Abstract
Background
Metabolic syndrome (MetS) includes a group of metabolic irregularities, including insulin resistance (IR), atherogenic dyslipidemia, central obesity, and hypertension. Consistent evidence supports IR and ongoing low-grade inflammation as the main contributors to MetS pathogenesis. However, the association between the triglyceride-glucose (TyG) index and mortality in people with MetS remains uncertain. The objective of this study was to examine the correlation between the baseline TyG index and all-cause and cardiovascular (CV) mortality in rural Northeast Chinese individuals with MetS.
Methods
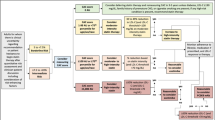
For the Northeast China Rural Cardiovascular Health Study, 3918 participants (mean age, 55 ± 10; 62.4% women) with MetS at baseline were enrolled in 2012–2013 and followed up from 2015 to 2017. The TyG index was calculated using the equation TyG index = ln [fasting TG (mg/dL) × fasting glucose (mg/dL)/2] and subdivided into tertiles [Q1(< 8.92); Q2 (8.92–9.36); Q3 (≥ 9.36)]. Multivariate Cox proportional hazards models were developed to examine the correlations between mortality and the baseline TyG index.
Results
During a median of 4.66 years of follow-up, 196 (5.0%) all-cause deaths and 108 (2.8%) CV disease-related deaths occurred. The incidence of all-cause mortality was significantly different among TyG index tertiles of the overall population (P = 0.045). Kaplan–Meier analysis demonstrated a significantly increased risk of all-cause mortality in rural Chinese patients with a higher TyG index (log-rank P < 0.05). After adjusting for possible confounders, Cox proportional hazard analysis revealed that the TyG index could effectively predict all-cause mortality (HR for the third vs. first tertile of TyG was 1.441 [95% confidence interval, 1.009–2.059]), but not CV mortality, in rural Chinese patients with MetS.
Conclusions
The TyG index is an effective predictor of all-cause mortality in rural Chinese patients with MetS. This indicates that the TyG index may be useful for identifying rural Chinese individuals with MetS at a high risk of death.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) includes a cluster of conditions associated with metabolic dysregulation, such as insulin resistance (IR), atherogenic dyslipidemia, central obesity, and hypertension. Cumulative evidence has confirmed that IR and persistent low-grade inflammation are the primary pathogenic factors of MetS [1]. SPECT-China, a population-based cross-sectional survey of Chinese individuals ≥ 18 years of age, reveals a 22.0% age-standardized prevalence of MetS in East China [2]. Similarly, our previous study found that MetS has gradually become more prevalent among participants from rural China (39.0%) [3]. If left untreated, MetS considerably increases morbidity and mortality [1]. It is thus crucial to investigate an effective predictor of mortality in patients with MetS to reduce the substantial disease burden.
Recently, the triglyceride-glucose (TyG) index has garnered increasing global attention, as it is associated with all-cause and cardiovascular (CV) mortality in the general population [2), encompassing both work-related and recreational pursuits, was evaluated and divided into three categories [3].
Definition
The TyG index was calculated using the following equation: ln [fasting triglycerides (mg/dL) × fasting glucose (mg/dL)/2] [9]. Body mass index (BMI) was determine using the following equation: BMI = \(\frac{weight \left(kg\right)}{{\left(height\right)}^{2} \left(m\right)}\). Figure 3 provides the definition of the ATPIII-modified criteria [10].
Statistical analyses
The individuals were divided into three groups based on their TyG index levels, with each group representing one-third of the total participants, as follows: tertile 1 (n = 1309, TyG index < 8.92), tertile 2 (n = 1299, 8.92 ≤ TyG index < 8.92–9.36), and tertile 3 (n = 1310, TyG index ≥ 9.36), with the depicted characteristics. Categorical variables have been quantified using numerical values (n) and percentages (%) and were evaluated using the chi-squared test. Continuous variables with a normal distribution are expressed as the mean ± standard deviation; non-normal distributions are represented as the median (interquartile range). Data were analyzed using one-way analysis of variance for a normal distribution and the Kruskal–Wallis test for a non-normal distribution. We investigated the precise correlation between the TyG index and mortality from all causes and CVDs in people with MetS using multivariate Cox proportional hazards models. Three sets of models were constructed in this study. Model 1 included only the TyG index, whereas Model 2 incorporated demographic attributes such as age, sex, and race. Model 3 further adjusted for education, current smoking and drinking habits, annual income, sleep duration, physical activity, and cardiovascular history. A stratified analysis was conducted to examine the impact of putative effect modifiers, including age, sex, BMI, and current smoking and drinking status, on relevant variables. The statistical analyses were conducted using R software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria), and statistical significance was defined as P < 0.05.
