Abstract
Backgrounds
Unilateral high myopia (uHM), commonly observed in patients with retinal diseases or only with high myopia, is frequently associated with amblyopia with poor prognosis. This study aims to reveal the clinical and genetic spectrum of uHM in a large Chinese cohort.
Methods
A total of 75 probands with simplex uHM were included in our Pediatric and Genetic Eye Clinic. Patients with significant posterior anomalies other than myopic fundus changes were excluded. Variants were detected by exome sequencing and then analyzed through multiple-step bioinformatic and co-segregation analysis and finally confirmed by Sanger sequencing. Genetic findings were correlated with associated clinical data for analysis.
Results
Among the 75 probands with a mean age of 6.21 ± 4.70 years at the presentation, myopic fundus of C1 and C2 was observed in 73 (97.3%) probands. Surprisingly, specific peripheral changes were identified in 63 eyes involving 36 (48.0%) probands after extensive examination, including peripheral retinal avascular zone (74.6%, 47/63 eyes), neovascularization (54.0%), fluorescein leakage (31.7%), peripheral pigmentary changes (31.7%), and others. Exome sequencing identified 21 potential pathogenic variants of 13 genes in 20 of 75 (26.7%) probands, including genes for Stickler syndrome (COL11A1 and COL2A1; 6/20), FEVR (FZD4, LRP5, and TSPAN12; 5/20), and others (FBN1, GPR179, ZEB2, PAX6, GPR143, OPN1LW, FRMD7, and CACNA1F; 9/20). For the peripheral retinal changes in the 20 probands, variants in Stickler syndrome-related genes were predominantly associated with retinal pigmentary changes, lattice degeneration, and retinal avascular region, while variants in genes related to FEVR were mainly associated with the avascular zone, neovascularization, and fluorescein leakage.
Conclusions
Genetic defects were identified in about one-fourth of simplex uHM patients in which significant consequences may be hidden under a classic myopic fundus in up to half. To our knowledge, this is the first systematic genetic study on simplex uHM to date. In addition to routine care of strabismus and amblyopia, careful examination of the peripheral retina and genetic screening is warranted for patients with uHM in order to identify signs of risk for retinal detachment and other complications and provide meaningful genetic counseling.
Similar content being viewed by others
Background
Anisometropia is a condition characterized by imbalanced ocular development of the two eyes, despite both eyes being exposed to similar genetic and environmental influences. Unilateral high myopia (uHM), a specific form of anisometropia, refers to patients with cycloplegic spherical equivalents of at least − 5.00 to − 6.00D in the highly myopic eye and a difference of 5.00 diopters or more between the two eyes [1], although variable criteria have also been used [2]. Unilateral high myopia is easily missed by their parents because of the relatively normal contralateral eye, although it is generally early-onset and frequently associated with serious consequences such as amblyopia with poor treatment outcomes, strabismus, or retinal detachment [2,3,4]. It may be classified into complex and simplex forms depending on whether it is associated with other obvious ocular or systemic diseases. Complex uHM is known to be associated with corneal opacity, congenital lens abnormalities, uveal coloboma, morning glory syndrome, Straatsma syndrome, Stickler syndrome, familial exudative vitreoretinopathy, retinopathy of prematurity, and other complications that are usually easily recognizable [2, 5,6,7,8,9,10]. On the other hand, simplex uHM refers to uHM without noticeable ocular or systemic changes except for myopic posterior fundus on routine examination. Clinical analysis of simplex uHM has been rare [1, 4, 11]. Simplex uHM is often missed and rarely described in children not only because of insignificant myopic posterior fundus but also due to the compensatory visual acuity provided by the better eye. However, the disastrous diseases may hide in presumed simplex uHM, in addition to amblyopia or strabismus. Therefore, the systematic evaluation of simplex uHM may contribute to the early detection of risk signs and further improvements in clinical management.
