Abstract
Depression is a common mood disorder characterized by a range of clinical symptoms, including prolonged low mood and diminished interest. Although many clinical and animal studies have provided significant insights into the pathophysiology of depression, current treatment strategies are not sufficient to manage this disorder. It has been suggested that connexin (Cx)-based hemichannels are candidates for depression intervention by modifying the state of neuroinflammation. In this study, we investigated the antidepressant-like effect of a recently discovered selective Cx hemichannel inhibitor, a small organic molecule called D4. We first showed that D4 reduced hemichannel activity following systemic inflammation after LPS injections. Next, we found that D4 treatment prevented LPS-induced inflammatory response and depressive-like behaviors. These behavioral effects were accompanied by reduced astrocytic activation and hemichannel activity in depressive-like mice induced by repeated low-dose LPS challenges. D4 treatment also reverses depressive-like symptoms in mice subjected to chronic restraint stress (CRS). To test whether D4 broadly affected neural activity, we measured c-Fos expression in depression-related brain regions and found a reduction in c-Fos+ cells in different brain regions. D4 significantly normalized CRS-induced hypoactivation in several brain regions, including the hippocampus, entorhinal cortex, and lateral septum. Together, these results indicate that blocking Cx hemichannels using D4 can normalize neuronal activity and reduce depressive-like symptoms in mice by reducing neuroinflammation. Our work provides evidence of the antidepressant-like effect of D4 and supports glial Cx hemichannels as potential therapeutic targets for depression.
Similar content being viewed by others
Introduction
Major depression is one of the most prevalent mood disorders worldwide. Individuals with depression display a variety of symptoms marked by prolonged depressed mood and diminished interest in activities [1, 2]. Although decades of clinical and basic research have provided significant insights into the pathophysiology of depression, the underlying neural basis remains unclear [3, 4]. Current treatment strategies are not sufficient to alleviate the burden caused by depression. For example, classic antidepressant drugs targeting traditional neurotransmitter systems, like serotonin, have delayed onsets [5]. Novel fast-acting antidepressants, such as ketamine, offer rapid relief from depressive symptoms but face restricted clinical use due to adverse effects, including safety in long-term treatment [6]. The future development of antidepressant medications still requires a better understanding of the neural mechanisms involved in depression and the identification of effective molecular targets.
In the central nervous system, hemichannels formed by connexins (Cxs) or pannexins are essential for neuron-glia communication and maintenance of brain homeostatic balance [7, 8]. In normal conditions, glial Cx-based hemichannels have relatively low activity. Under physiological conditions, neuronal pannexin hemichannels are permeable to small molecules like ATP and are preferentially permeable to Cl– [9, 10]. However, exacerbated neuronal hemichannel activity has been observed in diverse pathological conditions [51]. Overall, D4 did not change depressive-like z scores in unstressed mice (Vehicle, 0.00 ± 0.16; D4, − 0.24 ± 0.20) (Fig. 2A, G). Repeated LPS challenge induced significantly higher depressive-like z scores in mice, suggesting the expression of depressive-like behavior. This behavioral effect induced by LPS was reversed by D4 (Saline/vehicle, 0.00 ± 0.09; LPS/vehicle, 1.15 ± 0.11; LPS/D4, 0.28 ± 0.12) (Fig. 2N). Taken together, these results demonstrate that blocking preferentially Cx hemichannels with D4 exerts antidepressant-like effects in mice.
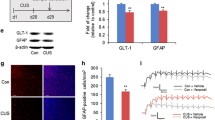
D4 suppresses LPS-induced astrocytic activation and hemichannel activity
Reactive glial cells are hallmarks of neuroinflammation and have been implicated in the pathophysiology of depression [52,53,54,55]. Having demonstrated the antidepressant-like properties of D4, we first investigated the effect of D4 on LPS-mediated inflammation. We injected adult mice intraperitoneally with a low dose of LPS (0.75 mg/kg, i.p.) or saline (control) daily for 1 week. Mice were orally fed with either D4 (5 mg/kg, p.o.) or vehicle after each LPS injection. Four hours after the last LPS injection, plasma and hippocampi were extracted for ELISA assay. We found that repeated LPS injections significantly increased pro-inflammatory cytokine IL-1β levels in the plasma and hippocampi. Notably, D4 significantly attenuated LPS-induced increases in the plasma and hippocampal IL-1β levels (Additional file 4: Fig. S4). We next tested whether D4 can affect reactive glia in LPS-induced depressive-like mice. After LPS injections and D4 treatment, mice were sacrificed after the last behavioral testing. We used GFAP as a marker to label astrocytes. Injections of LPS significantly increased astrocyte density in the hippocampus. Importantly, administration of D4 significantly decreased the number of GFAP+ astrocytes in the hippocampus (Saline/vehicle, 400.6 ± 26.3; LPS/vehicle, 464.6 ± 16.7; LPS/D4, 343.5 ± 17.5) (Fig. 3A, B). However, LPS-mediated hippocampal reactive microgliosis (Iba1+ microglia) was unaffected by D4 (Saline/vehicle, 85.2 ± 12.8; LPS/vehicle, 152.9 ± 21.0; LPS/D4, 149.2 ± 17.8) (Fig. 3C, D).
