Abstract
Background
People diagnosed with neurological pathology may experience gait disorders that affect their quality of life. In recent years, research has been carried out on a variety of exoskeletons in this population. However, the satisfaction perceived by the users of these devices is not known. Therefore, the objective of the present study is to evaluate the satisfaction perceived by users with neurological pathology (patients and professionals) after the use of overground exoskeletons.
Methods
A systematic search of five electronic databases was conducted. In order to be included in this review for further analysis, the studies had to meet the following criteria: [1] the study population was people diagnosed with neurological pathology; [2] the exoskeletons had to be overground and attachable to the lower limbs; and [3]: the studies were to include measures assessing either patient or therapist satisfaction with the exoskeletons.
Results
Twenty-three articles were selected, of which nineteen were considered clinical trials. Participants diagnosed with stroke (n = 165), spinal cord injury (SCI) (n = 102) and multiple sclerosis (MS) (n = 68). Fourteen different overground exoskeleton models were analysed. Fourteen different methods of assessing patient satisfaction with the devices were found, and three ways to evaluate it in therapists.
Conclusion
Users’ satisfaction with gait overground exoskeletons in stroke, SCI and MS seems to show positive results in safety, efficacy and comfort of the devices. However, the worst rated aspects and therefore those that should be optimized from the users’ point of view are ease of adjustment, size and weight, and ease of use.
Similar content being viewed by others
Background
Stroke, spinal cord injury (SCI), multiple sclerosis (MS), Parkinson or cerebral palsy (CP) are some of the main causes of paresis around the world [1]. Lack of mobility and loss of independence to perform basic activities of daily living limit patients to a sedentary lifestyle, increasing the likelihood of chronic diseases [2]. Gait recovery in people with neurological disorders has a significant impact on quality of life and gait training is a relevant target of rehabilitation [3, 4].
In recent years, with the advent of improved electro-mechanical technology, faster data processing and the reduction of equipment size, exoskeletons have emerged as a new option in the field of rehabilitation that can enable walking around the environment [5]. Recent technology developments make overground gait exoskeletons increasingly available to rehabilitation facilities and patients with gait disorders. These technological innovations have become an alternative to manual gait rehabilitation [6]. Compared to conventional therapy, robotic gait rehabilitation can offer highly controlled, repetitive and intensive training in an engaging environment, reducing the physical burden on the therapist and providing objective and quantitative assessments of patient progression [7].
Currently, there are two types of exoskeletons for walking assistance in people with neurological pathology: body-weight-supported treadmill training (BWSTT) exoskeletons and overground exoskeletons [6, 8]. BWSTT exoskeletons included treadmill gait training, while overground exoskeletons included gait training that involved moving across the floor with or without body-weight support [9]. Recent narrative reviews [5, 10] of overground exoskeletons describe the current state of the art with device-specific features and limitations. Overground exoskeletons are emerging as revolutionary devices for gait rehabilitation due to the active participation required from the patient, which promotes physical activity [3, 11, 12], and the possibility of being used as an assistive device in the community [13]. Therefore, it is expected that the exoskeletons will be further developed to be used as a mobility device in the daily life by people with gait disorders [14].
Satisfaction can be defined as the extent to which the user’s physical, cognitive and emotional responses that result from the use of a system, product or service meet the user’s needs and expectations [15]. As research on wearable robotic exoskeletons in rehabilitation facilities has increased, recent studies have assessed patient and therapist satisfaction with the therapy. However, some studies refer that only 8% of the scientific literature regarding robotic exoskeletons has included considerations of patient satisfaction [16,17,18]. Considering that the satisfaction of an assistive device is highly dependent on the user’s perspective [19, 20], it is surprising that little research has focused on participant perceptions of these powered exoskeletons and their learning process [16,17,18]. Assessing overall patients and therapists satisfaction can help measure the aggregate quality of a product/service [21] and tracking patient satisfaction can help developers and researchers to improve the product/service for users. In patient engagement management, satisfaction is the extent to which a product/service meets patients’ expectations [22]. Focusing on the health care sector, quality of care and patient satisfaction are major issues [23]. Therefore, the evaluation of any device/service from patient’s and professional’s perspective is crucial [24,25,26].
In response to this lack of information about gait exoskeletons satisfaction, this paper aimed to systematically review the literature to assess the evidence concerning gait exoskeleton satisfaction in people with neurological pathology. The specific objectives are to: [1] Assess the satisfaction of gait overground exoskeleton interventions for people with neurological pathology and [2]: Describe the exoskeleton, participants and methodology used to assess satisfaction in this population. To the best of our knowledge, no such research has previously been carried out or published.
Methods
Selection process
The search focused on scientific articles which provided relevant clinical information aimed at studying participant or therapist’s satisfaction of interventions using a gait overground exoskeleton. In order to be included in the analysis, each article had to meet the following conditions: [1] the study population was people diagnosed with neurological pathology; [2] the exoskeletons had to be overground and attachable to the lower limbs; and [3]: the studies were to include measures assessing patient or therapist satisfaction or perception with the exoskeletons. There were no limitations regarding study design, year of publication, language, participants’ age or gender. Studies that used treadmills were excluded with the purpose of focusing only on studies that solely investigated overground exoskeleton effects without body weight support.
Search strategy
We searched for scientific publications in five online databases: PubMed, Cochrane, Web of Science (WOS), Scopus and Springer Link using the following terms: (“usability” or “satisfaction”) AND “exoskeleton” AND “gait” AND (“stroke” or “spinal cord injury” or “multiple sclerosis” or “Parkinson” or “acquired brain injury” or “traumatic brain injury” or “cerebral palsy” or “neurology” or “neurological conditions” or “neurological pathology”). The term “usability” was used in the search strategy according to Earthy et al. [27], usability can be considered as “quality in use” and defined as “the extent to which a system, product or service can be used by specified users to achieve specific goals with effectiveness, efficiency and satisfaction in a specified context” (ISO 9241 − 210:2009). The final search was completed in February 2022.
The identification, screening and eligibility check of the studies were all done by the same author. In case of uncertainty during the screening or the classification process, a decision was reached in agreement with the authors of this manuscript.
Methodological quality assessment
After determining which articles met the inclusion criteria, the same author conducted the level of evidence for the articles using the Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence [28], which is characterized by assessing the evidence according to the subject area or clinical setting and the type of study involving the clinical problem in question. To assess methodological quality, a modified version of the quantitative McMasters Critical Appraisal Tool (MMCAT) [29] was used. The MMCAT assessed a range of domains as a part of the appraisal process including purpose, literature review, design, sampling, outcomes, intervention, results and conclusion with implications for practice. The original MMCAT was modified following the methodology used by Bunge et al. [30], in order to provide a numerical score as a result of the critical appraisal process. One point was given for every “yes” answer, while no points were obtained for “no” or “not addressed” answers. In some instances, the “N/A” criterion was selected (as the criterion did not apply to some study designs), which then altered the overall scoring. Therefore, the overall scores for individual studies were converted to percentages for easing the interpretation and comparison purposes.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline [31] was used as a quality control tool for the review.