Genetic factors have been shown to play a major role in early-onset high myopia (eoHM), [12] in which variants in RetNet genes could be detected in about 24% of eoHM families [13, 14] while those in eoHM genes contributed to about 9% of eoHM families [15,16,17] based on our previous studies on bilateral eoHM. Although genetic factors have also been suggested for uHM [2, 11, 18,19,20], the exact genetic defects rarely have been reported for uHM, especially for simplex uHM. Recently, high myopia in one eye has been found in 11% of obligate female carriers with unique haplotypes in OPN1LW that are a common cause of bilateral eoHM in males [16], suggesting that systematic analysis of other genes associated with ocular diseases may uncover additional genetic defects associated with uHM.
In the current study, a total of 75 unrelated probands with simplex uHM were recruited from the pediatric and genetic eye clinic at the Zhongshan Ophthalmic Center. Systematic ocular examinations were performed to reveal subtle clinical risk signs and exome sequencing of genomic DNA was performed to detect genetic variants. Potential pathogenic variants in 13 genes were identified in 26.7% (20/75) of probands, with majority being related to FEVR or Stickler syndrome. Notably, the molecular diagnostic rate of uHM is comparable to that of bilateral eoHM, indicating a significant role of monogenetic factors in the development of uHM. Those probands, with typical C1 (77.3%) and C2 (20.0%) myopic fundus without noticeable additional signs, were presumed to have simplex uHM at the initial routine clinical examination. Surprisingly, extensive additional examination including ultra-wide-field imaging and fundus fluorescein angiography (FFA) imaging revealed peripheral fundus changes in 48% of these cases, including color changes (pigmentary changes, hypopigmentation, degeneration), vascular abnormalities (avascular zone, neovascularization, fluorescein leakage), and fibrous proliferation. Our data highlight the importance of performing comprehensive peripheral retinal examinations and clinical genetic testing in patients with simplex or complex uHM in order to promptly detect the potential risk factors, such as retinal detachment and provide genetic counseling when needed, in addition to care of the strabismus and amblyopia.
Methods
Patient enrollment
This study was approved by the institutional review board of Zhongshan Ophthalmic Center. All patients with uHM and their family members in the study were enrolled from the Pediatric and Genetic Eye Clinic, Zhongshan Ophthalmic Center, Guangzhou, and evaluated by a senior ophthalmologist (Zhang Q). Based on the initial complaint and routine ophthalmic examination the preliminary diagnosis of probands from 75 unrelated families was simplex uHM that was defined as follows [1, 2]: (1) the highly myopic eye had a cycloplegic refraction (spherical equivalent) ≤ − 6.00 diopters (D) (or ≤ − 5.00 D when the age of a proband is less than 7 years old) while the fellow eye did not have such refraction; (2) The absolute difference of refraction between highly myopic eye and the fellow eye should be at least 5 diopters (≥ 5 D); (3) Exclusion of diseases involved in cornea, iris, and lens; (4) Exclusion of retinal diseases except for myopic fundus changes based on major complaint reported by the patient or their legal guardians and routine fundus examination; (5) Individuals with eoHM in both eyes were excluded. Prior to collecting peripheral venous blood samples and clinical data, all participants or their legal guardians underwent informed consent in accordance with the principles of the Declaration of Helsinki. The genomic DNA was prepared from the peripheral venous blood as previously described [13].
Variants identification and evaluation
Whole-exome sequencing (WES), or targeted exome-sequencing (TES) on 736 eye-panel genes was conducted on the genomic DNA from the 75 probands with uHM, using methods described in our previous study [17]. For WES, the Agilent SureSelect Human All Exon Enrichment Kit (Agilent, Santa Clara, CA, United States) was used to construct the whole-exome library. The library quality assessment was performed using Agilent Technologies 2100 Bioanalyzer and library sequencing was conducted using Illumina HiSeq platform (Illumina, San Diego, CA, United States) with an average depth of at least 125-fold. The clean reads were mapped to the human reference genome hg19 with Burrows-Wheeler Aligner (BWA) software (http://bio-bwa.sourceforge.net/). Subsequently, variant calling was performed using GATK (https://gatk.broadinstitute.org/hc.en-us) and SAMTOOLS (http://samtools.sourceforge.net/) implementing Bayesian approaches, and annotation of the detected variants was performed using ANNOVAR (http://annovar.openbioinformatics.org/en/latest/).