Results
Population characteristics stratified using the TyG index
As presented in Table 1, baseline participant characteristics were stratified using TyG index tertiles (Q) as follows: Q1, < 8.92; Q2, 8.92–9.36, and Q3 ≥ 9.36. The mean TyG index levels in these tertiles were 8.54 ± 0.29, 9.13 ± 0.122, and 9.87 ± 0.48, respectively. The participants with higher TyG index values were generally male, older, and current smokers and drinkers, had a longer sleep duration, and exhibited a higher prevalence of CV comorbidities. Additionally, they experienced a higher incidence of all-cause mortality than those with lower TyG index values (4.1% vs. 4.7% vs. 6.2%, P = 0.045). Moreover, the higher TyG index group exhibited higher levels of HbA1C, LDL-C, TC, TG, uric acid, FPG, AST, and ALT, along with lower eGFR and HDL-C levels (Table 2).
Associations between the TyG index and all‑cause and CV mortality
Cox proportional hazard analysis revealed a significant association between the TyG index and all-cause mortality, but not CV mortality, in both crude and adjusted models [crude: all-cause mortality HR, 1.303 (95% CI, 1.061–1.600), P = 0.012; CV mortality HR, 1.224 (95% CI, 0.923–1.623), P = 0.160]; Model 2: all-cause mortality HR, 1.330 (95% CI, 1.075–1.646), P = 0.009; CV mortality HR, 1.249 (95% CI, 0.931–1.674), P = 0.138; Model 3: all-cause mortality HR, 1.288 (1.033–1.605), P = 0.025; CV mortality HR, 1.194 (95% CI, 0.879–1.622), P = 0.257]. In both the crude and adjusted models, upward trends were observed between the TyG index and all-cause mortality (Table 3, both P < 0.05). The participants in the Q3 TyG index tertile had a significantly higher incidence of all-cause mortality than those in the Q1 and Q2 tertiles [HR, 1.441 (95% CI, 1.009–2.059), P = 0.016].
Subgroup analysis of the association between the TyG index and all‑cause and CV mortality
Stratification was performed based on age, sex, BMI, and current smoking and drinking status to evaluate the impact of the TyG index on the primary endpoints (Fig. 4). Except for the age subgroup (age subgroup: all-cause mortality, P for interaction < 0.001), no significant interactions were observed in most subgroups. The TyG index was associated with all-cause mortality in patients < 65 years of age [all-cause mortality: HR, 1.374 (95% CI, 1.036–1.823)]; however, this was not the case for participants ≥ 65 years of age [all-cause mortality: HR, 1.121 (95%, CI 0.782–1.606)].
Subgroup analysis of the association between Triglyceride glucose Index (TyG) index and all-cause mortality (A), cardiovascular disease (CV) mortality (B) among rural Chinese with Metabolic syndrome (MetS). Each subgroup analysis is adjusted if not stratified for age, gender, race, BMI, SBP, DBP, current smoking and drinking, education, annual income, sleep duration, physical activity, cardiovascular history
Discussion
In this study, a substantial relationship between the TyG index and all-cause mortality, but not CV mortality, was found in a rural Chinese population with MetS. Moreover, a significant interaction effect was identified between age and the TyG index, suggesting that the correlation between TyG levels and mortality was particularly pronounced among patients who were younger in age. Recently, the incidence of MetS has considerably increased in rural Chinese areas. A meta-analysis indicated a 24.5% prevalence of MetS among individuals 15 years and older in mainland China, with 19.2% residing in rural areas [11]. Our previous study held in rural Northeast China revealed a cumulative incidence of newly diagnosed MetS at 24.0% [12]. Given that metabolic disorders often increase insidiously, all-cause and CV mortalities increase at the time of detection in this population [13]. Hence, proactive strategies and tasks should be undertaken to prevent risk factors such as hypertension, hyperlipidemia, elevated blood glucose, and obesity, along with effective measures to predict mortality among patients with MetS. IR is a crucial pathological factor in MetS, contributing to CVDs and poor clinical outcomes in various ways via mechanisms such as endothelial dysfunction, low-level inflammation, and disruptions in systemic glucose–lipid metabolism [14]. Although various indicators are used to measure IR, including the hyperinsulinemic euglycemic (HIEG) clamp test, homeostatic model assessment for insulin resistance (HOMA-IR), and quantitative insulin sensitivity check index, most of these indicators are time-consuming and expensive to determine, limiting their use in rural areas. Consequently, the TyG index has gradually gained attention in recent years owing to its cost-effectiveness compared to other parameters. In comparison to the gold standard (HIEG clamp test), Won et al. demonstrated that the TyG index has high sensitivity (96.5%) and specificity (85.0%) for IR detection [15]. Furthermore, when compared to the HOMA-IR, the TyG index was confirmed to perform better in assessing IR [7]. The merits of our investigation encompassed a substantial sample size and a longitudinal retrospective approach. This study is the first known attempt to determine the correlation between the TyG index and mortality in a rural Chinese population with MetS. However, the present study had some limitations. First, the participants were from one province in Northeast China, limiting the generalizability of the findings. Second, the TyG index is based on a single blood test, which could introduce potential bias. Third, even after controlling for potential confounding variables, residual confounding factors might have endured. Finally, the influence of prescribed medications on triglyceride and glucose levels could have affected the findings.