Data extraction and synthesis
The process of data extraction was performed by the same author. All the extracted data from studies were entered into tables for easy comparison and grou**. The following information was collected from the included studies: author and year details; exoskeleton used in the article; study design; sample characteristics; training details (number of sessions, frequency, and length); outcome measures used and results. If the data from any study was identified as missing, an attempt was made to contact the authors for the missing data, but if the authors did not respond, that information was not considered.
To ease understanding and facilitate easy comparison of satisfaction levels between different devices, a standardised score (%) is shown for the maximum score of each questionnaire. In the case that the results derived from the article only show averages score per item, the standardised score will be calculated using the maximum score of the items assessed.
Results
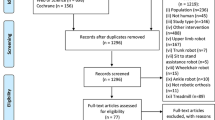
The search strategy implemented generated a total of 234 articles in online databases. The bibliographic manager Mendeley® was used to eliminate duplicate articles, removing 55 studies from our search. Finally, the remaining 179 publications were screened by their title and abstract according to the PICO model. 100 publications were full-text assessed for eligibility and 23 were finally selected (Fig. 1) to be analysed in detail (Table 1). Selected studies were published between 2012 and 2021 and all included studies were written in English. In total, there were 11 studies [13, 32,33,34,35,36,37,38,39,40,41] that included participants with SCI (n = 95), 8 studies [42,43,44,45,46,47,48,49] included participants diagnosed with stroke (n = 146), two studies [50, 51] included participants with MS (n = 67), 1 study [52] participants with SCI and stroke (SCI n = 7 and stroke n = 16), and other [53] with stroke and MS (stroke = 3, and MS = 1). In addition, in Fig. 2 is showed the volume of analysed studies per year.
Synthesis of the included studies
The sample size of the included articles was 8.9 ± 5.4 (range 3–20) people for SCI studies, 14.5 ± 12.8 (range 3–44) people for stroke studies and 16.3 ± 20.8 (range 1–40) people for MS studies. The included studies enrolled a total of 107 participants with SCI, 145 with stroke and 49 with MS. Male participants with SCI and stroke were 68.2% and 63.4% respectively, while in MS studies the sample was split 50% according to the gender. The age range of SCI participants was 16–68, 17–83 for stroke and 38–62 years for MS. The range of time since injury varied from 7 days to 29 years in people with SCI and from 3.6 days to 32 years in people with stroke.
Regarding the levels of SCI, the participants were: 11% cervical level; 25% thoracic level (Th) 1–6; 46% Th7-12 level and 18% lumbar level. And the classification of the participants according to the AIS scale was: 52% A; 18% B; 18% C; and 5% D. Regarding the classification and/or functionality of participants with stroke or MS, no homogeneous classification methods or descriptions were found that considered the origin or functionality of the stroke, or the type or functionality of MS.
This review identified 14 exoskeletons from which nine have FDA approval and/or CE certificate and are commercially available: ReWalk™ (ReWalk Robotics, Inc. USA); ReStore™ (ReWalk Robotics, Inc. USA); Ekso™ (Ekso Bionics Inc, USA); Ekso GT™ (Ekso Bionics Inc, USA); Indego™ (Parker Hannifin Corporation, Human & Control, USA); HAL™ (Cyberdine, Inc, Japan); REX™ (REX Bionics Ltd, New Zealand); MAK™ (Marsi Bionics S.L., Spain); and Exowalk™ (HMH Corp, Korea). The other exoskeletons included in the studies were: T-FLEX, ALLOR, Achilles, Kinesis and H2. The following exoskeletons were evaluated in more than one study: ReWalk (n = 5), Ekso GT (n = 5), Ekso (n = 2) and H2 (n = 2).
We found that 9 of 14 exoskeletons (64%) were completely bilateral and 8 exoskeletons actively assisted two or more joints (5: hip-knee, 3: hip-knee-ankle), while the rest actively aids in a single joint (3: knee, 3: ankle). The number of actuated degrees of freedom (DOF) in included exoskeletons ranges from one to three per leg in the sagittal plane (except for REX, which also enables movement in transverse and coronal planes).
The control strategies used in the exoskeletons included were mostly through assist-as-needed with stiffness variations algorithms, defined as the device ability to include the user’s capabilities as part of its control strategy. The exoskeletons that do not use this control system are Rewalk™ and REX, the latter having obstacle avoidance capabilities.
Every study analysed was carried out in hospital facilities, except for one intervention that was carried out in the community (outdoor and indoor) and at home on a free-to-use basis [13]. The total number of sessions performed with the exoskeletons ranged from one [39, 42, 45, 53] to 25 for SCI [35] and 20 for stroke and MS [43, 51]. Total study duration ranged from 1 day [39, 42, 45, 53] to 62 days for SCI [52] and 56 for stroke [49], with a weekly frequency between 0.8 [52] and 0.9 [52] sessions for stroke and SCI respectively and 5 sessions per week [35, 36, 43]. The time of use of the devices in the sessions of the selected studies ranged from 6 min [41] to 75 min [36] for SCI and between 27.5 [52] and 60 min per session for stroke [44, 49]. See Fig. 3 for the total number of sessions performed per exoskeleton and pathology.
Regarding to the tools used to assess the participant and therapist satisfaction with the exoskeletons, the included articles used 14 different methods. The most commonly used questionnaires to assess participant satisfaction were the Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST 2.0.) [13, 32, 40,41,42, 45, 46, 50, 51] and the Participant Satisfaction Questionnaire (PSQ) [33,34,35, 37, 49] used in 44% (n = 10) and 22% (n = 5) of the included studies, respectively. One of the included studies used the QUEST questionnaire with the services Sect. [13]. One of them added two additional items to the PSQ: “I could imagine using the device as an assistive walking aid at home” and “I would use the device as rehabilitation device in the future” [49].
Two studies [39, 52] used questionnaires developed by the company that produces the exoskeletons (Ekso, REX), one study developed a specific questionnaire for their study [36], other study used the Client Satisfaction Questionnaire (CSQ-8) [50], two conducted interviews with relatives [47] and patients [36, 47], and several assessed satisfaction parameters using Likert scales of: satisfaction, usefulness, disadvantages, willingness to repeat [44] and ease of use [48].In general, the issues most frequently evaluated through the participant satisfaction questionnaires were parameters related to: effectiveness (22% of the items in all questionnaires), overall satisfaction and recommendation (17%), ease of use (12%) and safety (10%).
