation between VV and Aβ42 was only present in the male group (rs = − 0.22, p = 0.038). Further, Aβ42 correlated positively with tau (rs = 0.30, p = 0.004) and p-tau (rs = 0.26, p = 0.012) in males but not in females.
Conclusions
The findings did not support a major dilution effect in iNPH, at least not in females. The only result in favor for dilution was a weak negative correlation between VV and Aβ42 but not with the other lumbar CSF biomarkers. The different results between males and females suggest that future investigations of the CSF pattern in iNPH would gain from sex-based subgroup analysis.
Similar content being viewed by others
Background
Idiopathic normal pressure hydrocephalus (iNPH) is characterized by impairment of balance, gait, and cognition as well as urinary incontinence [1, 2]. The cerebral ventricles are enlarged and deformed [3]. INPH is more common than previously thought with a prevalence of 3.7% in the general population above 65 years [4]. The only effective treatment is shunt surgery to divert cerebrospinal fluid (CSF), with 70–80% of patients improving afterwards [5]. The CSF composition differ between patients with iNPH, Alzheimer’s Disease (AD) and healthy controls (HC). The CSF levels of amyloid β 1–42 (Aβ42) have been reported to be lower in iNPH than in HC. Accordingly, iNPH patients had decreased or similar levels of tau and phosphorylated tau (p-tau) compared with HC [6,7,8,26,27,28,29,30]. Females with AD or mild cognitive impairment have a higher atrophy rate [31] and suffer a faster disease progression rate [32]. In a cognitively healthy population, no relationship was seen between gender and tau/p-tau while a complex relationship between age, gender and APO-E carriership was seen for Aβ42 [33]. In iNPH, less is known about potential sex differences and although a few iNPH studies include sex-matched controls, subgroup analysis and comparisons are rare. This leaves the question of sex differences in the pathophysiology of iNPH largely unanswered, a question of special interest when investigating Aβ42, tau and p-tau due to the sex differences in AD and possible overlap between the diseases.
The volume of intracranial CSF can be measured by magnetic resonance imaging (MRI), with different methods available from manual to automatic segmentation of the images [34,35,36]. A reduction in VV have been reported after shunt surgery and clinical symptoms have been seen to correlate with the reduction in volume, implicating the volume change in the pathophysiology of the disease [37, 38]. Given the hypothesis of dilution of the biomarkers in a larger amount of CSF [19, 20], a negative correlation is expected between VV and the concentration Aβ42, tau and p-tau. The objective of this study was to investigate whether such a correlation exists in an attempt to shed further light on the pathophysiology of iNPH.
Method
Participants
One hundred and thirty-six patients (47 females and 89 males with a median age of 76 and 75 years, respectively) who underwent surgery due to possible or probable iNPH [39] were consecutively recruited from Sahlgrenska University Hospital (n = 108) during 2013–2016 and Östersund Regional Hospital (n = 28) during 2017–2020. The mean time between imaging and CSF collection was 38.3 days (SD = 72.8). Two patients were excluded because of > 1 year between imaging and lumbar puncture.
A positive shunt response was defined as at least one of the following (a – c) [40, 41]:
a. A 20% reduction in the time or the number of steps in at least one of the two motor function tests (Timed Up and Go, and 10 m walk).
b. Four points increase in the Mini-Mental State Examination.
c. One level increase in the continence scale and two points increase in the Mini-Mental State Examination.
Ventricular volumetry
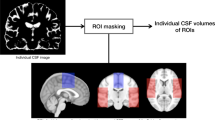
MRI images were acquired before operation with the resolution of 1mm3/voxel. The MRI used at Sahlgrenska University hospital was either a 1.5 T Intera (Philips Medical Systems, Best, The Netherlands) or a 1.5 T Achieva dStream (Philips Medical Systems, Best, The Netherlands) whereas the MRI used at Östersund Regional Hospital was either a 1.5 T GE Optima MR450w (GE Healthcare, Wausheka, WI, USA) or a 1.5 T GE Signa Voyager (GE Healthcare, Wausheka, WI, USA). The images were exported using DICOM format. The lateral and third ventricle volumes were semi-automatically segmented using the ITK-SNAP software [34], a method used in a recent study of volumetric change after shunt surgery [42] and exemplified in fig. 1.