D4 reduces astrocytic proliferation and hemichannel activity induced by repeated low-dose LPS injections. A Immunostaining for astrocyte (GFAP) in the hippocampus. GFAP, red; DAPI, blue. Scale bar, 100 µm. B Quantification of GFAP+ astrocytes in the hippocampus. n = 15 hippocampal sections from 5 mice for all groups. C Immunostaining for microglia (Iba1) in the hippocampus. Iba1, red; DAPI, blue. Scale bar, 100 µm. D Quantification of Iba1+ cells in the hippocampus. n = 15 hippocampal sections from 5 mice for all groups. E Representative images showing astrocytes (GFAP) and CBF uptake in the hilus of the hippocampal DG from the LPS-injected mice. GFAP, red; CBF, green; DAPI, blue. Scale bar, 10 µm. F Quantification of GFAP+CBF+ area in the stained DG sections. Saline/vehicle, n = 8 sections from 3 mice; LPS/vehicle, n = 10 DG sections from 5 mice; LPS/D4, n = 10 DG sections from 4 mice. G Representative images showing microglia (Iba1) and CBF uptake in the hilus of the hippocampal DG the LPS-injected mice. Iba1, red; CBF, green; DAPI, blue. Scale bar, 10 µm. H Quantification of Iba1+CBF+ area in the stained DG sections. Saline/vehicle, n = 9 sections from 3 mice; LPS/vehicle, n = 25 DG sections from 9 mice; LPS/D4, n = 18 DG sections from 6 mice. I Representative images showing neurons (Nissl) and CBF uptake in the granule cell layer of the hippocampal DG the LPS-injected mice. Nissl, magenta; CBF, green; DAPI, blue. Scale bar, 10 µm. J Quantification of Nissl+CBF+ area in the stained DG sections. Saline/vehicle, n = 8 sections from 3 mice; LPS/vehicle, n = 11 DG sections from 5 mice; LPS/D4, n = 9 DG sections from 4 mice. Saline/vehicle (Ctrl), white; LPS/vehicle, red; LPS/D4, blue. The open circle shows the value from each DG. One-way ANOVA followed by LSD post hoc test (B). Kruskal–Wallis test with Bonferroni correction (D, F, H, J). *p < 0.05, **p < 0.01, ***p < 0.001
To directly interrogate whether D4 has a stronger impact on the hemichannel activity of astrocytes or microglia, we co-stained for GFAP or Iba1 after CBF dye loading in LPS mice. In the DG, D4 drastically suppressed LPS-induced CBF uptake in GFAP-positive astrocytes compared to saline/vehicle control (Saline/vehicle, 100.0 ± 23.9%; LPS/vehicle, 609.6 ± 145.6%; LPS/D4, 122.2 ± 67.9%), suggesting that D4 inhibits dye uptake in astrocytes triggered by LPS (Fig. 3E, F). By contrast, D4 only had a minimal effect on CBF uptake evoked by LPS in the Iba1-positive microglia (Saline/vehicle, 100.0 ± 14.4%; LPS/vehicle, 246.1 ± 46.0%; LPS/D4, 207.5 ± 26.3%) (Fig. 3G, H). In addition, we found that D4 significantly reduced CBF uptake induced by LPS in Nissl-positive neurons in the hippocampal DG (Saline/vehicle, 100.0 ± 20.6%; LPS/vehicle, 1566.0 ± 461.3%; LPS/D4, 236.5 ± 97.8%) (Fig. 3I, J).
Together, these data suggest that D4 can preferentially block astrocytic Cx hemichannels, and that LPS-induced increases in membrane permeability of microglia might be mediated by other pathways not inhibited by D4, such as pannexin1 hemichannels [7].
D4 improves depressive-like behaviors in mice subjected to chronic restraint stress
To further validate the antidepressant-like potentials of D4, we asked whether D4 could reverse behavioral changes induced by CRS, a stress paradigm used for develo** depressive-like phenotypes in mice [56, 57]. We first assessed the behavioral effects of D4 on unstressed control mice using an alternative testing protocol. Mice were orally fed with either a vehicle or D4 (5 mg/kg) 16–18 h before each behavioral test (Fig. 4A). We found no differences in the OFT center time (Vehicle, 31.8 ± 3.0 s; D4, 31.2 ± 3.7 s) and total distance (Vehicle, 47.4 ± 2.4 m; D4, 48.2 ± 2.0 m) between vehicle and D4 treated mice (Fig. 4B, C). The latter could confirm that D4 had no detectable effect on anxiety-like behavior and locomotor activity in healthy control mice. In the TST, D4 induced a significant reduction in immobility (by 34.5%) at 18 h after treatment (Vehicle, 48.1 ± 9.5 s; D4, 31.5 ± 6.1 s) (Fig. 4D). D4-treated mice showed a small but insignificant decrease (by 17.8%) in FST immobility (Vehicle, 96.1 ± 6.5 s; D4, 79.1 ± 12.6 s) (Fig. 4E). In the SPT, treatment with D4 did not change sucrose preference in unstressed mice (Vehicle, 70.3 ± 6.7%; D4, 64.0 ± 6.2%) (Fig. 4F).
D4 improves depressive-like behaviors induced by chronic restraint stress (CRS). A Schematic of drug treatment and behavioral testing. B Center time and (C) total distance in the OFT. D Time immobile in the TST. E Time immobile in the FST. F Sucrose preference in the SPT. G Depressive-like z score of the unstressed mice treated with vehicle or D4. n = 13 mice (5 male and 8 female) for each group. H Schematic of the design of CRS, drug treatment, and behavioral testing. I Center time and J total distance in the OFT. K Time immobile in the TST. L Time immobile in the FST. M Sucrose preference in the SPT. N Normalized depressive-like z score of control/vehicle, CRS/vehicle, and CRS/D4 treated mice. Control/vehicle, n = 10 mice, 5 male and 5 female; CRS/vehicle, n = 18 mice, 9 male and 9 female; CRS/D4, n = 19 mice, 10 male and 9 female. The filled dot indicates the value of each mouse. Data are mean ± s.e.m. Unpaired Student's t-test (B, C, E–G). Mann–Whitney U test (D). One-way ANOVA followed by LSD post hoc test (L–N). Kruskal–Wallis test with Bonferroni correction (I–K). *p < 0.05, **p < 0.01, ***p < 0.001
Next, we tested whether D4 could affect depressive-like behaviors in the mice subject to CRS. In the CRS-induced mouse model of depression, mice were exposed to 6-h restraint stress daily for 5 weeks. After the CRS paradigm, mice were orally treated with either vehicle or D4 (5 mg/kg, p.o.) eighteen hours before each behavioral test (Fig. 4H). In the OFT, we observed no significant differences in center time (Control/vehicle, 49.8 ± 7.1 s; CRS/vehicle, 48.2 ± 5.4 s; CRS/D4, 41.1 ± 4.7 s) or total distance (Control/vehicle, 35.4 ± 1.8 m; CRS/vehicle, 38.0 ± 1.9 m; CRS/D4, 39.2 ± 1.2 m) across groups following only a single dose of D4 (Fig. 4I, J). We then measured behavioral despair in the TST and FST. D4 significantly reduced LPS-induced increases in the TST immobility in CRS-exposed mice (Control/vehicle, 57.8 ± 9.6 s; CRS/vehicle, 108.4 ± 10.4; CRS/D4, 58.5 ± 9.4 s) (Fig. 4K). Again, D4 induced a significant decrease in FST immobility time in CRS-exposed mice 18 h after treatment (Control/vehicle, 85.7 ± 9.2 s; CRS/vehicle, 129.5 ± 8.5; CRS/D4, 95.2 ± 8.1 s) (Fig. 4K). In the SPT, CRS led to a reduction in the sucrose preference. D4 treatment also significantly increased the sucrose preference of CRS-exposed mice (Control/vehicle, 69.9 ± 6.9%; CRS/vehicle, 51.1 ± 6.2%; CRS/D4, 67.0 ± 4.5%) (Fig. 4L).
