Abstract
Background
African swine fever virus (ASFV) is one of the most fatal swine etiological agents and has a huge economic impact on the global pork industry. Given that no effective vaccines or anti-ASFV drugs are available, there remains a pressing need for novel anti-ASFV drugs. This study aimed to investigate the anti-African swine fever virus (ASFV) activity of brequinar, a DHODH inhibitor.
Methods
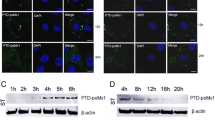
The anti-ASFV activity of brequinar was investigated using IFA, HAD, HAD50, qRT-PCR, and western blotting assays. The western blotting assay was used to investigate whether brequinar inhibits ASFV replication by killing ASFV particles directly or by acting on cell factors. The confocal microscopy and western blotting assays were used to investigate whether brequinar inhibits ASFV replication by activating ferroptosis.
Results
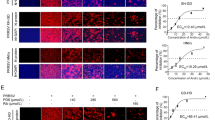
In this study, brequinar was found to effectively inhibit ASFV replication ex vivo in porcine alveolar macrophages (PAMs) in a dose-dependent manner. In kinetic studies, brequinar was found to maintain ASFV inhibition from 24 to 72 hpi. Mechanistically, the time-of-addition assay showed that brequinar exerted anti-ASFV activity in all treatment modes, including pre-, co-, and post-treatment rather than directly killing ASFV particles. Notably, FerroOrange, Mito-FerroGreen, and Liperfluo staining experiments showed that brequinar increased the accumulation of intracellular iron, mitochondrial iron, and lipid peroxides, respectively. Furthermore, we also found that ferroptosis agonist cisplatin treatment inhibited ASFV replication in a dose-dependent manner and the inhibitory effect of brequinar on ASFV was partially reversed by the ferroptosis inhibitor ferrostatin-1, suggesting that brequinar activates ferroptosis to inhibit ASFV replication. Interestingly, exogenous uridine supplementation attenuated the anti-ASFV activity of brequinar, indicating that brequinar inhibits ASFV replication by inhibiting DHODH activity and the depletion of intracellular pyrimidine pools; however, the induction of ferroptosis by brequinar treatment was not reversed by exogenous uridine supplementation, suggesting that brequinar activation of ferroptosis is not related to the metabolic function of pyrimidines.
Conclusions
Our data confirm that brequinar displays potent antiviral activity against ASFV in vitro and reveal the mechanism by which brequinar inhibits ASFV replication by activating ferroptosis, independent of inhibiting pyrimidine synthesis, providing novel targets for the development of anti-ASFV drugs.
Similar content being viewed by others
Background
African swine fever (ASF) is a major economically important infectious disease caused by the African swine fever virus (ASFV), which threatens the global pork industry and has high mortality rate [1]. In the 1920s, ASF was first reported in Kenya and was limited to Africa [2]. In the 1950s, it spread to Europe, including Spain, Portugal, Italy, and France [3]. Europe (except for Sardinia) has eradicated ASFV using drastic control and eradication programs. Unfortunately, the disease reemerged in the Caucasus region in 2007 and rapidly spread to the eastern territory of the European Union in 2014 [4]. Next, the disease was reported on August 3, 2018, in China, one of the largest pork industries in the world. Between 2018 and 2022, 204 ASF outbreaks across 32 Chinese provinces were reported by the Chinese Ministry of Agriculture and Rural Affairs, causing huge economic losses, with estimates of at least 1.2 million sick and culled pigs [5, 6].
ASFV is the only member of the Asfarviridae family. It is a large, enveloped, double-stranded DNA virus, with 151–167 open reading frames, encoding more than 150 proteins [7]. ASFV is highly restricted to macrophages and monocytes, especially porcine alveolar macrophages (PAMs), which are the primary targets of ASFV in vivo [8]. Owing to the limited cell tropism and complex viral particle structure of ASFV, research on ASFV is exceedingly difficult, and there are no commercial vaccines or drugs to control ASFV infection [9]. So far, the main strategy to control ASF includes disinfection of vehicles and transit areas, strengthening of biosafety management on pig farms, and stricter vigilance programs [37]. In this context, we attempted to develop an antiviral drug therapy against ASFV. The DHODH inhibitor brequinar is a broad-spectrum antiviral inhibitor, we explored whether brequinar possesses anti-ASFV activity.