Only three of the included studies assessed therapists’ satisfaction with the device [46, 50, 52]. The studies used different tools: an interview [52], the Physical Therapist Satisfaction Questionnaire (PTSQ) [46, 50] and the Ekso therapist feasibility survey [52], which was more focused on assessing the knowledge acquired after training and ease to use of the device. The PTSQ assessed aspects such as the amount of time to adjust the device and settings, safety, understandable device feedback and therapist satisfaction with the device.
Overall, the most frequently evaluated aspects of the therapists’ questionnaires were related to: ease of use (33% of the items in all questionnaires), ease of adjustment (17%), and effectiveness (17%). Other aspects assessed in the questionnaires were fatigue, safety and overall satisfaction with the device.
Main results: participants’ satisfaction with overground exoskeletons
One study did not provide its results on satisfaction, but the authors decided to include it in the analysis of the review in order to specify its satisfaction assessment methodology [47]. Participants’ satisfaction with the use of the exoskeleton in people with SCI, stroke and MS was generally positive, exceeding the average cut-off score in all questionnaires. The worst evaluated characteristics of the devices, and therefore the aspects to be optimised for development were: ease of adjustment and removal of the device [36, 40, 41, 46, 49], size and weight [13, 36, 39, 41, 42], and ease of use [13, 35]. On the other hand, the most positive exoskeletons features were: safety [32, 41, 46, 53], efficacy [41, 44] and comfort [35, 49, 53].
Concerning the QUEST questionnaire, the average score per item was 3.7 ± 0.5 points out of 5.0. The average per pathology was: stroke 3.7 ± 0.8 [42, 46, 53]; SCI 3.7 ± 0.3 [13, 32, 41]; and MS 3.7 ± 0.2 [50, 51]. The three parameters considered most important in a overground exoskeleton in the QUEST questionnaire were: effectiveness [13, 46], ease of use [13, 46], safety [13, 45], weight [13, 45] and comfort [45, 46].
Regarding the participants satisfaction questionnaire, which was shown just by the average results per item for the SCI studies, 4.1 ± 0.6 points per item [33, 34, 49] out of 5.0 were obtained. Participants self-reported improvements in spasticity [33, 34, 37], in bowel regulation [33, 37, 49] and in breathing difficulties [33,34,35]. These last three issues were included in the analysis of this review because they were part of the participants’ self-perceived satisfaction questionnaires, and not as outcomes related to the efficacy assessment by professionals.
Only two studies showed quantitative data regarding therapist satisfaction using the physical therapist questionnaire, in stroke the average was 3.7 out of 5.0 per item [46], and in MS was 4.3 out of 5.0 per item [50]. Difficulties related to donning/doffing were shown in the three studies that evaluated therapist satisfaction [46, 50, 52].
Methodological quality of the included studies
There were 19 studies identified as clinical trials according to the Clinical Trial definition proposed by the National Institutes of Health (NIH) [54] (see Additional file 1 for a detailed view on the clinical trial identification assessment). Table 2 provide an overview of the methodological quality and the level of evidence of the included studies. Most of the studies in this review are Non-Randomized Studied of Interventions (NRSI).
Only three Randomized Controlled Trials (RCT) were found [36, 38, 43]. 86% of the included studies had IV level (out of five) of methodological quality according to the OCEBM [28]. The methodological quality of the included studies was considered as poor to moderate. The raw scores were converted to a percentage for ease of comparison across the included studies as some criteria did not apply to all study designs. For example, the criterion for contamination was no applicable because the majority of the included studies were either cases series or case studies, there were no control or comparator groups. The highest critical appraisal was awarded to Fernández-Vázquez et al. [50] and the lowest to Chihara et al. [47]. While most of the included studies scored well for criterion two, three, six, eleven and twelve, there were a number of methodological concerns. These included measurement bias due to lack of sample size justification (criterion 4) and co-intervention bias (criterion 9). While the lack of participants’ blinding, therapists and measures increases the risk of placebo, Hawthorne effect [55] and measurement bias, given the nature of the intervention, these biases could not be entirely avoidable.
Discussion
The main objective of the present study is to assess satisfaction with gait exoskeletons in people with neurological pathology. This objective is particularly relevant in the field of exoskeleton development since feedback from patients and therapists is essential for further device optimization. In addition, the positive and negative aspects are critical to consider from the design stage and can be part of the technical requirements. This impacts not only companies that already have exoskeletons on the market, but also start-ups in the product development phase and research groups working in this field. However, regarding the articles published on gait exoskeletons, only few papers show the analysis of this key variable [16,17,18]. This lack of information in this field may also be due to confidentiality within these companies, whose aim could be to improve the device compared to the competitors, making this information highly sensitive to potential competitors in this technological field. Nevertheless, it is important to note that there seems to be an upward trend in the number of studies analysing satisfaction variables, with 70% of the studies included in this study having been published in the last 5 years. This increase of satisfaction-related studies may also be due to the exponential growth of publications concerning gait exoskeletons in recent years [12].
The search of published papers was carried out to find a variety of neurological pathologies, however only three were found: stroke, SCI and MS. Although it is true that these pathologies are the most studied in gait exoskeletons, there is published information in the literature on at least five other pathologies [10]: CP [14, 56,57,58], poliomyelitis [59], traumatic brain injury [60], spinocerebellar degeneration [61] and brain tumour surgery [62]. There is currently no information on usability satisfaction in these five pathologies.
Satisfaction with the use of the exoskeletons was generally positive among users. However, based on the results found, it appears that the exoskeletons need to be optimised to improve the ease of fitting and removal of the device, its weight and size, and its ease of use, among others. Concerning the ease of adjustment, Bryce et al. [63] suggested that individuals should be able to don and doff an exoskeleton independently in five minutes or less. However, other studies indicated that 84% of patients were able to don and doff independently, with a mean of 9:01 and 2:44, respectively [63,64,65]. The size and weight of the wearable exoskeleton are still heavy and bulky, due to their rigid structures, actuators and batteries. Rodríguez-Fernández et al. [10] report that the average weight of hip-knee exoskeletons is 14.3 kg (7.1 kg/leg), which approximately corresponds to the weight of an average adult human leg (i.e. 10.9 kg) [66]. Finally, the ease of use of the walking exoskeletons is another outstanding aspect to be improved. The next steps in the development of these devices could be directed towards improving usability in non-clinical settings and their access to the community. The transition from hospital facilities to community use requires a well-trained caregiver [67,68,69]. It is sometimes challenging for people with neurological pathology to identify a caregiver who will dedicate time and effort to support their partner during ambulation with the exoskeleton. Designing systems that do not require, or decrease, reliance on the caregiver could be a future goal for the development of exoskeleton usability in the community. In this review only one exoskeleton is used outside clinical environment, specifically in home and in the community [13]. Most participants do not perceive community use of these devices to be viable as a practical mobility solution. However, they also point to the therapeutic value of robotic exoskeletons in improving many aspects of functionality.