Next, we compared depressive-like behavior using the depressive-like z scores. Oral administration of D4 in a different treatment paradigm did not affect depressive-like z scores in the unstressed control mice (Vehicle, 0.00 ± 0.12; D4, − 0.22 ± 0.15) (Fig. 4G). Indeed, mice exposed to CRS exhibited significantly higher depressive-like z scores than the control/vehicle group. The CRS-induced depressive-like phenotype in mice was again ameliorated by D4 (Control/vehicle, 0.00 ± 0.14; CRS/vehicle, 1.02 ± 0.15; CRS/D4, 0.22 ± 0.13) (Fig. 4N). Given that two doses of D4 were administered before the beneficial effects in TST were observed, the earliest positive effects exerted by D4 were approximately two days. These results could consolidate the antidepressant-like effects of D4 and demonstrate its fast-acting potential using a different mouse model of depression.
D4 reduces CRS-mediated hemichannel activity and reactive glia
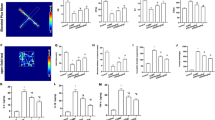
Chronic stress-induced changes in glial cell density and functions have been proposed to contribute to mood disorders, including depression [58]. Previous work reported that restraint stress increases glial activation and hemichannel activity in the hippocampus, which may be associated with behavioral deficits induced by chronic stress [39]. In the LPS-induced mouse model of depression, we found that D4 decreases hemichannel-mediated dye uptake (Fig. 1) and the number of reactive astrocytes in LPS-induced depressive-like mice (Fig. 3). To corroborate these results in the CRS-induced mouse model of depression, we measured hemichannel activity in the hippocampal DG from mice treated with either the vehicle or D4 by using CBF uptake. Consistent with results from the LPS model, treatments with D4 induced significant reductions in the number of GFAP+ astrocytes (Control/vehicle, 100.0 ± 23.0%; CRS/vehicle, 418.6 ± 62.0%; CRS/D4, 199.7 ± 48.8%), as well as a decrease in the CBF uptake in GFAP+ astrocytes (Control/vehicle, 100.0 ± 39.0%; CRS/vehicle, 444.5 ± 81.7%; CRS/D4, 267.6 ± 38.8) in the hippocampal DG (Fig. 5A–C). Compared to vehicle-treated unstressed control mice, CRS led to an increase in the DG Iba1+ microglia. CRS mice treated with D4 also showed a significant decrease in the number of Iba1+ microglia (Control/vehicle, 100.0 ± 39.0%; CRS/vehicle, 303.9 ± 37.6%; CRS/D4, 160.3 ± 16.5), and a tendency to reduce CBF uptake in the Iba1+ microglia (Control/vehicle, 100.0 ± 31.4%; CRS/vehicle, 417.2 ± 58.6%; CRS/D4, 347.8 ± 57.6) in the hippocampal DG (Fig. 5D–F). In the CRS-exposed mice, D4 also reduced CBF uptake (Control/vehicle, 100.0 ± 31.4%; CRS/vehicle, 917.6 ± 221.1%; CRS/D4, 341.4 ± 77.4) and dye uptake in Nissl+ neurons (Control/vehicle, 100.0 ± 12.1%; CRS/vehicle, 329.1 ± 51.1%; CRS/D4, 147.1 ± 43.1) in the hippocampal DG from mice subjected to CRS (Fig. 5G–I). Thus, these results suggest that D4 can suppress dye uptake through hemichannel and glial activation induced by CRS, indicating potential mechanisms that contribute to antidepressant-like behavioral outcomes of D4.
D4 decreases CRS-mediated hemichannel activity and glial activation. A Representative images showing astrocyte (GFAP) and CBF in the DG from the CRS-exposed mice. GFAP, red; CBF, green; DAPI, blue. Scale bar, 10 µm. B Quantification of GFAP+ astrocytes and (C) GFAP+CBF+ area in the stained DG sections. Control/vehicle, n = 9 DG sections from 3 mice; CRS/vehicle, n = 15 DG sections from 5 mice; CRS/D4, n = 13 DG sections from 5 mice. D Representative images showing microglia (Iba1) and CBF in the DG from the CRS-exposed mice. Iba1, red; CBF, green; DAPI, blue. Scale bar, 10 µm. E Quantification of Iba1+ microglia and F Iba1+CBF+ area in the stained DG sections. Control/vehicle, n = 8 DG sections from 4 mice; CRS/vehicle, n = 15 DG sections from 5 mice; CRS/D4, n = 15 DG sections from 5 mice. G Representative images showing CBF uptake in the hippocampal DG from the CRS-exposed mice treated with vehicle (Left) and D4 (Right). Nissl, magenta; CBF, green; DAPI, blue. Scale bar, 20 µm. H Quantification of CBF+ area and I Nissl+CBF+ area in the hippocampal DG sections. Control/vehicle, n = 8 DG sections from 3 mice; CRS/vehicle, n = 12 DG sections from 5 mice; CRS/D4, n = 13 DG sections from 5 mice. The open circle represents the value from each section. Control/vehicle (Ctrl), white; CRS/vehicle, red; CRS/D4, blue. Kruskal–Wallis test with Bonferroni correction (B, C, E, H, I). One-way ANOVA followed by LSD post hoc test (F). *p < 0.05, **p < 0.01, ***p < 0.001
D4 restores neuronal activity in CRS-induced depressive-like mice
Alterations in neural activity and brain network connectivity are consistently associated with depression [59,60,61,62]. Glial cells are important for supporting the functions of neurons [63, 64]. In addition, astroglial Cx hemichannels have been shown to regulate behavior by modulating neuronal activity [65, 66]. Therefore, we asked whether the antidepressant-like effects of hemichannel blockade with D4 could be accompanied by changes in neural activity.