Conclusion
The TyG index is a prominent risk predictor of all-cause mortality in participants with MetS in rural China. Our findings indicated that this simple and inexpensive index facilitates the early prediction of mortality in individuals with MetS, aiding village doctors in stratifying high-risk participants and implementing timely interventions.
Data availability
Data is available upon the reasonable request of the corresponding author.
Abbreviations
- BMI:
-
body mass index
- CV:
-
cardiovascular
- CVD:
-
cardiovascular disease
- FPG:
-
fasting plasma glucose
- HDL-C:
-
high-density lipoprotein cholesterol
- HIEG:
-
hyperinsulinemic euglycemic
- HOMA-IR:
-
homeostatic model assessment for insulin resistance
- IR:
-
insulin resistance
- LDL-C:
-
low-density lipoprotein cholesterol
- MetS:
-
metabolic syndrome
- TyG:
-
triglyceride-glucose
References
Fahed G, Aoun L, Bou Zerdan M, Allam S, Bou Zerdan M, Bouferraa Y, Assi HI. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23(2).
Jiang B, Zheng Y, Chen Y, Chen Y, Li Q, Zhu C, Wang N, Han B, Zhai H, Lin D, et al. Age and gender-specific distribution of metabolic syndrome components in East China: role of hypertriglyceridemia in the SPECT-China study. Lipids Health Dis. 2018;17(1):92.
Yu S, Guo X, Yang H, Zheng L, Sun Y. An update on the prevalence of metabolic syndrome and its associated factors in rural northeast China. BMC Public Health. 2014;14:877.
Chen J, Wu K, Lin Y, Huang M, **e S. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc Diabetol. 2023;22(1):320.
Liao Y, Zhang R, Shi S, Zhao Y, He Y, Liao L, Lin X, Guo Q, Wang Y, Chen L, et al. Triglyceride-glucose index linked to all-cause mortality in critically ill patients: a cohort of 3026 patients. Cardiovasc Diabetol. 2022;21(1):128.
Zhang Q, **ao S, Jiao X, Shen Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22(1):279.
Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride glucose (TyG) index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022;72(5):986–8.
Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr. 2022;16(8):102581.
Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr. et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13(6):322–327.
Li R, Li W, Lun Z, Zhang H, Sun Z, Kanu JS, Qiu S, Cheng Y, Liu Y. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health. 2016;16:296.
Yu S, Guo X, Li G, Yang H, Sun G, Zheng L, Sun Y. Gender discrepancy of incidence and risk factors of metabolic syndrome among rural Chinese from 2012–2013 to 2015–2017. Diabetol Metab Syndr. 2020;12:48.
Report on Cardiovascular Health and Diseases in China 2021: An Updated Summary. Biomed Environ Sci. 2022;35(7):573–603.
Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14(5):575–85.
Won KB, Kim YS, Lee BK, Heo R, Han D, Lee JH, Lee SE, Sung JM, Cho I, Park HB, et al. The relationship of insulin resistance estimated by triglyceride glucose index and coronary plaque characteristics. Med (Baltim). 2018;97(21):e10726.
Tam CS, **e W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35(7):1605–10.
Sasaki K, Shoji T, Kabata D, Shintani A, Okute Y, Tsuchikura S, Shimomura N, Tsujimoto Y, Nakatani S, Mori K, et al. Oxidative stress and inflammation as predictors of mortality and cardiovascular events in hemodialysis patients: the DREAM cohort. J Atheroscler Thromb. 2021;28(3):249–60.
Ballard-Hernandez J, Sall J. Dyslipidemia update. Nurs Clin North Am. 2023;58(3):295–308.