Analysis of CSF
Analyses of Aβ42, tau and p-tau were performed as part of clinical routine workup and the results were retrieved from the participants’ patient charts. Like most Swedish hospitals, both the hospitals in Gothenburg and Östersund purchase this service from the clinical Neurochemistry Laboratory at Sahlgrenska University Hospital, Mölndal, Sweden. CSF was collected in polypropylene tubes during the diagnostic tap test after discarding the initial 3-5 mL to avoid contamination of the sample by small initial bleeding, then centrifuged at 2000 g for 10 min before being frozen at − 70 °C awaiting transportation. Biochemical analysis was performed by the standard clinical procedures at the laboratory using commercially available kits for quantitative solid-phase enzyme immunoassay: INNOTEST hTAU Ag, INNOTEST PHOSPHO-TAU(181P), INNOTEST β-AMYLOID (Fujirebio Europe N.V, Gent, Belgium).
Statistical analysis
Spearman's rank order correlation was used to analyze associations due to non-normal distribution of the data and the presence of outliers. Confidence intervals for spearman’s rho were acquired by bootstrap** with 3000 samples using the bias correction accelerated method [43]. Between-group comparisons were made using the Mann–Whitney U Test. One participant had a tau value of < 75 pg/mL which was included in the statistical analysis as the value 75 pg/mL. Because of the non-parametric tests used and it being the single lowest value in the data set this should not have impacted the reliability of results. The level of statistical significance was set to p < 0.05. Statistical analysis was performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Power calculation was made using G*Power 3.1.9.4. [44]. The sample size was large enough to have discovered correlations of effect size 0.3 or larger with 95.4% power, per Cohen’s convention a medium effect [45].
Results
For the total sample (n = 136) VV had a negative correlation with Aβ42 (rs = − 0.17, p = 0.042) which is visualized in Fig. 2, but not with tau or p-tau. However, the correlation between Aβ42 and VV was slightly stronger in the male subgroup (rs = − 0.22, p = 0.039), visualized in Fig. 3. For males there was also a positive correlation between Aβ42 and tau (rs = 0.302 p = 0.004) as well as p-tau (rs = 0.264 p = 0.001). In females, no significant correlation between VV and the CSF biomarkers was found (Table 1). The relationship between Aβ42 and VV for females is shown in Fig. 4 and the interrelationship of the biomarkers for the subgroups in Fig. 5 and 6.
Median VV and CSF biomarker concentrations are shown in Table 2. Males had significantly larger VV than females, but no significant differences were found in CSF biomarker concentration, age nor in improvement rate. Neither did the two clinics differ significantly in these variables nor in sex distribution. Detailed clinical information was available for a subset of the participants consisting of 83 individuals. At the first follow-up, 63 (76%) had improved after surgery.
Discussion
Considering previous reports of generally low CSF concentrations of Aβ42, tau and p-tau in iNPH [6,7,8,27,28,29,30] and a high comorbidity between AD pathology and iNPH has been found in perioperative brain biopsies [52, 53]. With this in mind, one possible explanation for our discrepant findings between the sexes could be a higher prevalence of preclinical AD pathology in the female group. However, this explanation is contradicted by the lack of difference between the sexes regarding absolute concentration of Aβ42, t-tau and p-tau. Another explanation is that there are sex differences in the complex pathophysiology of iNPH affecting the pattern of CSF-biomarkers. In a recent study, Graff-Radford et al. [54] underlined the need to take CSF disorders like iNPH into account in the interpretation of Aβ42, tau and p-tau levels. Further studies on a possible dilution effect would gain from complementary information on AD pathology, for example APO-E genotype and AD pathology in perioperative brain biopsies, to explore interaction between the effects of sex, iNPH and AD on CSF biomarker patterns.
In a previous study, an increase in Aβ42 has been observed in patients with iNPH one year after shunt surgery, though only in a low tau-subgroup [55]. In the high tau-subgroup, Aβ42 remained unchanged. Due to a cognitive decline in the high tau group the authors considered the possibility of AD pathology in the group, this could in theory cause a progressive lowering of Aβ42 canceling out the effects of shunting at follow-up. A recent longitudinal study on CSF biomarkers before and after shunt surgery found a postsurgical decrease in Aβ42 and simultaneous increase in tau, p-tau [52]. The authors argued that decrease in Aβ42 was mainly driven by APOE epsilon 4 carriers, indicating a possible role of AD pathology in the evolution of biomarker concentrations. In other studies, an increase in the levels of different lumbar CSF biomarkers was seen after shunt insertion, except for Aβ42 that was unchanged [8, 56]. The latter findings seem to contradict the dilution theory, given that the expected outcome of a decrease in VV following shunting would be an overall increase in the concentrations of CSF biomarkers, including Aβ42. However, lumbar CSF composition of brain-derived proteins is affected by absorption and metabolism along the spinal canal [57] and as such it susceptible to the changes in fluid dynamics introduced by a shunt and effects of surgery on CSF content should be interpreted with caution.