For TES, through the Bioruptor Plus (Diagenode, Liege, Belgium), genomic DNA Fragments of approximately 200 base-pair (bp) were obtained. Subsequently, the fragments were subject to the KAPA HTP Library Preparation Kit (Roche, Basel, Switzerland) to generate a paired-end library. For the library capture, the NimbleGen SeqCap EZ Choice Library SR V5 kit (Roche) was utilized, and then the libraries were sequenced using NextSeq550 Mild output v2 kit (150 bp paired-end) on an Illumina Nextseq550 Analyzer (Illumina, San Diego, CA). Data analysis, including variant calling, annotation, and screening was conducted utilizing Strand NGS software (Karnataka, India). The genes included in the target gene panels are listed in Additional file 1: Table S1 as described in our previous study [17].
The detected variants were filtered through multiple steps of bioinformatic analysis as previously described. Firstly, variants with low sequencing quality (coverage depth less than 5) or with minor allele frequency (MAF) of more than 1% in the gnomAD database (https://gnomad.broadinstitute.org/) were excluded. Subsequently, the potential effect of missense variants was evaluated using five in silico prediction tools, including SIFT (http://sift.jcvi.org/), Polyphen-2 (http://genetics.bwh.harvard.edu/pph2/), PROVEAN (http://provean.jcvi.org/seq_submit.php), CADD (http://cadd.gs.washington.edu), and REVEL (https://sites.google.com/site/revelgenomics/). Then, the influence of variants on splicing signals was computationally examined by an online splicing prediction tool: Human splicing finder program (HSF, https://hsf.genomnis.com/mutation/analysis). Additionally, these variants were evaluated by a comparison analysis of the gnomAD database and the Human Gene Mutation Database (HGMD). The variants were evaluated according to the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) criteria. Then, the residual variants, including pathogenic, likely pathogenic, and variants of uncertain significance, were confirmed by Sanger sequencing as described previously [21], for which the primers were designed using primer 3 (http://primer3.ut.ee/). Finally, co-segregation analysis was performed on available family members.
Clinical data collection
General clinical information of all participants including age, sex, and first symptom was collected. Participants included in this study underwent routine ophthalmological examinations and necessary additional specific tests, including fundus photography, RetCam digital photography, scanning-laser ophthalmology (SLO), optical coherence tomography (OCT), fundus fluorescein angiography (FFA), and the electroretinogram (ERG). Based on fundus photography, SLO, Retcam, FFA, and OCT results, the peripheral retinal abnormalities were evaluated by two ophthalmologists. The evaluation on peripheral retina mainly considered color changes (pigmentary changes, hypopigmentation, degeneration), and vascular morphology (avascular zone, neovascularization, vascular leakage), and fibrous proliferation, as described in previous studies [22,23,24,25]. The comprehensive list of peripheral retinal changes observed in this study and represent image of each peripheral retinal changes could be found in Additional file 3: Fig. S3 and Table S6. In addition, grading of high myopia was based on the classification proposed by Ohno-Matsui et al. [26], where the myopic fundi were classified into five categories: Category 0 (no myopic retinal degenerative lesion), Category 1 (tessellated fundus), Category 2 (chorioretinal atrophy), Category 3 (patchy chorioretinal atrophy), Category 4 (macular atrophy). Foveal hypoplasia grading was performed according to the Leicester Grading System [27]. Patients with FEVR were staged based on the FEVR classification scheme proposed by Pendergast and Trese [28].