In this study, we demonstrated that brequinar strongly inhibits ASFV in a dose-dependent manner (Figs. 2 and 3) and sustain inhibition of ASFV from 24 to 72 hpi (Fig. 3). In general, the compounds exhibit antiviral activity in two ways: either the compound directly targets the virus itself, or the compound impairs host cell factors that are essential for the viral life cycle [38]. Therefore, we investigate whether brequinar interacts directly with ASFV thereby killing ASFV particles. However, the results showed that brequinar did not directly interact with ASFV; instead, brequinar inhibited ASFV replication in different treatment modes, including pre-, co-, and post-treatment, suggesting that brequinar inhibited ASFV replication by acting on cell factors (Fig. 4). DHODH, the fourth enzyme in the de novo pyrimidine biosynthesis pathway, is a popular target for antiviral and anticancer activities. Uridine is the precursor of the pyrimidine nucleotide. Exogenous uridine can be taken up by human ovarian cancer cell line 2008 cells and the uptake rate is essentially linear during the first 30 min [39]. Additionally, exogenous uridine has been used to supplement the consumption of endogenous uridine by the drugs in vitro and in vivo studies [40, 41]. As previously mentioned, brequinar exerted a broad-spectrum antiviral activity by inhibiting DHODH activity and depleting intracellular pyrimidine pools. Consistent with the reported results, we also found that exogenous supplementation with pyrimidines reversed the anti-ASFV activity of brequinar, demonstrating that brequinar suppressed ASFV replication by inhibiting DHODH activity and depleting intracellular pyrimidine pools (Fig. 5).
DHODH is thought to be an enzyme required for the de novo synthesis of pyrimidine nucleotides, but recently Mao et al. found that brequinar activates ferroptosis by inhibiting DHODH activity independently of GPX4 or FSP1 [30]. Ferroptosis is a recently discovered form of cell death characterized by massive iron accumulation and lipid peroxidation [42]. The virus-induced cell death has long been recognized as a double-edged sword that inhibits or exacerbates viral replication [43]. Cheng et al. found that SIV promotes viral replication by activating GPX4-mediated ferroptosis [44]. However, the effect of ASFV infection on ferroptosis and whether brequinar inhibits ASFV replication through ferroptosis are still unknown. Therefore, we investigated the impact of ASFV infection on ferroptosis and the results showed that ASFV infection did not induce ferroptosis; however, brequinar treatment induced ferroptosis and the accumulation of intracellular Fe2+, mitochondrial Fe2+ or lipid peroxides, which were not reversed by exogenous uridine supplementation. We further explored the effect of ferroptosis on ASFV replication and, as expected, treatment with the ferroptosis agonist cisplatin inhibited ASFV replication and found that the inhibitory effect of brequinar on ASFV was partially reversed by the ferroptosis inhibitor ferrostatin-1 (Fig. 5). Hence, the ferroptosis pathway may be a target for the development of anti-ASFV compounds.
It is important to note that there were some side effects with brequinar. Thrombocytopenia was the main side effect, but it was dose-limiting [45]. Given that all drugs have side effects, these clinical observations are acceptable. Importantly, the selectivity index > 20 of brequinar and the effective concentration of brequinar used in our study are much lower than the doses used in previous clinical trials [46]. Although inhibitors can negatively affect cellular function and may lead to deleterious long-term and broad consequences by targeting host cell signaling pathways, the safety and pharmacology of brequinar have already been tested in clinical trials [47, 48]. Moreover, Li and colleagues showed that brequinar inhibited FMDV replication and provided a 25% survival rate in FMDV-infected mice in vivo, suggesting that brequinar could be an effective anti-FMD antiviral agent [27]. Therefore, develo** brequinar as an anti-ASFV inhibitor has advantages over develo** new drugs or identifying new antiviral strategies.
Conclusions
In summary, our data confirm that brequinar displays potent antiviral activity against ASFV in vitro and reveal the mechanism by which brequinar inhibits ASFV replication by activating ferroptosis, independent of inhibiting pyrimidine synthesis. Therefore, brequinar has potential as a novel drug or adjuvant therapeutic option to combat ASFV infection, and the ferroptosis pathway can be used as a novel target for anti-ASFV drug development.