In summary, our results do not support dilution to be the major mechanism behind the lower lumbar CSF concentrations of Aβ42, t-tau and p-tau found in iNPH. Due to the unexplained sex differences, however, the possibility of a minor dilution effect cannot be dismissed. As our study focused on whether dilution could explain the lower concentrations of Aβ42, tau and p-tau in iNPH, our results provide no further insight regarding other possible pathophysiological explanations.
Strengths and limitations
That our material consists of patients undergoing routine clinical care for iNPH with a shunt response rate and levels of Aβ42, tau and p-tau in accordance with previous reports supports generalizability. Lumbar CSF was used which is a methodological limitation shared with most previous studies on CSF composition in iNPH. Levels of biomarkers of ventricular CSF would probably have been more relevant to relate with VV and better reflect the pathophysiology of iNPH. While there is a known difference between ventricular and lumbar CSF composition [48, 57], how predictable the difference is is largely unknown. One study showed a strong correlation between ventricular and lumbar CSF composition, although based on only five participants [58]. Further research in this area would be valuable, however ventricular CSF from healthy individuals is seldom available.
In our study, neither the spinal nor other extraventricular CSF was covered by the volumetric measurements. As the largest increase in CSF volume in iNPH is intraventricular this is unlikely to have masked a dilution effect.
The material provided sufficient power to detect a medium or larger effect supporting its reliability. For the subgroup analyses the power was weaker, though considering the small overlap between the confidence intervals of rs for the correlation between Aβ42 and tau in particular, a true difference between the subgroups is likely to exist.
Conclusion
A major dilution effect was not found when analyzing the correlation between VV and lumbar CSF levels of Aβ42, tau and p-tau biomarker in the total sample of 136 iNPH patients. Subgroup analyses revealed sex-based differences which highlights a need for stratification when investigating iNPH pathophysiology.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author after appropriate ethical review board approval.
Abbreviations
- AD:
-
Alzheimer's Disease
- Aβ42:
-
Amyloid-β 1–42
- CSF:
-
Cerebrospinal fluid
- HC:
-
Healthy Controls
- iNPH:
-
Idiopathic normal pressure hydrocephalus
- MRI:
-
Magnetic resonance imaging
- Md:
-
Median
- P-tau:
-
Phosphorylated tau
- SD:
-
Standard Deviation
- VV:
-
Ventricular volume
References
Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with normal cerebrospinal-fluid pressure. N Engl J Med. 1965;273(3):117–26.
Agerskov S, Hellström P, Andrén K, Kollén L, Wikkelsö C, Tullberg M. The phenotype of idiopathic normal pressure hydrocephalus-a single center study of 429 patients. J Neurol Sci. 2018;391:54–60.
Kockum K, Lilja-Lund O, Larsson E-M, Rosell M, Söderström L, Virhammar J, et al. The idiopathic normal-pressure hydrocephalus Radscale: a radiological scale for structured evaluation. Eur J Neurol. 2018;25(3):569–76.
Andersson J, Rosell M, Kockum K, Lilja-Lund O, Söderström L, Laurell K. Prevalence of idiopathic normal pressure hydrocephalus: a prospective, population-based study. PLoS ONE. 2019;14(5): e0217705.
Toma AK, Papadopoulos MC, Stapleton S, Kitchen ND, Watkins LDJAN. Systematic review of the outcome of shunt surgery in idiopathic normal-pressure hydrocephalus. Acta Neurochir. 2013;155(10):1977–80.
Craven CL, Baudracco I, Zetterberg H, Lunn MPT, Chapman MD, Lakdawala N, et al. The predictive value of T-tau and AB1-42 levels in idiopathic normal pressure hydrocephalus. Acta Neurochir. 2017;159(12):2293–300.