After CRS and the battery of four behavioral tests (OFT, TST, FST, and SPT), mice were subjected to one last TST, which acted as an acute stressor, and were sacrificed 1 h later to evaluate neuronal activation (Fig. 6A). Similar to previous results (Fig. 4), CRS increased depressive-like z scores in mice, and D4 treatment (5 mg/kg, p.o.) reduced mouse depressive-like z scores (Depressive-like z score, ctrl/vehicle, 0.00 ± 0.17; CRS/vehicle, 0.86 ± 0.24; CRS/D4, − 0.26 ± 0.21) (Fig. 6B). In the TST, D4 (5 mg/kg, p.o.) decreased immobility time, and significantly increased struggling time compared to CRS/vehicle group (TST immobility, ctrl/vehicle, 85.0 ± 23.3 s; CRS/vehicle, 161.1 ± 9.1 s; CRS/D4, 92.3 ± 36.5 s. TST struggling, ctrl/vehicle, 7.2 ± 3.1 s; CRS/vehicle, 1.1 ± 0.4 s; CRS/D4, 22.3 ± 9.0 s) (Fig. 6C, D). This suggests that D4 reduces depressive-like behaviors in the CRS-exposed mice.
D4 rescues hypofunction of depression-related brain regions in the CRS-induced depressive-like mice. A Schematic of the design of CRS, drug treatment, behavioral testing, and c-Fos immunostaining after tail suspension test (TST). B Depressive-like z score of mice used for the TST and c-Fos experiments. C Time immobile and struggling (D) in the TST. The filled dot shows the value of each mouse. Ctrl/vehicle, white; CRS/vehicle, red; CRS/D4, blue. n = 5 mice for all groups. Data are mean ± s.e.m. One-way ANOVA followed by LSD post hoc test (B). Kruskal–Wallis test with Bonferroni correction (C, D). E Immunostaining for c-Fos expression (red) in the depression-associated brain regions of ctrl/vehicle, CRS/vehicle, and CRS/D4 treated mice (from top to bottom). The nuclei were stained with DAPI (blue). Scale bar, 100 µm. dDG: dorsal dentate gyrus; vDG: ventral dentate gyrus; vCA1: ventral cornu ammonis 1; vSub: ventral subiculum; vEC: ventral entorhinal cortex; LS: lateral septum; Amy: amygdala; NAc: nucleus accumbens. F Violin plot of c-Fos+ cells in each region after the TST. Ctrl/vehicle, white; CRS/vehicle, red; CRS/D4, blue. The open circle indicates the value from each section. n = 15 sections from 5 mice for all groups. One-way ANOVA followed by LSD post hoc test (LS and NAc). Kruskal–Wallis test with Bonferroni correction (dDG, vDG, vCA1, vSub, EC, and Amy). *p < 0.05, **p < 0.01, ***p < 0.001
Subsequently, we assessed neuronal activation using c-Fos immunostaining following acute exposure to stress (TST). In mice subjected to CRS, there were significantly fewer c-Fos+ cells in several depression-related brain regions, including the dorsal and ventral hippocampus (dDG, vDG, vCA1, vSub), ventral entorhinal cortex, lateral septum, and nucleus accumbens (NAc). CRS-exposed mice treated with D4 (5 mg/kg, p.o.) exhibited increases in the number of c-Fos+ cells in the hippocampus (dDG, vDG, vCA1), entorhinal cortex, and lateral septum. No significant change in the number of c-Fos+ cells was found in the amygdala (Fig. 6E, F). These data suggest that D4 may improve behavioral deficits in depressive-like mice by restoring neural activity in various brain regions.
Discussion
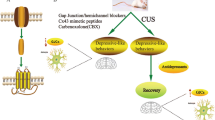
Hemichannels are important for regulating cellular communication and maintaining homeostasis [8]. Uncontrolled hemichannel activation has been proposed to disrupt neuron-glia communication, promote inflammation, and lead to cell damage in various brain diseases [7]. Recent evidence suggests that elevated hemichannel activity is associated with pathological changes in depression [67]. Therefore, inhibiting hemichannel activity may provide therapeutic benefits, as previously suggested [67,68,69].
Accordingly, in the present study, we demonstrated the rapid, antidepressant-like effects of D4, which reduced depressive-like symptoms in mice exposed to repeated systemic LPS challenges or CRS. We also showed that blocking hemichannels with D4 inhibits stress-mediated reactive astrogliosis and induces a brain-wide restoration of neural activity in the depressive-like mice subjected to CRS, which may contribute to the behavioral effects of D4. Together, our findings indicate that Cx hemichannels can constitute effective therapeutic targets for depression, and that D4 can be a new molecule mediating this therapeutic effect.
The pathogenesis of depression is highly heterogeneous and is associated with intricate interplays across multiple neurotransmitter systems, neuroimmune systems, neural circuity, and brain networks [70,71,72]. Considering that neuroinflammatory responses are likely to affect the ontogeny of the central nervous system [73], outcomes under these conditions could be irreversible, and could generate a radical difference compared to the effects of neuroinflammation that only occurs in adulthood. These differences limit current treatment outcomes and dramatically impede the rational design of successful therapies.
Cx hemichannels have been suggested as potential targets for depression [24, 67, 74]. Several agents, including small molecules, antibodies, and peptides, are available for inhibiting Cx hemichannels, with most studies carried out in vitro [18]. However, the behavioral benefits of hemichannel inhibition in depression in vivo remain elusive. In the present study, using two well-established mouse models of depression, we provide substantive evidence for the first time that D4 treatment can improve stress-induced depressive-like symptoms in adult mice. In addition, adult mice that received either acute or chronic applications of D4 did not show evident side effects. The latter could suggest that D4 is a relatively safe Cx hemichannel inhibitor with therapeutic effects. The antidepressant-like potential of D4 still needs to be substantiated for future translational research and clinical studies. Future work is also required to determine its bioavailability and druggability.
It has been shown that hemichannel activity can increase due to inflammation and chronic stress in adult animals [39, 67]. One of the prevailing hypotheses is that abnormal hemichannel activity may amplify detrimental effects on several cellular processes and promote the pathogenesis of a range of neurological and neurodegenerative diseases [10, 16, 43, 75, 76]. For example, in animal models of brain injury, such as ischemic stroke, hemichannels are activated in response to injury and pro-inflammatory cytokines. Dysregulated hemichannel activity can facilitate excitotoxicity due to the excessive release of gliotransmitters and may lead to neuronal death [77]. During epileptogenesis, seizures increase glial hemichannel activity [16, 78]. In turn, such heightened hemichannel activity promotes seizure generation and propagation through neuroinflammation and neuronal hyperexcitability [15, 79]. In animal studies, currently available hemichannel inhibitors have been used to control diseases like ischemia and epilepsy [12, 15]. Consistent with a previous study [41], we found that a single high-dose LPS injection increases glial hemichannel activity in the hippocampus. In addition, we found that repeated low doses of LPS challenges can increase Cx hemichannel activity in the hippocampal DG. Blocking hemichannels with D4 also prevented LPS-mediated reactive astrogliosis, probably due to reduced hemichannel activity in astrocytes, as evidenced by decreased CBF dye uptake. Thus, decreases in Cx hemichannel activity may contribute to antidepressant-like behavioral effects exerted by D4 in the LPS-induced depression model. These findings are consistent with the hypothesis that inhibition of Cx hemichannels by small molecules or other agents can improve depressive-like behaviors.