Data Availability
The datasets used and/or analyzed in this study are obtained and available from the corresponding authors upon a reasonable request.
References
De la Torre A, Bosch J, Iglesias I, Muñoz MJ, Mur L, Martínez-López B, Martínez M, Sánchez-Vizcaíno JM. Assessing the risk of African swine fever introduction into the European Union by wild boar. Transbound Emerg Dis. 2015;62:272–9.
Arzt J, White WR, Thomsen BV, Brown CC. Agricultural diseases on the move early in the third millennium. Vet Pathol. 2010;47:15–27.
Iglesias I, Rodríguez A, Feliziani F, Rolesu S, de la Torre A. Spatio-temporal analysis of African swine fever in Sardinia (2012–2014): trends in domestic pigs and wild boar. Transbound Emerg Dis. 2017;64:656–62.
Gallardo MC, Reoyo AT, Fernández-Pinero J, Iglesias I, Muñoz MJ, Arias ML. African swine fever: a global view of the current challenge. Porcine Health Manag. 2015;1:21.
Gong L, Xu R, Wang Z, Deng Q, Wang H, Zhang G. African swine fever recovery in China. Vet Med Sci. 2020;6:890–3.
Liu Y, Zhang X, Qi W, Yang Y, Liu Z, An T, Wu X, Chen J. Prevention and control strategies of African swine fever and progress on pig farm repopulation in China. Viruses. 2021;13.
Dixon LK, Chapman DA, Netherton CL, Upton C. African swine fever virus replication and genomics. Virus Res. 2013;173:3–14.
Nunes JF, Vigário JD, Terrinha AM. Ultrastructural study of African swine fever virus replication in cultures of swine bone marrow cells. Arch Virol. 1975;49:59–66.
Zhang Y, Zhang Z, Zhang F, Zhang J, Jiao J, Hou M, Qian N, Zhao D, Zheng X, Tan X. ASFV transcription reporter screening system identifies ailanthone as a broad antiviral compound. Virol Sin. 2023.
Qiu Z, Li Z, Yan Q, Li Y, **ong W, Wu K, Li X, Fan S, Zhao M, Ding H, Chen J. Development of diagnostic tests provides technical support for the control of African swine fever. Vaccines (Basel). 2021;9.
Hakobyan A, Arabyan E, Avetisyan A, Abroyan L, Hakobyan L, Zakaryan H. Apigenin inhibits African swine fever virus infection in vitro. Arch Virol. 2016;161:3445–53.
Arabyan E, Hakobyan A, Kotsinyan A, Karalyan Z, Arakelov V, Arakelov G, Nazaryan K, Simonyan A, Aroutiounian R, Ferreira F, Zakaryan H. Genistein inhibits African swine fever virus replication in vitro by disrupting viral DNA synthesis. Antiviral Res. 2018;156:128–37.
Huang Z, Gong L, Zheng Z, Gao Q, Chen X, Chen Y, Chen X, Xu R, Zheng J, Xu Z, et al. GS-441524 inhibits African swine fever virus infection in vitro. Antiviral Res. 2021;191:105081.
Galindo I, Hernáez B, Berná J, Fenoll J, Cenis JL, Escribano JM, Alonso C. Comparative inhibitory activity of the stilbenes resveratrol and oxyresveratrol on African swine fever virus replication. Antiviral Res. 2011;91:57–63.
Fabregas J, García D, Fernandez-Alonso M, Rocha AI, Gómez-Puertas P, Escribano JM, Otero A, Coll JM. In vitro inhibition of the replication of haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) by extracts from marine microalgae. Antiviral Res. 1999;44:67–73.
Cuesta-Geijo M, Chiappi M, Galindo I, Barrado-Gil L, Muñoz-Moreno R, Carrascosa JL, Alonso C. Cholesterol flux is required for endosomal progression of African swine fever virions during the initial establishment of infection. J Virol. 2016;90:1534–43.
de León P, Bustos MJ, Torres E, Cañas-Arranz R, Sobrino F, Carrascosa AL. Inhibition of porcine viruses by different cell-targeted antiviral drugs. Front Microbiol. 2019;10:1853.