Jeppsson A, Zetterberg H, Blennow K, Wikkelsø C. Idiopathic normal-pressure hydrocephalus. Pathophysiology and diagnosis by CSF biomarkers. Neurology. 2013;80(15):1385–92.
Agren-Wilsson A, Lekman A, Sjoberg W, Rosengren L, Blennow K, Bergenheim AT, et al. CSF biomarkers in the evaluation of idiopathic normal pressure hydrocephalus. Acta Neurol Scand. 2007;116(5):333–9.
Chen Z, Liu C, Zhang J, Relkin N, **ng Y, Li Y. Cerebrospinal fluid Aβ42, t-tau, and p-tau levels in the differential diagnosis of idiopathic normal-pressure hydrocephalus: a systematic review and meta-analysis. Fluids Barriers CNS. 2017;14(1):13.
Kapaki EN, Paraskevas GP, Tzerakis NG, Sfagos C, Seretis A, Kararizou E, et al. Cerebrospinal fluid tau, phospho-tau181 and β-amyloid1−42 in idiopathic normal pressure hydrocephalus: a discrimination from Alzheimer’s disease. Eur J Neurol. 2007;14(2):168–73.
Schirinzi T, Sancesario GM, Ialongo C, Imbriani P, Madeo G, Toniolo S, et al. A clinical and biochemical analysis in the differential diagnosis of idiopathic normal pressure hydrocephalus. Front Neurol. 2015;6:86.
Manniche CS-H, Hejl A-M, Hasselbalch SG, Simonsen AH. Cerebrospinal fluid biomarkers in idiopathic normal pressure hydrocephalus versus Alzheimer’s disease and subcortical ischemic vascular disease: a systematic review. J Alzheimer’s Dis. 2019. https://doi.org/10.3233/JAD-180816.
Momjian S, Owler BK, Czosnyka Z, Czosnyka M, Pena A, Pickard JD. Pattern of white matter regional cerebral blood flow and autoregulation in normal pressure hydrocephalus. Brain J Neurol. 2004;127(Pt 5):965–72.
Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33(46):18190–9.
Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab. 2018;39(7):1355–68.
Hasan-Olive MM, Enger R, Hansson HA, Nagelhus EA, Eide PK. Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia. 2018;67(1):91–100.
Graff-Radford NR. Alzheimer CSF biomarkers may be misleading in normal-pressure hydrocephalus. Neurology. 2014;83(17):1573–5.
Schirinzi T, Sancesario GM, Di Lazzaro G, D’Elia A, Imbriani P, Scalise S, et al. Cerebrospinal fluid biomarkers profile of idiopathic normal pressure hydrocephalus. J Neural Transm. 2018;125(4):673–9.
**gami N, Asada-Utsugi M, Uemura K, Noto R, Takahashi M, Ozaki A, et al. Idiopathic normal pressure hydrocephalus has a different cerebrospinal fluid biomarker profile from Alzheimer’s disease. J Alzheimers Dis. 2015;45(1):109–15.
van Waalwijk van Doorn LJ, Gispert JD, Kuiperij HB, Claassen JA, Arighi A, Baldeiras I, et al. Improved cerebrospinal fluid-based discrimination between Alzheimer’s disease patients and controls after correction for ventricular volumes. J Alzheimers Dis. 2017;56(2):543–55.
Vanninen A, Nakajima M, Miyajima M, Rauramaa T, Kokki M, Musialowicz T, et al. Elevated CSF LRG and decreased Alzheimer’s disease biomarkers in idiopathic normal pressure hydrocephalus. J Clin Med. 2021;10(5):1105.
Holm A, Savolainen S, Alafuzoff I. Brain biopsy prior to treatment of Alzheimer’s disease. Minim Invasive Neurosurg MIN. 2003;46(3):161–4.
Pomeraniec IJ, Bond AE, Lopes MB, Jane JA. Concurrent Alzheimer’s pathology in patients with clinical normal pressure hydrocephalus: correlation of high-volume lumbar puncture results, cortical brain biopsies, and outcomes. J Neurosurg. 2016;124(2):382.
Yasar S, Jusue-Torres I, Lu J, Robison J, Patel MA, Crain B, et al. Alzheimer’s disease pathology and shunt surgery outcome in normal pressure hydrocephalus. PLoS ONE. 2017;12(8): e0182288.