Follow-up observation on progress of visual acuity after intervention
Follow-up observation on progress of visual acuity was through medical record review, telephone interviews, and outpatient follow-up visits. Of the 75 uHM probands, 38 had follow-up clinical data on wear of glasses together with patching therapy. The baseline date was defined as the date of the proband’s first treatment and the last follow-up date was defined as the date of the last measurement of best corrected visual acuity (BCVA). Each participant was advised to continuously wear spectacles for optical correction, with replacement of glasses in follow-up visit, if necessary. Additionally, the relative healthy eye was recommended to be patched for at least 4 h each day for at least five days a week, while in the meantime the highly myopic eye was encouraged to perform amblyopia training exercises once the healthy eye was patched. The patients were also advised to have regular follow up visit every six months. The mean follow-up time was 24.55 ± 20.17 months.
Statistical analysis
All statistical analyses were performed using IBM SPSS statistics software v26.0 (IBM Corporation, Armonk, NY, USA). Clinical data were compared between the highly myopic eye and the fellow eye using a Wilcoxon signed-rank test or Fihser’s exact test. The comparison of the BCVA within the same uHM patient group at different time points was evaluated using the Wilcoxon signed-rank test or paired Student’s t-test. The refractive difference between the highly myopic eye and the fellow eye with the corresponding difference in total axial length was compared by linear regression. A P value less than 0.05 is taken to indicate statistical significance.
Results
The clinical characteristics of uHM
The 75 unrelated probands initially diagnosed as uHM were recruited in the current study. The general clinical findings of these 75 uHM patients were presented in Fig. 1 and Additional file 1: Table S2. Of the 75 probands, 38 were female (50.7%) and 37 were male (49.3%), with a mean age at onset of 4.42 ± 2.07 years (median 3.83, interquartile range (IQR) 3.08 to 5.33 years) (Fig. 1A and Additional file 1: Table S2). At the first examination, the majority of the probands (71/75) were children except for four who were more than 12 years of age. The mean age at examination was 6.21 ± 4.70 years (median 5.08, IQR 4.00 to 6.42) (Fig. 1A). The most common initial symptoms were HM (40%, 30/75) and impaired vision in the poor eye (45.3%, 34/75). Based on the available clinical data, the prevalence of strabismus in this cohort was 13.8% (9/65).
The demographic characteristics and clinical data of 75 pedigrees with unilateral high myopia in this study. A Demographic data and clinical characteristics including sex, age, visual acuity, refraction error, axial length, first symptom, myopic maculopathy classification, and FEVR stage classification. B Correlation of refraction error difference (diopters) and axial length difference (millimeters) between the highly myopic and contralateral eyes (R2 = 0.79, P < 0.0001). C Distribution of available refraction data in diopters of patients with unilateral high myopia in this study. D Distribution of available axial length in millimeters of patients with unilateral high myopia in this study. The red circles represent the highly myopic eyes, and the gray circles represent the fellow eye
In the 75 probands, the highly myopic eyes had a mean spherical equivalent refraction of − 10.20 ± 3.30 diopters (median − 9.75 diopters, IQR − 11.75 to − 8.0 diopters) while the fellow eyes had a mean spherical equivalent refraction of − 0.70 ± 1.88 diopters (median − 0.25 diopters, IQR − 2.06 to 0.75 diopters). A difference of at least − 5.00 diopters was observed in all patients (Fig. 1C, D). Consistent with this, the mean total axial length of the highly myopic eye was 26.18 ± 1.70 mm (median 25.87 mm, IQR 25.26 to 26.98 mm) while that of the fellow eye was 22.90 ± 1.09 mm (median 22.75 mm, IQR 22.25 to 23.73 mm) (Fig. 1A). The relationship between the intraocular difference in axial length and the intraocular difference in refractive error is shown in Fig. 1B.