Galindo I, Garaigorta U, Lasala F, Cuesta-Geijo MA, Bueno P, Gil C, Delgado R, Gastaminza P, Alonso C. Antiviral drugs targeting endosomal membrane proteins inhibit distant animal and human pathogenic viruses. Antiviral Res. 2021;186:104990.
Freitas FB, Frouco G, Martins C, Leitão A, Ferreira F. In vitro inhibition of African swine fever virus-topoisomerase II disrupts viral replication. Antiviral Res. 2016;134:34–41.
Wang J, Ji M, Yuan B, Luo A, Jiang Z, Zhu T, Liu Y, Kamau PM, ** L, Lai R. Peptide OPTX-1 from ornithodoros papillipes tick inhibits the pS273R protease of African swine fever virus. Front Microbiol. 2021;12:778309.
Ivanov V, Efremov EE, Novikov BV, Balyshev VM, Tsibanov S, Kalinovsky T, Kolbasov DV, Niedzwiecki A, Rath M. Vaccination with viral protein-mimicking peptides postpones mortality in domestic pigs infected by African swine fever virus. Mol Med Rep. 2011;4:395–401.
Chen S, Ding S, Yin Y, Xu L, Li P, Peppelenbosch MP, Pan Q, Wang W. Suppression of pyrimidine biosynthesis by targeting DHODH enzyme robustly inhibits rotavirus replication. Antiviral Res. 2019;167:35–44.
Boukalova S, Hubackova S, Milosevic M, Ezrova Z, Neuzil J, Rohlena J. Dihydroorotate dehydrogenase in oxidative phosphorylation and cancer. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165759.
Schnellrath LC, Damaso CR. Potent antiviral activity of brequinar against the emerging Cantagalo virus in cell culture. Int J Antimicrob Agents. 2011;38:435–41.
Qing M, Zou G, Wang QY, Xu HY, Dong H, Yuan Z, Shi PY. Characterization of dengue virus resistance to brequinar in cell culture. Antimicrob Agents Chemother. 2010;54:3686–95.
Luthra P, Naidoo J, Pietzsch CA, De S, Khadka S, Anantpadma M, Williams CG, Edwards MR, Davey RA, Bukreyev A, et al. Inhibiting pyrimidine biosynthesis impairs Ebola virus replication through depletion of nucleoside pools and activation of innate immune responses. Antiviral Res. 2018;158:288–302.
Li SF, Gong MJ, Sun YF, Shao JJ, Zhang YG, Chang HY. Antiviral activity of brequinar against foot-and-mouth disease virus infection in vitro and in vivo. Biomed Pharmacother. 2019;116:108982.
Fu H, Zhang Z, Dai Y, Liu S, Fu E. Brequinar inhibits enterovirus replication by targeting biosynthesis pathway of pyrimidines. Am J Transl Res. 2020;12:8247–55.
**ong R, Zhang L, Li S, Sun Y, Ding M, Wang Y, Zhao Y, Wu Y, Shang W, Jiang X, et al. Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein Cell. 2020;11:723–39.
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee H, Koppula P, Wu S, Zhuang L, Fang B, et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature. 2021;593:586–90.
Ait-Ali T, Wilson AD, Westcott DG, Clapperton M, Waterfall M, Mellencamp MA, Drew TW, Bishop SC, Archibald AL. Innate immune responses to replication of porcine reproductive and respiratory syndrome virus in isolated swine alveolar macrophages. Viral Immunol. 2007;20:105–18.
Carrascosa AL, Bustos MJ, de Leon P. Methods for growing and titrating African swine fever virus: field and laboratory samples. Curr Protoc Cell Biol. 2011;Chap. 26:26.14.21–26.14.25.
Long F, Zhang M, Yang X, Liang X, Su L, An T, Zhang G, Zeng Z, Liu Y, Chen W, Chen J. The antimalaria drug artesunate inhibits porcine reproductive and respiratory syndrome virus replication by activating AMPK and Nrf2/HO-1 signaling pathways. J Virol. 2022;96:e0148721.
Jiang M, Song Y, Liu H, ** Y, Li R, Zhu X. DHODH inhibition exerts synergistic therapeutic effect with cisplatin to induce ferroptosis in cervical cancer through regulating mTOR pathway. Cancers (Basel). 2023;15.