Elobeid A, Laurell K, Cesarini KG, Alafuzoff I. Correlations between mini-mental state examination score, cerebrospinal fluid biomarkers, and pathology observed in brain biopsies of patients with normal-pressure hydrocephalus. J Neuropathol Exp Neurol. 2015;74(5):470–9.
Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease. Arch Gen Psychiatry. 1998;55(9):809.
Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies theAPOE-related risk of develo** Alzheimer disease. Ann Neurol. 2014;75(4):563–73.
Mielke M, Vemuri P, Rocca W. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014. https://doi.org/10.2147/CLEP.S37929.
Chêne G, Beiser A, Au R, Preis SR, Wolf PA, Dufouil C, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015;11(3):310–20.
Ferretti MT, Martinkova J, Biskup E, Benke T, Gialdini G, Nedelska Z, et al. Sex and gender differences in Alzheimer’s disease: current challenges and implications for clinical practice. Eur J Neurol. 2020;27(6):928–43.
Hua X, Hibar DP, Lee S, Toga AW, Jack CR, Weiner MW, et al. Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans. Neurobiol Aging. 2010;31(8):1463–80.
Tschanz JT, Corcoran CD, Schwartz S, Treiber K, Green RC, Norton MC, et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with Alzheimer dementia: the cache county dementia progression study. Am J Geriatr Psychiatry. 2011;19(6):532–42.
Li G, Shofer JB, Petrie EC, Yu C-E, Wilkinson CW, Figlewicz DP, et al. Cerebrospinal fluid biomarkers for Alzheimer’s and vascular disease vary by age, gender, and APOE genotype in cognitively normal adults. Alzheimer’s Res Ther. 2017. https://doi.org/10.1186/s13195-017-0271-9.
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116–28.
Warntjes JBM, Leinhard OD, West J, Lundberg P. Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reason Med. 2008;60(2):320–9.
Lehmann M, Douiri A, Kim LG, Modat M, Chan D, Ourselin S, et al. Atrophy patterns in Alzheimer’s disease and semantic dementia: a comparison of FreeSurfer and manual volumetric measurements. Neuroimage. 2010;49(3):2264–74.
Virhammar J, Laurell K, Cesarini KG, Larsson EM. Increase in callosal angle and decrease in ventricular volume after shunt surgery in patients with idiopathic normal pressure hydrocephalus. J Neurosurg. 2018:1–6.
Hiraoka K, Yamasaki H, Takagi M, Saito M, Nishio Y, Iizuka O, et al. Changes in the volumes of the brain and cerebrospinal fluid spaces after shunt surgery in idiopathic normal-pressure hydrocephalus. J Neurol Sci. 2010;296(1):7–12.
Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(suppl_3):S2-4-S2-16.
Kockum K, Virhammar J, Riklund K, Söderström L, Larsson E-M, Laurell K. Diagnostic accuracy of the iNPH Radscale in idiopathic normal pressure hydrocephalus. PLoS ONE. 2020;15(4): e0232275.
Virhammar J, Laurell K, Cesarini KG, Larsson EM. Preoperative prognostic value of MRI findings in 108 patients with idiopathic normal pressure hydrocephalus. Am J Neuroradiol. 2014;35(12):2311–8.
Neikter J, Agerskov S, Hellström P, Tullberg M, Starck G, Ziegelitz D, et al. Ventricular volume is more strongly associated with clinical improvement than the evans index after shunting in idiopathic normal pressure hydrocephalus. Am J Neuroradiol. 2020;41(7):1187–92.
Haukoos JS. Advanced statistics: bootstrap** confidence intervals for statistics with “difficult” distributions. Acad Emerg Med. 2005;12(4):360–5.
Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60.
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988.
Miyajima M, Nakajima M, Ogino I, Miyata H, Motoi Y, Arai H. Soluble amyloid precursor protein α in the cerebrospinal fluid as a diagnostic and prognostic biomarker for idiopathic normal pressure hydrocephalus. Eur J Neurol. 2013;20(2):236–42.
Michael Malekahmadi AT. Differences in cerebrospinal fluid biomarkers between clinically diagnosed idiopathic normal pressure hydrocephalus and Alzheimer’s disease. J Alzheimer’s Dis Parkinsonism. 2014. https://doi.org/10.4172/2161-0460.1000150.
Tullberg M, Blennow K, Månsson JE, Fredman P, Tisell M, Wikkelsö C. Ventricular cerebrospinal fluid neurofilament protein levels decrease in parallel with white matter pathology after shunt surgery in normal pressure hydrocephalus. Eur J Neurol. 2007;14(3):248–54.