The intraocular difference in axial length in these patients is consistent with the intraocular difference in refraction error (R2 = 0.79, P < 0.0001) (Fig. 1B), based on correlation analysis of 60 of 75 probands with both data of refraction and axial length, in which the average axial length difference between the highly myopic eye and the fellow eye was 3.27 ± 1.31 mm and the average refractive error between the highly myopic eye and the fellow eye was 9.30 ± 3.31 diopters. The linear regression coefficient of the relationship was 0.3505, indicating that each 1 mm intraocular difference in axial length corresponds to the intraocular difference of 2.81 diopters.
According to the myopic maculopathy classification system [26], a tessellated fundus (Category 1), diffuse chorioretinal atrophy (Category 2), and macular atrophy (Category 4) were observed in 77.3% (58/75), 20.0% (15/75), and 2.7% (2/75) of patients, respectively (Fig. 1A). The available posterior fundus photographs revealed a normal-appearing or typical myopic appearance in the 75 probands (Fig. 2). However, extensive ocular examinations including SLO, OCT, FFA, and ERG, ultra-widefield fundus photography and FFA images revealed specific fundus changes in 48% (36/75) of these probands (Additional file 1: Table S2). The distribution of peripheral fundus changes in the probands were demonstrated in the Additional file 1: Table S3-1. Of the 63 eyes in 36 probands with peripheral retinal changes, the most common abnormality was the peripheral retinal avascular zone (74.6%, 47/63), followed by neovascularization (54.0%, 34/63), fluorescein leakage (31.7%, 20/63), peripheral pigmentary changes (31.7%, 20/63), peripheral retinal degeneration (30.2%, 19/63), and followed by several others (Additional file 1: Table S3-1). In addition, there was no significant difference in the distribution of peripheral retinal degeneration between the highly myopic eye (36/75) and the fellow eye (27/75) (Fisher’s exact test, P = 0.558).
The posterior fundus photographs of bilateral eyes of six patients in this cohort. The uHM presents unilateral high myopia. The uHM symbol is in the lower-left corner of the fundus image of the highly myopic eye. The traditional fundus images of six patients show typical myopic fundus. A–C the fundus images of three probands (F13-II:2; F18-II:1; F4-II:1) reveal the tessellated fundus (C1) in the highly myopia eye and normal appearance (C0) in the fellow eye. D the fundus image of the proband (F15-II:2) shows diffuse chorioretinal atrophy (C2) in the highly myopic eye and normal appearance in the fellow eye. E the fundus images of the proband (F12-II:2) shows diffuse chorioretinal atrophy (C2) in both eyes. F the fundus photography of the proband (F20-II:2) demonstrates the macular atrophy (C4) in the highly myopic eye and the tessellated fundus (C1) in the fellow eye
Available OCT findings demonstrate Grade 1 to Grade 2 foveal hypoplasia in eight probands and normal structures in 36 probands. Similarly, the available ERG examination results of 18 eyes from nine probands exhibited normal cone response in six eyes and normal rod response in nine eyes. The remaining eyes demonstrated a mild reduction in cone response in four eyes, moderate reduction in one eye, and severe reduction in seven eyes. Additionally, there was a mild reduction in rod response in five eyes, moderate reduction in one eye, and severe reduction in three eyes (Additional file 2: Fig. S1).
Molecular findings
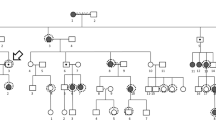
The results of the genetic screening of the 75 probands with uHM from unrelated families are shown in Additional file 1: Table S2. After the detected variants were systematically evaluated with reference to the ACMG/AMP criteria, 21 potential pathogenic variants in 13 genes were identified in 20 of 75 (26.67%) probands, including two in-frame variants, nine truncation variants, and ten missense variants (Fig. 3 and Table 1; Additional file 1: Table S2). These variants in the 20 probands could be classified as pathogenic (6 probands), likely pathogenic (6), and variant of uncertain significance (9) based on ACMG/AMP criteria (Table 1). In addition, these variants are predicted to be deleterious by at least five in-silico prediction tools or were predicted to have an impact on splicing sites, as determined by the HSF tool. Finally, the majority of these variants (15/21) were absent in the gnomAD database, while the other four variants were extremely rare, with allele frequencies below 0.0001 and the last two were compound heterozygous variants in GPR179. The 21 variants were confirmed by the Sanger sequencing (Additional file 2: Fig. S2).