For the transition of exoskeletons out of the community environment, it is essential to review the cost of this equipment. The current price of these devices is high, although it seems to be starting to come down with the emergence of a multitude of start-up companies in the sector and studies demonstrating their effectiveness [68]. The financial cost of the devices was only addressed in a questionnaire, in which 90% of patients strongly agreed that they would like the exoskeleton to be more affordable in order to be able to access it [39]. Related to this, a qualitative review of patient experiences with walking exoskeletons [70] indicated that participants felt that purchasing this technology for personal use was not feasible given the cost; therefore, their access to robotic exoskeletons has typically been through a research project or in a clinical setting [71,72,73], in line with the research literature.
Regarding therapists’ satisfaction, the lack of evaluation of this parameter is noteworthy [74], as nowadays it is essential to evaluate the therapists’ perception of these devices in order for them to continue to evolve [75, 76]. In addition, it should be noted that currently exoskeletons are mostly indicated for clinical use, so therapists are the potential customers of the companies that manufacture these devices.
As reflected in the results section above, various ways of assessing satisfaction in patients and therapists have been found. Currently, there is no clear way of assessing people’s satisfaction with this type of device. However, among the scales included in this study, there are some that address more diverse dimensions than others, such as the QUEST scale, the Participant Satisfaction Questionnaire scale, the Usability Evaluation Questionnaire of Walking Devices and the REX Acceptability Questionnaire. Considering that the last two were created for a specific study and by a commercial brand focused on a specific exoskeleton model respectively, it is thought that the most appropriate for assessing participant satisfaction at present are the QUEST and the Participant Satisfaction Questionnaire. Furthermore, these two were the most widely used in the included studies. Moreover, it could be interesting in future studies to use both scales, as it could complement the information, since the QUEST scale addresses its items in a more general way and the Participant Satisfaction Questionnaire goes into more specific details of the device use.
It is essential to highlight the importance of ensuring that the satisfaction assessments of both patients and therapists are not influenced by the commercial brands of the exoskeletons, as can be seen in some of the studies included in this review [39, 52], which use self-completed questionnaires designed by the companies that develop the devices. This could introduce biases in the evaluation derived from conflicts of interest of these brands.
In addition, it should be noted that no studies have been found where the satisfaction of the child population and/or their parents with the use of exoskeletons has been assessed. However, there is not much literature available on the use of these types of portable devices in the paediatric population. In the current literature available, to the best of our knowledge, only one overground device in the market has been found to be indicated for the paediatric population [14, 77]. Therefore, robotic exoskeletons should be considered within the developmental stages to assist children with SCI and other clinical populations.
Limitations and considerations for future studies
We should be cautious in generalizing the results of this study since most of the studies included were Non-Randomized Studies on Intervention (NRSI). According to the Cochrane Handbook, it was decided to include NRSI following Reeves et al. [78] instructions for the inclusion of NRSI and RCT, since the objectives of our work cannot be answered by RCT alone at present. In the current literature, most publications on gait exoskeletons are NRSI, due to the fact that it is a new approach used for gait rehabilitation. Accordingly, all but one study included in this systematic review were published in the last six years.
Thus, further high-quality studies with larger sample sizes are needed to show more evidence in this area. All included studies show positive results of gait exoskeleton therapy satisfaction, but further investigation is needed to show solid conclusions about the efficacy of walking exoskeleton therapy. In addition, it is believed that the questionnaires evaluated, at least for the most part, have not passed the validation process for use in the studies. Therefore, it would be interesting to validate this type of questionnaires in order to increase the reliability of the results.
Conclusion
The satisfaction of users with gait overground exoskeletons in stroke, SCI and MS seems to show positive results in satisfaction parameters related to the safety, efficacy and comfort of the devices. However, the worst rated aspects and therefore those that should be optimized from the users’ point of view are ease of adjustment, size, weight, and ease of use. However, due to the heterogeneity of procedures to assess satisfaction and the low quality of the studies, the design of studies with high methodological quality and more homogeneous satisfaction outcome measures is recommended.
Implications for future: a new approach to exoskeletons design
At the moment, for the reasons mentioned above, there are people who still see the field of walking exoskeletons as under development: due to their weight, ease of use, ease of adjustment and removal, their economic cost and their adaptation to different pathologies. But what can be done about this problem?
Nowadays, the physical appearance of exoskeletons is rigid and closed to adaptations. The proposal is to change the trend by creating simpler devices customized to the patient’s functional capacity and therapeutic needs [75]. Therefore, it would be appropriate to take into consideration the proposal of some developers [79] to consider exoskeletons as a set of modules, each one being a single exoskeleton for a joint of the lower extremity (hips, knees and ankles) [80,81,82]. These systems could be assembled in a specific configuration to respond to a gait deficit in a particular patient. Therefore, therapists or users could choose the configuration of modules needed depending on their pathology and functional capacity. This type of system could provide multiple benefits over the current ones:
-
1)
The weight of the device would be reduced by having only the joints needed in each case, instead of the entire bilateral exoskeleton.
-
2)
By acting only on the joints where the user needs additional force, exoskeleton energy consumption would be optimized. Less current will be consumed by having fewer actuators and therefore the battery will last longer.
-
3)
The active work by the patient is increased by avoiding the support given to healthy joints.
-
4)
By adjusting to the number of joints the user needs, the cost of the device could be proportional to the number of joints needed.
-
5)
For rehabilitation centres or hospitals, a single device could allow them to cover more pathologies, for example: people with complete cervical SCI could use the entire exoskeleton bilaterally; people with stroke could use only the single leg configuration; people with MS could use the exoskeleton using only one knee, only one hip, or only knee and hip without the structure of the entire exoskeleton. Furthermore, if hospitals and rehabilitation centres were to purchase a complete modular exoskeleton, they could have the possibility to treat several patients simultaneously with the same device using different joints, improving the cost-effectiveness of the device.
-
6)
The use and progressive implantation of modules could help reduce the psychological impact of exoskeleton use in degenerative diseases.
-
7)
The time and ease of fitting and removal of the device to the patient could be optimized by only fitting the necessary joints.
Therefore, based on the above and from the clinical and practical point of view, it is thought that the proposed design could have additional benefits and improvements over the current exoskeletons. Nevertheless, the design of a modular exoskeleton requires to pay special attention to some aspects in order to ensure the expected functioning. As the structure of the device may not discharge to the ground in some phases of gait, e.g. in the single knee joint configuration; or in the full cycle, e.g. in the single hip joint configuration; it is necessary for the device to be as light as possible. Furthermore, this weight should be distributed in such a way that it does not affect the symmetry of the step during the walking process, by locating it wherever possible at the waist or trunk. On the other hand, fittings must consider that in any of the configuration’s torque is transmitted effectively and joint alignments are maintained.