Wahlund L-O, Blennow K. Cerebrospinal fluid biomarkers for disease stage and intensity in cognitively impaired patients. Neurosci Lett. 2003;339(2):99–102.
Ott BR, Cohen RA, Gongvatana A, Okonkwo OC, Johanson CE, Stopa EG, et al. Brain ventricular volume and cerebrospinal fluid biomarkers of Alzheimer’s disease. J Alzheimers Dis. 2010;20(2):647–57.
Sutphen CL, Jasielec MS, Shah AR, Macy EM, **ong C, Vlassenko AG, et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 2015;72(9):1029.
Lukkarinen H, Tesseur I, Pemberton D, Van Der Ark P, Timmers M, Slemmon R, et al. Time trends of cerebrospinal fluid biomarkers of neurodegeneration in idiopathic normal pressure hydrocephalus. J Alzheimer’s Dis. 2021. https://doi.org/10.3233/JAD-201361.
Leinonen V, Koivisto AM, Alafuzoff I, Pyykko OT, Rummukainen J, von Und Zu Fraunberg M, et al. Cortical brain biopsy in long-term prognostication of 468 patients with possible normal pressure hydrocephalus. Neurodegener Dis. 2012;10(14):166–9.
Graff-Radford J, Jones DT, Wiste HJ, Cogswell PM, Weigand SD, Lowe V, et al. Cerebrospinal fluid dynamics and discordant amyloid biomarkers. Neurobiol Aging. 2022;110:27–36.
Akiba C, Nakajima M, Miyajima M, Ogino I, Motoi Y, Kawamura K, et al. Change of amyloid-β 1–42 toxic conformer ratio after cerebrospinal fluid diversion predicts long-term cognitive outcome in patients with idiopathic normal pressure hydrocephalus. J Alzheimer’s Dis JAD. 2018;63(3):989–1002.
Tullberg M, Blennow K, Mansson JE, Fredman P, Tisell M, Wikkelso C. Cerebrospinal fluid markers before and after shunting in patients with secondary and idiopathic normal pressure hydrocephalus. Cereb Fluid Res. 2008;5:9.
Brandner S, Thaler C, Lelental N, Buchfelder M, Kleindienst A, Maler JM, et al. Ventricular and lumbar cerebrospinal fluid concentrations of Alzheimer’s disease biomarkers in patients with normal pressure hydrocephalus and posttraumatic hydrocephalus. J Alzheimers Dis. 2014;41(4):1057–62.
Serot J-M, Peltier J, Fichten A, Ledeme N, Bourgeois A-M, Jouanny P, et al. Reduced CSF turnover and decreased ventricular Aβ42 levels are related. BMC Neurosci. 2011;12(1):42.
Acknowledgements
We would like to thank the staff at the neurology and radiology departments at Sahlgrenska University Hospital and Östersund’s Hospital.
Funding
Open access funding provided by Uppsala University. The study received governmental funding from Uppsala University and Region Jämtland Härjedalen as well as donations from the following foundations: Forskningsfonden för klinisk neurovetenskap Norrlands Universitetssjukhus, Jämtlands läns cancer- och omvårdnadsfond, Syskonen Perssons Donationsfond.
None of the funding bodies had any say in the design of the study, writing of the manuscript nor the collection, analysis and interpretation of the data.
Author information
Authors and Affiliations
Contributions
SL was primarily responsible for data collection, data analysis as well as writing and revision of the manuscript while contributing substantially to interpretation of data. DF contributed substantially to study conceptualization, data collection, interpretation of data and manuscript revision. KL contributed substantially to study conceptualization, data collection, interpretation of data, manuscript writing and revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All participants received written and verbal information. All included participants signed a written consent form. The study was performed according to the Helsinki Declaration. It was approved by the local ethical committees in Gothenburg, D-number 328–14, T-number 439–15; Umeå, D-number 254–31; Swedish Ethical Review Authority, application number 2019–05842.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lidén, S., Farahmand, D. & Laurell, K. Ventricular volume in relation to lumbar CSF levels of amyloid-β 1–42, tau and phosphorylated tau in iNPH, is there a dilution effect?. Fluids Barriers CNS 19, 59 (2022). https://doi.org/10.1186/s12987-022-00353-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12987-022-00353-9