Our finding that blocking Cx hemichannels with D4 restored neuronal activity in CRS-exposed depressive-like mice provides another mechanistic insight into how hemichannel inhibition can contribute to behavioral improvement. Reduced neuronal activity in several regions of the limbic system has been identified in the etiology of depression [80]. Notably, post-mortem and neuroimaging studies have confirmed that the hippocampus is hypoactive in patients with depression [81, 82]. Antidepressant treatments can reverse depression-associated structural and functional changes in the hippocampus [83,84,85].
Similar to previous findings, we found that mice acutely exposed to CRS display significant reductions in neuronal activity in the hippocampus [86] and several other limbic regions, including the entorhinal cortex and the lateral septum. In contrast, D4 treatment reduces depressive-like behavior in the TST and enhances neural activation, as evidenced by the recovery of c-Fos+ cells in these regions. Although our dye uptake and glial density assays were restricted to the hippocampal DG regions, findings from our c-Fos screening results raise the possibility that D4 could impact the hemichannel and neural activity in multiple sites across the brain after systemic application via oral administration. Evidence for this possibility is supported by our recent study showing that oral treatment of D4 with optimized doses is sufficient to reduce seizure-induced glial cell density and hemichannel activity in different subfields of the hippocampus and anterior piriform cortex [16]. Data from clinical and preclinical studies suggest that the route of antidepressant administration may affect therapeutic efficacy and experience, especially for the novel rapid-acting antidepressant such as ketamine [6, 85]. Thus, specific mechanisms by which CRS reduces neuronal activation and D4 regulates neural activity remain to be studied with more sophisticated and precise targeted drug delivery strategies.
Cx43 is predominantly expressed in astrocytes in the adult mouse brain. Resting microglia rarely express Cx43, but its expression level can be increased in activated microglia [75, 87, 88]. Previously, it has been shown that D4 inhibits Cx43 hemichannels but not gap junction channels [29]. In this study, we showed that D4 reduces LPS-induced CBF uptake in astrocytes without affecting dye uptake in microglia. D4-induced reduction in astroglia-mediated hemichannel activity could indirectly reduce pannexin1 hemichannel activity in neurons, as observed under neuroinflammation triggered by a neurotoxic beta-amyloid peptide [89]. Based on existing studies suggesting that hemichannels can regulate gliotransmitter release, synaptic transmission, and neuronal firing [90,91,92,93,94], we speculate that the behavioral effect of D4 is likely mediated by the initial inhibition of astrocytic Cx43 hemichannel activity, which subsequently affects neuron–glia interaction and neuronal function. A limitation of our current study was the lack of detailed comparisons between sexes in their sensitivity to chronic stressors and compound D4. Sex could be a factor that affects the prognosis of depression and the treatment response of antidepressant interventions [2, 5]. It will be interesting to test whether D4 has a sex-specific effect on changes in hemichannel and behavioral activity induced by depressogenic factors. Given that Cx hemichannels can actively regulate neural activity and behavioral states via a cornucopia of cellular processes [64, 65], future studies are needed to elucidate the mechanisms of antidepressant-like behavioral effects of D4.
Conclusion
In conclusion, we have shown that a recently discovered hemichannel inhibitor, namely the small organic molecule D4, can exert antidepressant-like effects in mice subjected to repeated systemic LPS challenges or CRS. These behavioral benefits of D4 are accompanied by the blockade of hemichannel activity reduced inflammatory response, and a brain-wide restoration of neural activity in depressive-like mice. Our findings support the hypothesis that the hemichannel inhibitor is a promising pharmacophore for future antidepressant development focusing on glial cells. A better understanding of D4’s cellular mechanisms may help to identify its therapeutic potential and promote clinical uses of hemichannel inhibitors in mood disorders.
Availability of data and materials
All data presented in this study are available from the corresponding author upon reasonable request.
References
Maj M, Stein DJ, Parker G, Zimmerman M, Fava GA, De Hert M, Demyttenaere K, McIntyre RS, Widiger T, Wittchen HU. The clinical characterization of the adult patient with depression aimed at personalization of management. World Psychiatry. 2020;19:269–93.
Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, Mohr DC, Schatzberg AF. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065.
Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68.
Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902.
Harmer CJ, Duman RS, Cowen PJ. How do antidepressants work? New perspectives for refining future treatment approaches. Lancet Psychiatry. 2017;4:409–18.
Riggs LM, Gould TD. Ketamine and the future of rapid-acting antidepressants. Annu Rev Clin Psychol. 2021;17:207–31.
Giaume C, Naus CC, Sáez JC, Leybaert L. Glial connexins and pannexins in the healthy and diseased brain. Physiol Rev. 2021;101:93–145.
Spray DC, Ye ZC, Ransom BR. Functional connexin “hemichannels”: a critical appraisal. Glia. 2006;54:758–73.
Patel D, Zhang X, Veenstra RD. Connexin hemichannel and pannexin channel electrophysiology: how do they differ? FEBS Lett. 2014;588:1372–8.
Thompson RJ, Macvicar BA. Connexin and pannexin hemichannels of neurons and astrocytes. Channels. 2008;2:81–6.
**ng L, Yang T, Cui S, Chen G. Connexin hemichannels in astrocytes: role in CNS disorders. Front Mol Neurosci. 2019;12:23.
Ma D, Feng L, Cheng Y, **n M, You J, Yin X, Hao Y, Cui L, Feng J. Astrocytic gap junction inhibition by carbenoxolone enhances the protective effects of ischemic preconditioning following cerebral ischemia. J Neuroinflamm. 2018;15:198.
Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–7.
Fukuyama K, Fukuzawa M, Okada M. Upregulated and hyperactivated thalamic connexin 43 plays important roles in pathomechanisms of cognitive impairment and seizure of autosomal dominant sleep-related hypermotor epilepsy with S284L-mutant alpha4 subunit of nicotinic ACh receptor. Pharmaceuticals. 2020;13:99.