Data availability
All data generated or analysed during the study are included in this published article and its supplementary information files.
Abbreviations
- BWSTT:
-
Body-Weight-Supported Treadmill Training
- CP:
-
Cerebral Palsy
- CSQ-8:
-
Client Satisfaction Questionnaire
- DOF:
-
Degrees of Freedom
- KAFO:
-
Knee Ankle Foot Orthosis
- MAK:
-
Marsi Active Knee
- MMCAT:
-
McMasters Critical Appraisal Tool
- MS:
-
Multiple sclerosis
- N/A:
-
Not applicable
- NIH:
-
National Institutes of Health
- OCEBM:
-
Oxford Centre for Evidence-Based Medicine
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines
- PSQ:
-
Participant Satisfaction Questionnaire
- PTSQ:
-
Physical Therapist Satisfaction Questionnaire
- QUEST:
-
Quebec User Evaluation of Satisfaction with Assistive Technology
- RCT:
-
Randomized Controlled Trial
- SCI:
-
Spinal Cord Injury
- SUS:
-
System Usability Scale
- Th:
-
Thoracic
- WOS:
-
Web of Science
References
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet Neurol. 2021 Oct;20(10):795–820. https://doi.org/10.1016/s1474-4422(21)00252-0.
Booth FW, Roberts CK, Laye MJ. Lack of Exercise is a Major cause of Chronic Diseases. Compr Physiol. 2012 April;2:1143–211. https://doi.org/10.1002/cphy.c110025.
Duddy D, Doherty R, Connolly J, McNally S, Loughrey J, Faulkner M. The Effects of Powered Exoskeleton Gait Training on Cardiovascular Function and Gait Performance: A Systematic Review. Sensors. 2021 May 1;21. https://doi.org/10.3390/s21093207.
Calafiore D, Negrini F, Tottoli N, Ferraro F, Ozyemisci-Taskiran O, de Sire A. Efficacy of robotic exoskeleton for gait rehabilitation in patients with subacute stroke: a systematic review with meta-analysis. Eur J Phys Rehabil Med. 2021 July. https://doi.org/10.23736/s1973-9087.21.06846-5.
Esquenazi A, Talaty M, Jayaraman A. Powered Exoskeletons for Walking Assistance in Persons with Central Nervous System Injuries: A Narrative Review. PM&R. 2017 January 1;9:46–62. https://doi.org/10.1016/j.pmrj.2016.07.534.
Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Cochrane database Syst Rev. 2013 July;25. https://doi.org/10.1002/14651858.CD006185.pub3.
Bruni MF, Melegari C, De Cola MC, Bramanti A, Bramanti P, Salvatore R. What does best evidence tell us about robotic gait rehabilitation in stroke patients: A systematic review and meta-analysis.J Clin Neurosci. 2018 February1;48:11–17. https://doi.org/10.1016/j.jocn.2017.10.04.
Mehrholz J, Pohl M. Electromechanical-assisted gait training after stroke: a systematic review comparing end-effector and exoskeleton devices. J Rehabil Med. 2012;44(3):193. https://doi.org/10.2340/16501977-0943.
Mehrholz J, Pohl M, Elsner B. The Improvement of Walking Ability Following Stroke.Dtsch Arztebl Int. 2018 September28;115:639–645. https://doi.org/10.3238/arztebl.2018.0639.
Rodríguez-Fernández A, Lobo-Prat J, Font-Llagunes JM. Systematic review on wearable lower-limb exoskeletons for gait training in neuromuscular impairments. J Neuroeng Rehabil. 2021 December. https://doi.org/10.1186/s12984-021-00815-5. 1;18.
Lefeber N, Swinnen E, Kerckhofs E. The immediate effects of robot-assistance on energy consumption and cardiorespiratory load during walking compared to walking without robot-assistance: a systematic review.Disabil Rehabil Assist Technol.2017 October3;12:657–671. https://doi.org/10.1080/17483107.2016.1235620.
Cumplido C, Delgado C, Ramos E, Puyuelo J, Garcés G, Destarac E, Plaza MA, Hernández A, Gutiérrez M, García A. E. Gait Assisted Exoskeletons for Children with Cerebral Palsy or Spinal Muscular Atrophy: A Systematic Review. 2021;49(3):333–348. https://doi.org/10.3233/nre-210135.
van Dijsseldonk RB, van Nes IJWW, Geurts ACHH, Keijsers NLWNLW. Exoskeleton home and community use in people with complete spinal cord injury. Sci Rep. 2020 December. https://doi.org/10.1038/s41598-020-72397-6. 1;10.
Delgado E, Cumplido C, Ramos J, Garcés E, Puyuelo G, Plaza A, Hernández M, Gutiérrez A, Taverner T, Destarac MA et al. ATLAS2030 Pediatric Gait Exoskeleton: Changes on Range of Motion, Strength and Spasticity in Children With Cerebral Palsy. A Case Series Study. Front Pediatr. 2021 November 24;9. https://doi.org/10.3389/fped.2021.753226.
ISO 9241-11. :2018(en), Ergonomics of human-system interaction — Part 11: Usability: Definitions and concepts. [cited 2022 March 25]. Available from: https://www.iso.org/obp/ui/#iso:std:iso:9241:-11:ed-2:v1:en.
Contreras-Vidal JL, Bhagat NA, Brantley J, Cruz-Garza JG, He Y, Manley Q, Nakagome S, Nathan K, Tan SH, Zhu F et al. Powered exoskeletons for bipedal locomotion after spinal cord injury.J Neural Eng. 2016 April11;13. https://doi.org/10.1088/1741-2560/13/3/031001.
Lajeunesse V, Vincent C, Routhier F, Careau E, Michaud F. Exoskeletons’ design and usefulness evidence according to a systematic review of lower limb exoskeletons used for functional mobility by people with spinal cord injury.Disabil Rehabil Assist Technol. 2016 October2;11:535–547. https://doi.org/10.3109/17483107.2015.1080766.
Hill D, Holloway CS, Morgado Ramirez DZ, Smitham P, Pappas Y. What are user perspectives of exoskeleton technology? A literature review. Int J Technol Assess Health Care. 2017;33:160–7. https://doi.org/10.1017/s0266462317000460.
Biddiss E, Chau T. Upper-limb prosthetics: critical factors in device abandonment. Am J Phys Med Rehabil. 2007 December;86:977–87. https://doi.org/10.1097/phm.0b013e3181587f6c.
Phillips B, Zhao H. Predictors of assistive technology abandonment.Assist Technol. 1993 June30;5:36–45. https://doi.org/10.1080/10400435.1993.10132205.
Koumpouros Y. A systematic review on existing measures for the subjective Assessment of Rehabilitation and Assistive Robot Devices. J Healthc Eng. 2016. https://doi.org/10.1155/2016/1048964.