Madak JT, Bankhead A 3rd, Cuthbertson CR, Showalter HD, Neamati N. Revisiting the role of dihydroorotate dehydrogenase as a therapeutic target for cancer. Pharmacol Ther. 2019;195:111–31.
Halasa T, Boklund A, Bøtner A, Mortensen S, Kjær LJ. Simulation of transmission and persistence of African swine fever in wild boar in Denmark. Prev Vet Med. 2019;167:68–79.
Wang T, Sun Y, Huang S, Qiu HJ. Multifaceted Immune responses to African swine fever virus: implications for vaccine development. Vet Microbiol. 2020;249:108832.
Sirakanyan S, Arabyan E, Hakobyan A, Hakobyan T, Chilingaryan G, Sahakyan H, Sargsyan A, Arakelov G, Nazaryan K, Izmailyan R, et al. A new microtubule-stabilizing agent shows potent antiviral effects against African swine fever virus with no cytotoxicity. Emerg Microbes Infect. 2021;10:783–96.
Chan TC, Howell SB. Mechanism of synergy between N-phosphonacetyl-L-aspartate and dipyridamole in a human ovarian carcinoma cell line. Cancer Res. 1985;45:3598–604.
** L, Li Y, Pu F, Wang H, Zhang D, Bai J, Shang Y, Ma Z, Ma XX. Inhibiting pyrimidine biosynthesis impairs Peste des Petits Ruminants Virus replication through depletion of nucleoside pools and activation of cellular immunity. Vet Microbiol. 2021;260:109186.
Fukushima R, Kanamori S, Hirashiba M, Hishikawa A, Muranaka R, Kaneto M, Kitagawa H. Inhibiting the teratogenicity of the immunosuppressant leflunomide in mice by supplementation of exogenous uridine. Toxicol Sci. 2009;108:419–26.
Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: past, present and future. Cell Death Dis. 2020;11:88.
Wang MP, Joshua B, ** NY, Du SW, Li C. Ferroptosis in viral infection: the unexplored possibility. Acta Pharmacol Sin. 2022;43:1905–15.
Cheng J, Tao J, Li B, Shi Y, Liu H. Swine influenza virus triggers ferroptosis in A549 cells to enhance virus replication. Virol J. 2022;19:104.
de Forni M, Chabot GG, Armand JP, Fontana X, Recondo G, Domenge C, Carde P, Barbu M, Gouyette A. Phase I and pharmacokinetic study of brequinar (DUP 785; NSC 368390) in cancer patients. Eur J Cancer. 1993;29a:983–8.
Burris HA 3rd, Raymond E, Awada A, Kuhn JG, O’Rourke TJ, Brentzel J, Lynch W, King SY, Brown TD, Von Hoff DD. Pharmacokinetic and phase I studies of brequinar (DUP 785; NSC 368390) in combination with cisplatin in patients with advanced malignancies. Invest New Drugs. 1998;16:19–27.
Makowka L, Sher LS, Cramer DV. The development of Brequinar as an immunosuppressive drug for transplantation. Immunol Rev. 1993;136:51–70.
Aggarwal M, Leser GP, Lamb RA. Repurposing papaverine as an antiviral agent against influenza viruses and paramyxoviruses. J Virol. 2020;94.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Funding
This research was funded by the Guangzhou Basic and Applied Basic Research Foundation (202201010490), Science and Technology Program of Guangzhou, China (202206010036), Start-up Research Project of Maoming Laboratory (2021TDQD002), and China Agriculture Research System of MOF and MARA (cars-35).
Author information
Authors and Affiliations
Contributions
Y.S. and G.Z. designed the study and revised the manuscript. Y.C. per-formed the experiments and wrote the paper. Y.G. and H.C. performed the experiments. Z.S., Z.H. and Z.W. participated in the establishment of the methodology and data analysis. Z.W. and Z.Z. provided some critical reagents. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors agree to publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Y., Guo, Y., Chang, H. et al. Brequinar inhibits African swine fever virus replication in vitro by activating ferroptosis. Virol J 20, 242 (2023). https://doi.org/10.1186/s12985-023-02204-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-023-02204-x