Yang TT, Qian F, Liu L, Peng XC, Huang JR, Ren BX, Tang FR. Astroglial connexins in epileptogenesis. Seizure. 2021;84:122–8.
Guo A, Zhang H, Li H, Chiu A, García-Rodríguez C, Lagos CF, Sáez JC, Lau CG. Inhibition of connexin hemichannels alleviates neuroinflammation and hyperexcitability in temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2022;119: e2213162119.
Yin X, Feng L, Ma D, Yin P, Wang X, Hou S, Hao Y, Zhang J, **n M, Feng J. Roles of astrocytic connexin-43, hemichannels, and gap junctions in oxygen-glucose deprivation/reperfusion injury induced neuroinflammation and the possible regulatory mechanisms of salvianolic acid B and carbenoxolone. J Neuroinflamm. 2018;15:97.
Willebrords J, Maes M, Crespo Yanguas S, Vinken M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol Ther. 2017;180:144–60.
Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305–20.
Ernst C, Nagy C, Kim S, Yang JP, Deng X, Hellstrom IC, Choi KH, Gershenfeld H, Meaney MJ, Turecki G. Dysfunction of astrocyte connexins 30 and 43 in dorsal lateral prefrontal cortex of suicide completers. Biol Psychiatry. 2011;70:312–9.
Miguel-Hidalgo JJ, Wilson BA, Hussain S, Meshram A, Rajkowska G, Stockmeier CA. Reduced connexin 43 immunolabeling in the orbitofrontal cortex in alcohol dependence and depression. J Psychiatr Res. 2014;55:101–9.
Wang Y, Su Y, Yu G, Wang X, Chen X, Yu B, Cheng Y, Li R, Saez JC, Yi C, et al. Reduced oligodendrocyte precursor cell impairs astrocytic development in early life stress. Adv Sci. 2021;8: e2101181.
Portal B, Delcourte S, Rovera R, Lejards C, Bullich S, Malnou CE, Haddjeri N, Deglon N, Guiard BP. Genetic and pharmacological inactivation of astroglial connexin 43 differentially influences the acute response of antidepressant and anxiolytic drugs. Acta Physiol (Oxf). 2020;229: e13440.
Jeanson T, Pondaven A, Ezan P, Mouthon F, Charveriat M, Giaume C. Antidepressants impact connexin 43 channel functions in astrocytes. Front Cell Neurosci. 2015;9:495.
Shiroyama T, Fukuyama K, Okada M. Distinct effects of escitalopram and vortioxetine on astroglial L-glutamate release associated with Connexin43. Int J Mol Sci. 2021;22:10013.
Krystal JH, Charney DS, Duman RS. A new rapid-acting antidepressant. Cell. 2020;181:7.
Liu X, Gangoso E, Yi C, Jeanson T, Kandelman S, Mantz J, Giaume C. General anesthetics have differential inhibitory effects on gap junction channels and hemichannels in astrocytes and neurons. Glia. 2016;64:524–36.
Wang Q, Jie W, Liu JH, Yang JM, Gao TM. An astroglial basis of major depressive disorder? An overview. Glia. 2017;65:1227–50.
Cisterna BA, Vargas AA, Puebla C, Fernandez P, Escamilla R, Lagos CF, Matus MF, Vilos C, Cea LA, Barnafi E, et al. Active acetylcholine receptors prevent the atrophy of skeletal muscles and favor reinnervation. Nat Commun. 2020;11:1073.
Turovsky EA, Braga A, Yu Y, Esteras N, Korsak A, Theparambil SM, Hadjihambi A, Hosford PS, Teschemacher AG, Marina N, et al. Mechanosensory signaling in astrocytes. J Neurosci. 2020;40:9364–71.
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J Mice. Neuropsychopharmacology. 2013;38:1609–16.
Chiba S, Numakawa T, Ninomiya M, Richards MC, Wakabayashi C, Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–9.
Rodriguez A, Zhang H, Klaminder J, Brodin T, Andersson M. ToxId: an efficient algorithm to solve occlusions when tracking multiple animals. Sci Rep. 2017;7:14774.
Kraeuter AK, Guest PC, Sarnyai Z. The open field test for measuring locomotor activity and anxiety-like behavior. Methods Mol Biol. 2019;1916:99–103.
Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp. 2012. https://doi.org/10.3791/3769.
Yankelevitch-Yahav R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp. 2015. https://doi.org/10.3791/52587-v.
Liu MY, Yin CY, Zhu LJ, Zhu XH, Xu C, Luo CX, Chen H, Zhu DY, Zhou QG. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat Protoc. 2018;13:1686–98.
Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Bultynck G, Leybaert L. Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening. Neuropharmacology. 2013;75:506–16.
Orellana JA, Moraga-Amaro R, Diaz-Galarce R, Rojas S, Maturana CJ, Stehberg J, Saez JC. Restraint stress increases hemichannel activity in hippocampal glial cells and neurons. Front Cell Neurosci. 2015;9:102.
Sáez JC, Contreras-Duarte S, Gómez GI, Labra VC, Santibañez CA, Gajardo-Gómez R, Avendaño BC, Díaz EF, Montero TD, Velarde V, Orellana JA. Connexin 43 hemichannel activity promoted by pro-inflammatory cytokines and high glucose alters endothelial cell function. Front Immunol. 1899;2018:9.
Abudara V, Roux L, Dallerac G, Matias I, Dulong J, Mothet JP, Rouach N, Giaume C. Activated microglia impairs neuroglial interaction by opening Cx43 hemichannels in hippocampal astrocytes. Glia. 2015;63:795–811.
Siebert AP, Ma Z, Grevet JD, Demuro A, Parker I, Foskett JK. Structural and functional similarities of calcium homeostasis modulator 1 (CALHM1) ion channel with connexins, pannexins, and innexins. J Biol Chem. 2013;288:6140–53.
García-Rodríguez C, Bravo-Tobar ID, Duarte Y, Barrio LC, Sáez JC. Contribution of non-selective membrane channels and receptors in epilepsy. Pharmacol Ther. 2022;231: 107980.
Potter JA, Price GW, Cliff CL, Williams BM, Hills CE, Squires PE. Carboxyfluorescein dye uptake to measure connexin-mediated hemichannel activity in cultured cells. Bio Protoc. 2021;11: e3901.
Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511.
Beurel E, Toups M, Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–56.
Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6: e946.