**n F, He S, Zhou Y, Jia X, Zhao Y, Zhao H. The triglyceride glucose index trajectory is associated with hypertension: a retrospective longitudinal cohort study. Cardiovasc Diabetol. 2023;22(1):347.
Wang Y, Yang W, Jiang X. Association between triglyceride-glucose index and hypertension: a meta-analysis. Front Cardiovasc Med. 2021;8:644035.
Campos Muñiz C, León-García PE, Serrato Diaz A, Hernández-Pérez E. Diabetes mellitus prediction based on the triglyceride and glucose index. Med Clin (Barc). 2023;160(6):231–6.
Shi W, **ng L, **g L, Tian Y, Liu S. Usefulness of triglyceride-glucose index for estimating hyperuricemia risk: insights from a general population. Postgrad Med. 2019;131(5):348–56.
Gao JW, Hao QY, Gao M, Zhang K, Li XZ, Wang JF, Vuitton DA, Zhang SL, Liu PM. Triglyceride-glucose index in the development of peripheral artery disease: findings from the atherosclerosis risk in communities (ARIC) study. Cardiovasc Diabetol. 2021;20(1):126.
Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19(1):155.
Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20(1):146.
Wang X, Xu W, Song Q, Zhao Z, Meng X, **a C, **e Y, Yang C, ** P, Wang F. Association between the triglyceride-glucose index and severity of coronary artery disease. Cardiovasc Diabetol. 2022;21(1):168.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, Ma J, Zhao Y, Zhu W, Wang J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1):124.
Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride-to-high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. J Investig Med. 2014;62(2):345–9.
Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The triglyceride-glucose index, an insulin resistance marker, was non-linear associated with all-cause and cardiovascular mortality in the general population. Front Cardiovasc Med. 2020;7:628109.
Shen J, Feng B, Fan L, Jiao Y, Li Y, Liu H, Hou X, Su Y, Li D, Fu Z. Triglyceride glucose index predicts all-cause mortality in oldest-old patients with acute coronary syndrome and diabetes mellitus. BMC Geriatr. 2023;23(1):78.
Zhao M, **ao M, Tan Q, Lu F. Triglyceride glucose index as a predictor of mortality in middle-aged and elderly patients with type 2 diabetes in the US. Sci Rep. 2023;13(1):16478.
Li H, Jiang Y, Su X, Meng Z. The triglyceride glucose index was U-shape associated with all-cause mortality in population with cardiovascular diseases. Diabetol Metab Syndr. 2023;15(1):181.
Zhou Y, Wang C, Che H, Cheng L, Zhu D, Rao C, Zhong Q, Li Z, Wang X, Wu Z, et al. Association between the triglyceride-glucose index and the risk of mortality among patients with chronic heart failure: results from a retrospective cohort study in China. Cardiovasc Diabetol. 2023;22(1):171.
Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–97.
Cho YR, Ann SH, Won KB, Park GM, Kim YG, Yang DH, Kang JW, Lim TH, Kim HK, Choe J, et al. Association between insulin resistance, hyperglycemia, and coronary artery disease according to the presence of diabetes. Sci Rep. 2019;9(1):6129.
Zhou D, Liu XC, Kenneth L, Huang YQ, Feng YQ. A Non-linear association of triglyceride glycemic index with cardiovascular and all-cause mortality among patients with hypertension. Front Cardiovasc Med. 2022;8:778038.
Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, Zhao XQ, Li W, Li H. Predictive effect of triglyceride–glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. 2021;20(1):43.
Acknowledgements
No.
Funding
This study was supported by grants from the National Key Research and Development Program from the Ministry of Science and Technology of China (Project Grant # 2018 YFC 1312400, Sub-project Grant # 2018 YFC 1312403). This study was supported by grants from the ‘Support the high-quality development of science and technology fund projects of China Medical University’ project funds (grant no. 2023JH2/20200024).
Author information
Authors and Affiliations
Contributions
SSY and QYL drafted the manuscript. YXS obtained funding and designed the study. GXL were involved in data cleaning and analyzing. XFG and HMY collected the data. YXS and XFG contributed to the critical revision of the manuscript. All authors approved the final version of the manuscript. All authors have read and approved the final manuscript. Administrative, technical and logistic support was provided by all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of China Medical University (Shenyang, China AF-SDP-07-1, 0–01). Informed consent was obtained from all subjects involved in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, S., Li, Q., Yang, H. et al. Triglyceride-glucose index predicts all-cause mortality, but not cardiovascular mortality, in rural Northeast Chinese patients with metabolic syndrome: a community-based retrospective cohort study. Nutr Metab (Lond) 21, 27 (2024). https://doi.org/10.1186/s12986-024-00804-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12986-024-00804-0