The molecular testing results and genetic landscape of the unilateral high myopia cohort. A Pedigrees of 20 unilateral families with potential pathogenic variants in 13 genes. The phenotypes of the patients shown in the pedigrees were based on the first visit. A solid black pattern indicates high myopia, a half-filled pattern indicated unilateral high myopia. a striped pattern indicates mild to moderate myopia. Circles represent females, squares represent males, and circles with a dot in center indicate heterozygous female carriers. M indicates mutant allele, + indicates the normal allele. Black arrows point to probands. B The molecular test results of 75 unrelated pedigrees with unilateral high myopia. Potential pathogenic variants in 13 genes were identified in 26.73% of the in-house cohort (20/75). C The distribution of the contributions of 13 genes to unilateral high myopia in these patients
Of the 13 genes, variants in genes related to Stickler syndrome were most common, including COL11A1 (4/20, 20%) and COL2A1 (2/20, 10%). Variants in genes associated with FEVR were the second leading causative factor in solved uHM probands: FZD4 (2/20, 10%), LRP5 (2/20, 10%), and TSPAN12 (1/20, 5%). The variants in the remaining 9 probands were detected in the following genes, which contribute to other related diseases when mutated, including FBN1, GPR179, ZEB2, PAX6, GPR143, OPN1LW, FRMD7, and CACNA1F (Fig. 3C). Interestingly, three female probands with uHM had heterozygous variants in X-linked genes (GPR143, OPN1LW, and CACNA1F), suggesting a skewed inactivation of the X chromosome between their two eyes.
Genotype–phenotype correlation of uHM probands
The 20 probands with genetic variants identified in known myopia-related genes presented as simplex uHM without any other ocular conditions on their first visit to the general eye clinic. Additionally, while most parents only had mild to moderate myopia, so that the initial diagnosis of the 20 probands was presumed to be simplex uHM based on routine ocular examination (Fig. 2 and Table 2). There was no difference in population characteristics, including age, gender, visual acuity, refraction error, and axial length between the 20 probands with identified genetic defects and the remaining 55 probands without genetic variants (Additional file 1: Table S4). However, the myopic maculopathy in the highly myopic eye tended to be more severe in these 20 probands compared to the remaining probands (Fisher’s exact test, P = 0.003).
Extensive ophthalmological evaluation identified peripheral specific signs in these patients, corresponding to their genetic findings. Among these peripheral retinal changes, variants in genes related to Stickler syndrome were predominantly associated with peripheral retinal pigmentary changes (50%), lattice degeneration (25%), or peripheral avascular region (25%) in this cohort (Additional file 1: Table S3-2). For example, the peripheral fundus images of proband with variants in genes related to Stickler syndrome show lattice degeneration or peripheral retinal degeneration in the highly myopic eye only, and peripheral retinal pigmentary change in both eyes (Fig. 4A–F).