Hughes HK, Yang H, Lesh TA, Carter CS, Ashwood P. Evidence of innate immune dysfunction in first-episode psychosis patients with accompanying mood disorder. J Neuroinflamm. 2022;19:287.
Gururajan A, Reif A, Cryan JF, Slattery DA. The future of rodent models in depression research. Nat Rev Neurosci. 2019;20:686–701.
Ma L, Demin KA, Kolesnikova TO, Khatsko SL, Zhu X, Yuan X, Song C, Meshalkina DA, Leonard BE, Tian L, Kalueff AV. Animal inflammation-based models of depression and their application to drug discovery. Expert Opin Drug Discov. 2017;12:995–1009.
Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenoty** in mice: relevance to emotionality and sex. J Neurosci Methods. 2011;197:21–31.
Meyer JH. Neuroprogression and immune activation in major depressive disorder. Mod Trends Pharmacopsychiatry. 2017;31:27–36.
Haroon E, Miller AH, Sanacora G. Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology. 2017;42:193–215.
Rajkowska G, Miguel-Hidalgo JJ. Glial pathology in major depressive disorder: an approach to investigate the coverage of blood vessels by astrocyte endfeet in human postmortem brain. Methods Mol Biol. 2019;1938:247–54.
Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–58.
Seo JS, Wei J, Qin L, Kim Y, Yan Z, Greengard P. Cellular and molecular basis for stress-induced depression. Mol Psychiatry. 2017;22:1440–7.
Harro J. Animal models of depression: pros and cons. Cell Tissue Res. 2019;377:5–20.
Murphy-Royal C, Gordon GR, Bains JS. Stress-induced structural and functional modifications of astrocytes-Further implicating glia in the central response to stress. Glia. 2019;67:1806–20.
Pulcu E, Elliott R. Neural origins of psychosocial functioning impairments in major depression. Lancet Psychiatry. 2015;2:835–43.
Spellman T, Liston C. Toward circuit mechanisms of pathophysiology in depression. Am J Psychiatry. 2020;177:381–90.
Lv QY, Chen MM, Li Y, Yu Y, Liao H. Brain circuit dysfunction in specific symptoms of depression. Eur J Neurosci. 2021;55:2393.
Yang Z, Jian L, Qiu H, Zhang C, Cheng S, Ji J, Li T, Wang Y, Li J, Li K. Understanding complex functional wiring patterns in major depressive disorder through brain functional connectome. Transl Psychiatry. 2021;11:526.
Eroglu C, Barres BA. Regulation of synaptic connectivity by glia. Nature. 2010;468:223–31.
Lawal O, Ulloa Severino FP, Eroglu C. The role of astrocyte structural plasticity in regulating neural circuit function and behavior. Glia. 2022;70:1467.
Mazaud D, Capano A, Rouach N. The many ways astroglial connexins regulate neurotransmission and behavior. Glia. 2021;69:2527–45.
Oliveira JF, Araque A. Astrocyte regulation of neural circuit activity and network states. Glia. 2022;70:1455.
Bravo-Tobar ID, Fernández P, Sáez JC, Dagnino-Subiabre A. Long-term effects of stress resilience: hippocampal neuroinflammation and behavioral approach in male rats. J Neurosci Res. 2021;99:2493–510.
Charveriat M, Naus CC, Leybaert L, Sáez JC, Giaume C. Connexin-dependent neuroglial networking as a new therapeutic target. Front Cell Neurosci. 2017;11:174.
Okada M, Oka T, Nakamoto M, Fukuyama K, Shiroyama T. Astroglial Connexin43 as a potential target for a mood stabiliser. Int J Mol Sci. 2020;22:339.
Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25:321–38.
Gray JP, Muller VI, Eickhoff SB, Fox PT. Multimodal abnormalities of brain structure and function in major depressive disorder: a meta-analysis of neuroimaging studies. Am J Psychiatry. 2020;177:422–34.
Guo B, Zhang M, Hao W, Wang Y, Zhang T, Liu C. Neuroinflammation mechanisms of neuromodulation therapies for anxiety and depression. Transl Psychiatry. 2023;13:5.
Hantsoo L, Kornfield S, Anguera MC, Epperson CN. Inflammation: a proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biol Psychiatry. 2019;85:97–106.
**a CY, Wang ZZ, Yamakuni T, Chen NH. A novel mechanism of depression: role for connexins. Eur Neuropsychopharmacol. 2018;28:483–98.
Giaume C, Leybaert L, Naus CC, Sáez JC. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol. 2013;4:88.
Takeuchi H, Suzumura A. Gap junctions and hemichannels composed of connexins: potential therapeutic targets for neurodegenerative diseases. Front Cell Neurosci. 2014;8:189.
Bennett MV, Garré JM, Orellana JA, Bukauskas FF, Nedergaard M, Sáez JC. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012;1487:3–15.
Walrave L, Pierre A, Albertini G, Aourz N, De Bundel D, Van Eeckhaut A, Vinken M, Giaume C, Leybaert L, Smolders I. Inhibition of astroglial connexin43 hemichannels with TAT-Gap19 exerts anticonvulsant effects in rodents. Glia. 2018;66:1788–804.
Medina-Ceja L, Salazar-Sánchez JC, Ortega-Ibarra J, Morales-Villagran A. Connexins-based hemichannels/channels and their relationship with inflammation, seizures and epilepsy. Int J Mol Sci. 2019;20:5976.
Malhi GS, Mann JJ. Depression. Lancet. 2018;392:2299–312.
MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16:252–64.
MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry. 2008;64:880–3.
Sapolsky RM. Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci U S A. 2001;98:12320–2.
Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–49.
Yang C, Yang J, Luo A, Hashimoto K. Molecular and cellular mechanisms underlying the antidepressant effects of ketamine enantiomers and its metabolites. Transl Psychiatry. 2019;9:280.
Moretto JN, Duffy AM, Scharfman HE. Acute restraint stress decreases c-fos immunoreactivity in hilar mossy cells of the adult dentate gyrus. Brain Struct Funct. 2017;222:2405–19.
Eugenín EA, Eckardt D, Theis M, Willecke K, Bennett MV, Sáez JC. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc Natl Acad Sci U S A. 2001;98:4190–5.
Gajardo-Gómez R, Labra VC, Orellana JA. Connexins and pannexins: new insights into microglial functions and dysfunctions. Front Mol Neurosci. 2016;9:86.
Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Sáez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 2011;118:826–40.