Gustafsson A, Johnson MD, Roos I. The effects of customer satisfaction, relationship commitment dimensions, and triggers on customer retention. J Mark. 2005 October;69:210–8. https://doi.org/10.1509%2Fjmkg.2005.69.4.210.
Van Campen C, Sixma H, Friele RD, Kerssens JJ, Peters L. Quality of care and patient satisfaction: a review of measuring instruments. Med Care Res Rev. 1995;52:109–33. https://doi.org/10.1177/107755879505200107.
Vaughan-Graham J, Brooks D, Rose L, Nejat G, Pons J, Patterson K. Exoskeleton use in post-stroke gait rehabilitation: a qualitative study of the perspectives of persons post-stroke and physiotherapists.J Neuroeng Rehabil. 2020 September10;17. https://doi.org/10.1186/s12984-020-00750-x.
Shah SGS, Robinson I. Benefits of and barriers to involving users in medical device technology development and evaluation. Int J Technol Assess Health Care. 2007 January;23:131–7. https://doi.org/10.1017/s0266462307051677.
Cowan RE, Fregly BJ, Boninger ML, Chan L, Rodgers MM, Reinkensmeyer DJ. Recent trends in assistive technology for mobility. J Neuroeng Rehabil. 2012;9. https://doi.org/10.1186/1743-0003-9-20.
Earthy J, Sherwood Jones B, Bevan Nigel. Chapter 17. ISO Standards for User-Centered Design and the Specification of Usability - Usability in Government Systems [Book]. [cited 2022 March 23]. https://www.oreilly.com/library/view/usability-ingovernment/9780123910639/xhtml/CHP017.html
Jeremy Howick I, Chalmers P, Glasziou T, Greenhalgh C, Heneghan A, Liberati I, Moschetti B, Phillips, Thornton H. “Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence”. Oxford Centre for Evidence-Based Medicine. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
Law M, Stewart D, Pollock N, Letts L, Bosch J, Westmorland M. Guidelines for Critical Review Form - Quantitative Studies.McMaster University, Hamilton, Ontario. https://canchild.ca/system/tenon/assets/attachments/000/000/366/original/quantguide.pdf.
Rose Bunge L, Jade Davidson A, Roslyn Helmore B, Daniella Mavrandonis A, David Page T, Rochelle Schuster-Bayly T, KumarID S. Effectiveness of powered exoskeleton use on gait in individuals with cerebral palsy: A systematic review. 2021. https://doi.org/10.1371/journal.pone.0252193.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D, Barbour V, Barrowman N, Berlin JA, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6. https://doi.org/10.1371/journal.pmed.1000097.
Lopez-Larraz E, Trincado-Alonso F, Rajasekaran V, Perez-Nombela S, del-Ama AJ, Aranda J, Minguez J, Gil-Agudo A, Montesano L, López-Larraz E et al. Control of an Ambulatory Exoskeleton with a Brain-Machine Interface for Spinal Cord Injury Gait Rehabilitation. Front Neurosci. 2016 August 3;10. https://doi.org/10.3389/fnins.2016.00359.
Del-Ama AJ, Gil-Agudo Á, Bravo-Esteban E, Pérez-Nombela S, Pons JL, Moreno JC. Hybrid therapy of walking with Kinesis overground robot for persons with incomplete spinal cord injury: A feasibility study. Rob Auton Syst. 2015 November 1;73:44–58. https://doi.org/10.1016/j.robot.2014.10.014.
Sale P, Russo EF, Russo M, Masiero S, Piccione F, Calabrò RS, Filoni S. Effects on mobility training and de-adaptations in subjects with Spinal Cord Injury due to a Wearable Robot: A preliminary report.BMC Neurol. 2016 January28;16. https://doi.org/10.1186/s12883-016-0536-0.
Sale P, Russo EF, Scarton A, Calabrò RS, Masiero S, Filoni S. Training for mobility with exoskeleton robot in spinal cord injury patients: a pilot study. Eur J Phys Rehabil Med. 2018 October;154:745–51. https://doi.org/10.23736/s1973-9087.18.04819-0.
Platz T, Gillner A, Borgwaldt N, Kroll S, Roschka S. Device-Training for Individuals with Thoracic and Lumbar Spinal Cord Injury Using a Powered Exoskeleton for Technically Assisted Mobility: Achievements and User Satisfaction. Biomed Res Int. 2016;2016. https://doi.org/10.1155/2016/8459018.
Kwon SH, Lee BS, Lee HJ, Kim EJ, Lee JA, Yang SP, Kim TY, Pak HR, Kim HYHK, Kim HYHK, et al. Energy efficiency and patient satisfaction of Gait with knee-ankle-foot orthosis and Robot (ReWalk)-Assisted gait in patients with spinal cord Injury. Ann Rehabil Med. 2020 April;44:131–41. https://doi.org/10.5535/arm.2020.44.2.131.
Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk Powered Exoskeleton to restore ambulatory function to individuals with Thoracic-Level Motor-Complete spinal cord Injury. Am J Phys Med Rehabil. 2012;91:911–21. https://doi.org/10.1097/phm.0b013e318269d9a3.
Corbianco S, Cavallini G, Dini M, Franzoni F, D’Avino C, Gerini A, Stampacchia G. Energy cost and psychological impact of robotic-assisted gait training in people with spinal cord injury: effect of two different types of devices.Neurol Sci. 2021 August1;42:3357–3366. https://doi.org/10.1007/s10072-020-04954-w.
Birch N, Graham J, Priestley T, Heywood C, Sakel M, Gall A, Nunn A, Signal N. Results of the first interim analysis of the RAPPER II trial in patients with spinal cord injury: Ambulation and functional exercise programs in the REX powered walking aid. J Neuroeng Rehabil. 2017 June 19;14. https://doi.org/10.1186/s12984-017-0274-6.
Tamburella F, Tagliamonte NL, Pisotta I, Masciullo M, Arquilla M, Van Asseldonk EHF, Van Der Kooij H, Wu AR, Dzeladini F, Ijspeert AJ et al. Neuromuscular Controller Embedded in a Powered Ankle Exoskeleton: Effects on Gait, Clinical Features and Subjective Perspective of Incomplete Spinal Cord Injured Subjects.IEEE Trans Neural Syst Rehabil Eng. 2020 May1;28:1157–1167. https://doi.org/10.1109/tnsre.2020.2984790.
Villa-Parra AC, Lima J, Delisle-Rodriguez D, Frizera-Neto A, Bastos T. Stance control with the active knee orthosis ALLOR for post-stroke patients during walking. Biosyst Biorobotics. 2019;22:196–200. https://doi.org/10.3390/s20092452.