Montero TD, Orellana JA. Hemichannels: new pathways for gliotransmitter release. Neuroscience. 2015;286:45–59.
Chever O, Lee CY, Rouach N. Astroglial connexin43 hemichannels tune basal excitatory synaptic transmission. J Neurosci. 2014;34:11228–32.
Linsambarth S, Carvajal FJ, Moraga-Amaro R, Mendez L, Tamburini G, Jimenez I, Verdugo DA, Gomez GI, Jury N, Martinez P, et al. Astroglial gliotransmitters released via Cx43 hemichannels regulate NMDAR-dependent transmission and short-term fear memory in the basolateral amygdala. FASEB J. 2022;36: e22134.
Meunier C, Wang N, Yi C, Dallerac G, Ezan P, Koulakoff A, Leybaert L, Giaume C. Contribution of astroglial Cx43 hemichannels to the modulation of glutamatergic currents by D-serine in the mouse prefrontal cortex. J Neurosci. 2017;37:9064–75.
Moore AR, Zhou WL, Sirois CL, Belinsky GS, Zecevic N, Antic SD. Connexin hemichannels contribute to spontaneous electrical activity in the human fetal cortex. Proc Natl Acad Sci U S A. 2014;111:E3919-3928.
Funding
This work was partially supported by the Shenzhen General Basic Research Program JCYJ20190808182203591 (to CGL), Hong Kong Research Grants Council RGC/ECS 21103818 and RGC/GRF 11104320 and 11104521 (to CGL), internal funds from City University of Hong Kong (to CGL), two Grants from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) 1231523 (to JCS) and 3210284 (to MS), and a Grant ICM-ANID, Project P09-022 from the Centro Interdisciplinario de Neurociencia de Valparaíso (to JCS).
Author information
Authors and Affiliations
Contributions
CGL, JCS, and HL conceived the study. HL, AG, and MS performed experiments and analyzed data. HL wrote the first version of the manuscript. CGL supervised the study, provided funding, reviewed, and revised the manuscript. JCS provided the D4 compound and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the animal experiments and procedures were received prior approval from the Animal Research Ethics Sub-Committees of the City University of Hong Kong and the Department of Health of the Government of the Hong Kong Special Administrative Region.
Consent for publication
All authors of the manuscript have agreed to publish this article.
Competing interests
JCS is listed as an inventor on a patent (WO2015188288A1) entitled “Specific modulators of connexin hemichannels” related to the development of the small organic molecule D4. JCS is also a named inventor on a patent (CL2017002254A1) entitled “Selective hemichannel inhibitors formed by connexins for the treatment of epilepsy” related to the therapeutic potential of D4 in epilepsy. CGL and HL are co-inventors on a patent entitled “一种半通道抑制剂在药学上的应用 / Pharmaceutical application of a hemichannel inhibitor” covering the therapeutic application of D4 as an antidepressant using some of the results described in this manuscript (CN202211496351.7; filed on November 25, 2022). This patent is “preliminarily approved” by the China National Intellectual Property Administration (January 16, 2023). Other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
D4 does not inhibit TRPV2 channels. A–D HeLa parental cells (Cx45−/−) were transfected with pIRES2-EGFP-hTRPV2 vector (0.5 µg/105 cells) and 24 h later channel activity was assessed through calcium imaging with Fura-2. TRPV2 channel was opened with 50 µM 2-Aminoethoxydiphenyl borate (2-APB), an effect that was maintained even after 3 washes (A). When La3+ was added to TRPV2 channels already open with 2-APB, Ca2+ influx was blocked (B), whereas 500 nM D4 did not affect TRPV2 activity (C). D4 pre-incubation did not prevent the opening of TRPV2 induced by 2-APB (D). 10 µM A23187 ionophore was used as a positive control for Ca2+ influx. Representative graphs of at least 3 evaluations for each experiment. Each point represents the average of 20–30 cells recorded in each condition.
Additional file 2: Fig. S2.
D4 does not inhibit P2X7R channels. A–C HeLa parental cells (Cx45−/−) were transfected with pIRES2-EGFP-hP2X7R vector (0.5 µg/105 cells) and 24 h later channel activity was assessed through calcium imaging with Fura-2. In all assays, P2X7R channel was opened with 2 mM adenosine triphosphate (ATP) and then the effect of 100 nM (A), 500 nM (B) and 1 µM (C) of D4 was evaluated. Application of D4 (100 nM, 500 nM, or 1 µM) did not affect the opening of P2X7R induced by ATP, whereas 20 µM A740003 inhibited the ATP-mediated P2X7R channel opening in all experiments. Representative graphs of at least 2 evaluations for each experiment. Each point represents the average of 20–30 cells recorded in each condition.
Additional file 3: Fig. S3.
D4 does not inhibit CALHM1 channels. A HeLa parental cells (Cx45−/−) were transfected with human GFP-tagged CALHM1 vector (origene cat#: RG206902, 0.5 µg/105 cells) and 24 h later channel activity was assessed by DAPI uptake. B DAPI uptake rates calculated from values as shown in (A), considering basal segment (0–5 min), stimulation with divalent cation-free solution (DCFS) (5–10 min) and the effect of ruthenium red (RR, 20 µM) or 0.5 µM of D4 (10–15 min). The slope of dye uptake within the last minute in each condition was quantified (basal, 4–5 min; DCFS, 9–10 min; RR or D4, 14–15 min). RR but not D4 reduced the opening of CALHM1 induced by DCFS. Each point represents the average of 20–30 cells recorded in each condition. n = 6. Data are mean ± s.e.m. One-way ANOVA followed by LSD post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001. C HeLa cells stably expressing Cx43 were used as a positive control for D4 (0.5 µM) inhibition. n = 3.
Additional file 4: Fig. S4.
D4 mitigates inflammatory responses induced by LPS. A Timeline of LPS injection and sample collection. B ELISA analysis of plasma (left) and hippocampal (right) levels of pro-inflammatory cytokine IL-1β in the saline/vehicle control, LPS/vehicle, and LPS/D4 mice. n = 3 mice per group. The filled dot indicates the value of each mouse. Data are mean ± s.e.m. One-way ANOVA followed by LSD post hoc test. **p < 0.01, ***p < 0.001.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, H., Guo, A., Salgado, M. et al. The connexin hemichannel inhibitor D4 produces rapid antidepressant-like effects in mice. J Neuroinflammation 20, 191 (2023). https://doi.org/10.1186/s12974-023-02873-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-023-02873-z