Nam YG, Lee JW, Park JHJW, Lee HJ, Nam KY, Park JHJW, Yu CS, Choi MR, Kwon BS. Effects of Electromechanical Exoskeleton-Assisted Gait Training on Walking Ability of Stroke Patients: A Randomized Controlled Trial.Arch Phys Med Rehabil. 2019 January1;100:26–31. https://doi.org/10.1016/j.apmr.2018.06.020.
Hoyer E, Opheim A, Jorgensen V. Implementing the exoskeleton Ekso GT(TM) for gait rehabilitation in a stroke unit - feasibility, functional benefits and patient experiences. Disabil Rehabil Technol. 2020. https://doi.org/10.1080/17483107.2020.1800110.
Gomez-Vargas D, Ballen-Moreno F, Barria P, Aguilar R, Azorín JM, Munera M, Cifuentes CA, Azorin JM, Munera M, Cifuentes CA. The Actuation System of the ankle exoskeleton T-FLEX: First Use Experimental Validation in people with stroke. BRAIN Sci. 2021;11. https://doi.org/10.3390/brainsci11040412.
Awad LN, Esquenazi A, Francisco GE, Nolan KJ, Jayaraman A. The ReWalk ReStoreTM soft robotic exosuit: A multi-site clinical trial of the safety, reliability, and feasibility of exosuit-augmented post-stroke gait rehabilitation.J Neuroeng Rehabil. 2020 June18;17. https://doi.org/10.1186/s12984-020-00702-5.
Chihara H, Takagi Y, Nishino K, Yoshida K, Arakawa Y, Kikuchi T, Takenobu Y, Miyamoto S, Nlshino K, Yoshida K et al. Factors Predicting the Effects of Hybrid Assistive Limb Robot Suit during the Acute Phase of Central Nervous System Injury.Neurol Med Chir (Tokyo). 2016 January15;56:33–37. https://doi.org/10.2176/nmc.oa.2015-0178.
Bortole M, Venkatakrishnan A, Zhu F, Moreno JC, Francisco GE, Pons JL, Contreras-Vidal JL. The H2 robotic exoskeleton for gait rehabilitation after stroke: early findings from a clinical study. J Neuroeng Rehabil. 2015 June 17;12. https://doi.org/10.1186/s12984-015-0048-y.
Jyräkoski T, Merilampi S, Puustinen J, Kärki A. Over-ground robotic lower limb exoskeleton in neurological gait rehabilitation: user experiences and effects on walking ability. Technol Disabil. 2021;33:53–63. https://doi.org/10.3233/TAD-200284.
Fernández-Vázquez D, Cano-De-la-Cuerda R, Gor-García-fogeda MD, Molina-Rueda F. Wearable Robotic Gait Training in Persons with Multiple Sclerosis: A Satisfaction Study. Sensors. 2021 July 2;21. https://doi.org/10.3390/s21144940.
Kozlowski AJ, Fabian M, Lad D, Delgado AD. Feasibility and Safety of a Powered Exoskeleton for Assisted Walking for Persons With Multiple Sclerosis: A Single-Group Preliminary Study.Arch Phys Med Rehabil. 2017 July1;98:1300–1307. https://doi.org/10.1016/j.apmr.2017.02.010.
Swank C, Sikka S, Driver S, Bennett M, Callender L. Feasibility of integrating robotic exoskeleton gait training in inpatient rehabilitation.Disabil Rehabil Assist Technol. 2020 May18;15:409–417. https://doi.org/10.1080/17483107.2019.1587014.
Puyuelo-Quintana G, Cano-De-La-Cuerda R, Plaza-Flores A, Garces-Castellote E, Sanz-Merodio D, Goñi-Arana A, Marín-Ojea J, García-Armada E. A new lower limb portable exoskeleton for gait assistance in neurological patients: A proof of concept study. J Neuroeng Rehabil. 2020 May 6;17. https://doi.org/10.1186/s12984-020-00690-6.
NIH’s Definition of a. Clinical Trial | grants.nih.gov. Accessed 06 Mar 2022. Available from: https://grants.nih.gov/policy/clinical-trials/definition.htm.
Elston DM. The Hawthorne effect. J Am Acad Dermatol. 2021 Mar. https://doi.org/10.1016/j.jaad.2021.01.085. 30;S0190-9622(21)00245-0.
Mataki Y, Kamada H, Mutsuzaki H, et al. Use of Hybrid Assistive Limb (HAL®) for a postoperative patient with cerebral palsy: a case report. BMC Res Notes. 2018;11:201. https://doi.org/10.1186/s13104-018-3311-z.
Matsuda M, Iwasaki N, Mataki Y, Mutsuzaki H, Yoshikawa K, Takahashi K, Enomoto K, Sano K, Kubota A, Nakayama T et al. Robot-assisted training using Hybrid Assistive Limb® for cerebral palsy.Brain Dev. 2018 September; 40:642–648. https://doi.org/10.1016/j.braindev.2018.04.004.
Lerner ZF, Damiano DL, Park HS, Gravunder AJ, Bulea TC. A Robotic Exoskeleton for Treatment of Crouch Gait in Children with Cerebral Palsy: Design and Initial Application.IEEE Trans Neural Syst Rehabil Eng. 2017 June1;25:650–659. https://doi.org/10.1109/tnsre.2016.2595501.
Arazpour M, Bani MA, Samadian M, Mousavi ME, Hutchins SW, Bahramizadeh M, Curran S, Mardani MA. The physiological cost index of walking with a powered knee-ankle-foot orthosis in subjects with poliomyelitis: A pilot study.Prosthet Orthot Int. 2016 August1;40:454–459. https://doi.org/10.1177/0309364615592697.
Nolan KJ, Karunakaran KK, Ehrenberg N, Kesten AG. Robotic exoskeleton Gait Training for Inpatient Rehabilitation in a Young Adult with traumatic brain Injury. Proc Annu Int Conf IEEE Eng Med Biol Soc EMBS. 2018;2809–12. https://doi.org/10.1109/embc.2018.8512745. October 26;2018-July.
Tsukahara A, Yoshida K, Matsushima A, Ajima K, Kuroda C, Mizukami N, Hashimoto M. Effects of gait support in patients with spinocerebellar degeneration by a wearable robot based on synchronization control.J Neuroeng Rehabil. 2018 September19;15:1–12. https://doi.org/10.1186/s12984-018-0425-4.
Li L, Ding L, Chen N, Mao Y, Huang D, Li L. Improved walking ability with wearable robot-assisted training in patients suffering chronic stroke.Biomed Mater Eng. 2015 January1;26:S329–S340. https://doi.org/10.3233/bme-151320.
Bryce TN, Dijkers MP, Kozlowski AJ. Framework for Assessment of the Usability of Lower-Extremity Robotic Exoskeletal Orthoses.Am J Phys Med Rehabil. 2015 November1;94:1000–1014. https://doi.org/10.1097/phm.0000000000000321.
Tefertiller C, Hays K, Jones J, Jayaraman A, Hartigan C, Bushnik T, Forrest GF. Initial outcomes from a Multicenter Study utilizing the Indego Powered Exoskeleton in spinal cord Injury. Top Spinal Cord Inj Rehabil. 2018;24:78–85. https://doi.org/10.1310/sci17-00014.
Kandilakis C, Sasso-Lance E. Exoskeletons for Personal Use After Spinal Cord Injury.Arch Phys Med Rehabil. 2021 February1;102:331–337. https://doi.org/10.1016/j.apmr.2019.05.028.
Stanley Plagenhoef F, Gaynor, Evans, Thomas Abdelnour. & (1983) Anatomical Data for Analyzing Human Motion, Research Quarterly for Exercise and Sport, 54:2, 169–178, https://doi.org/10.1080/02701367.1983.10605290.
Asselin P, Knezevic S, Kornfeld S, Cirnigliaro C, Agranova-Breyter I, Bauman WA, Spungen AM. Heart rate and oxygen demand of powered exoskeleton-assisted walking in persons with paraplegia. J Rehabil Res Dev. 2015;52:147–58. https://doi.org/10.1682/jrrd.2014.02.0060.
Gorgey AS. Robotic exoskeletons: The current pros and cons., World JO. 2018 September 1 October 19];9:112–119. https://doi.org/10.5312/wjo.v9.i9.112.
Gorgey AS, Sumrell R, Goetz LL. Exoskeletal Assisted Rehabilitation After Spinal Cord Injury. Atlas Orthoses Assist Devices. 2019 January 1:440–447.e2. https://doi.org/10.1016/B978-0-323-48323-0.00044-5.
Kinnett-Hopkins D, Mummidisetty CK, Ehrlich-Jones L, et al. Users with spinal cord injury experience of robotic locomotor exoskeletons: a qualitative study of the benefits, limitations, and recommendations. J Neuroeng Rehabil. 2020;17:124. https://doi.org/10.1186/s12984-020-00752-9.
Heinemann AW, Jayaraman A, Mummidisetty CK, Spraggins J, Pinto D, Charlifue S, Tefertiller C, Taylor HB, Chang SH, Stampas A, et al. Experience of robotic exoskeleton use at four spinal cord Injury Model Systems Centers. J Neurol Phys Ther. 2018;42:256–67. https://doi.org/10.1097/npt.0000000000000235.
Palermo AE, Maher JL, Baunsgaard CB, Nash MS. Clinician-Focused Overview of Bionic Exoskeleton Use After Spinal Cord Injury.Top Spinal Cord Inj Rehabil. 2017 June1;23:234–244. https://doi.org/10.1310/sci2303-234.
Fritz H, Patzer D, Galen SS. Robotic exoskeletons for reengaging in everyday activities: promises, pitfalls, and opportunities.Disabil Rehabil. 2019 February27;41:560–563. https://doi.org/10.1080/09638288.2017.1398786.
Louie DR, Mortenson WB, Durocher M, Teasell R, Yao J, Eng JJ. Exoskeleton for post-stroke recovery of ambulation (ExStRA): study protocol for a mixed-methods study investigating the efficacy and acceptance of an exoskeleton-based physical therapy program during stroke inpatient rehabilitation. BMC Neurol. 2020 January;2820. https://doi.org/10.1186/s12883-020-1617-7.
Laffranchi M, D’Angella S, Vassallo C, Piezzo C, Canepa M, De Giuseppe S, Di Salvo M, Succi A, Cappa S, Cerruti G et al. User-Centered Design and Development of the Modular TWIN Lower Limb Exoskeleton.Front Neurorobot. 2021 October7;15:709731. https://doi.org/10.3389/fnbot.2021.709731.
Turchetti G, Vitiello N, Trieste L, Romiti S, Geisler E, Micera S. Why effectiveness of robot-mediated neurorehabilitation does not necessarily influence its adoption. IEEE Rev Biomed Eng. 2014;7:143–53. https://doi.org/10.1109/rbme.2014.2300234.
Garcia E, Sancho J, Sanz-Merodio D, Prieto MATLAS. 2020: The pediatric gait exoskeleton project. In: Human-Centric Robotics- Proceedings of the 20th International Conference on Climbing and Walking Robots and the Support Technologies for Mobile Machines, CLAWAR 2017. World Scientific Publishing Co. Pte. Ltd.; 2018. pp 29–38. https://doi.org/10.1142/10736.
Sterne JAC, Hernán MA, McAleenan A, Reeves BC, Higgins JPT. Chapter 25: Assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ WV, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). 2020.
Plaza A, Hernandez M, Gutierrez A, Ramos J, Puyuelo G, Cumplido C, Garces E. Marie Andre Destarac, Elena Delgado. And Elena García. Design of a modular exoskeleton based on distributed central pattern generators. IEEE Sys J. 2022. https://doi.org/10.1109/JSYST.2022.3169235.
Plaza A, Hernandez M, Puyuelo G, Garces E, Garcia E. Wearable rehabilitation exoskeletons of the lower limb: analysis of versatility and adaptability. Disabil Rehabil Assist Technol. 2020. October;19. https://doi.org/10.1080/17483107.2020.1858976.
Meijneke C, Van Oort G, Sluiter V, Van Asseldonk E, Tagliamonte NL, Tamburella F, Pisotta I, Masciullo M, Arquilla M, Molinari M, et al. Symbitron Exoskeleton: design, control, and evaluation of a modular exoskeleton for incomplete and complete spinal cord injured individuals. IEEE Trans Neural Syst Rehabil Eng. 2021;29:330–9. https://doi.org/10.1109/tnsre.2021.3049960.
Wang J, Fei Y, Chen W. Integration, Sensing, and Control of a Modular Soft-Rigid Pneumatic Lower Limb Exoskeleton.Soft Robot. 2020 April1;7:140–154. https://doi.org/10.1089/soro.2019.0023.
Acknowledgements
The authors would like to thank Fernando Aneiros (Marsi Bionics S.L., Madrid, Spain) for proofreading the final version of the manuscript.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Study design and conceptualization: CT and MR; Database searching and screening: CT and PQ; Data synthesis and interpretation: CT, PF and HM; Manuscript drafting: CT; Critical revision: CT, MR, PQ, PF, HM and BS; Study supervision: MR, DE and GA. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cumplido-Trasmonte, C., Molina-Rueda, F., Puyuelo-Quintana, G. et al. Satisfaction analysis of overground gait exoskeletons in people with neurological pathology. a systematic review. J NeuroEngineering Rehabil 20, 47 (2023). https://doi.org/10.1186/s12984-023-01161-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12984-023-01161-4