The specific ophthalmology examination results during follow-up for six probands in our cohort. The clinical findings of highly myopic eyes are boxed in red. A–F, the traditional fundus images of the three patients (F1-II:1; F2-II:2; F6-II:2) with variants in genes related to Stickler syndrome (COL2A1 and COL11A1) demonstrates the typical myopic fundus, while ultra-widefield fundus images show the specific peripheral fundus changes including lattice degeneration and peripheral retinal pigmentation in the highly myopic eyes. The corresponding magnified images of the local retinal region (outlined by white dashed lines in an ultra-widefield fundus image) illustrate the similar fundus changes. G–H, the posterior fundus images of the GPR143 carrier (F18-II:1) shows myopic fundus, and ultra-widefield fundus images and magnified images demonstrate the nonuniform fundus pigmentation in both eyes. I-L, the fundus images of two probands (F11-II:1; F9-II:1) with variants in genes related to FEVR (LRP5 and FZD4) illustrate classic myopic fundus in the posterior pole, the peripheral avascular region, and fluorescein leakage were observed in fluorescein angiography
On the other hand, the variants in genes related to FEVR were mainly associated with peripheral retinal avascular zone (100%), neovascularization (90%), and fluorescein leakage (60%) (Additional file 1: Table S3-2). The peripheral retinal degeneration was also found in both eyes of all patients with variants in LRP5 (Fig. 4I–J). The FFA images of proband F9-II:1 demonstrate bilateral peripheral avascular areas, fluorescein leakage, and neovascularization, despite the myopic or normal appearance in the posterior fundus, consistent with FEVR disease caused by the FZD4 variant c.328_330del (p.Ile110del) (Fig. 4K, L). A similar pattern of fundus changes was also observed in proband F11-II:1 with LRP5 variant 4466C > A (p.Thr1489Lys) (Fig. 4I, J). In comparison to the fellow eye, patients with uHM and FEVR tended to have a more advanced stage of FEVR in the highly myopic eye (P = 0.043) (Fig. 1A).
Moreover, the SLO images of GPR143 c.767 + 10C > G carrier (F17-II:1) showed bilateral mosaic fundus pigmentation with radially hypopigmentation streaks oriented in the peripheral region with a normal-like posterior pole consistent with the specific changes in OA1 carriers (Fig. 4G, H) in whom uHM is relatively rare. For the variants in other genes such as FBN1, ZEB2, GPR179, PAX6, OPN1LW, FRMD7, and CACNA1F, specific peripheral changes were relatively rare in probands carrying variants in these genes, which was indeed aligning with the genetic findings. In summary, the final diagnostic phenotypes of the 20 probands or their affected family members were based on genetic variants as well as comprehensive ocular ophthalmic examinations, especially the strong correlation between the varied gene and closely related specific phenotypes. However, for those probands with unspecific retinal changes without identified genetic variants, a final diagnosis rather than uHM would be difficult in clinic (Table 2; Additional file 1: Table S2; Additional file 2: Fig. S2).
Improvement of visual acuity in probands with uHM
The mean logMAR BCVA of the highly myopic eye and the fellow eye were 0.89 ± 0.63 (median 0.7, IQR 0.43 to 1.00) and 0.17 ± 0.19 (median 0.1, IQR 0.0 to 0.3), respectively. There was a statistically significant difference between the BCVA of the highly myopic eye and that of the fellow eye (Wilcoxon signed-rank test, P < 0.001). Based on results of the follow-up study, uHM probands demonstrated steady BCVA improvement and gradual progression of refractive error (Additional file 1: Table S5). There were similar BCVA improvements in both eyes of these probands (Fig. 5A, C), but different rates of refractive error progression were observed in the highly myopic eye and the fellow eye of uHM probands. Compared to the fellow eye, the highly myopic eye of uHM children had a higher progression rate of refraction error (Fig. 5B, D). According to the available follow-up study results for 38 uHM probands, the mean logMAR BCVA of the highly myopic eye improved from 0.84 to 0.56 within 6 to 12 months of clinical intervention (optical correction together with patch therapy) (Wilcoxon signed-rank test, P = 4.3E−5) (Fig. 5E). Similarly, based on the follow-up data available for 20 uHM probands, the mean logMAR BCVA of highly myopic eyes improved from 0.72 to 0.39 with clinical intervention over the period from 18 to 24 months (Wilcoxon signed-rank test, P = 0.003) (Fig. 5F). Moreover, the mean logMAR BCVA further improved from 0.53 to 0.40 with clinical intervention (Wilcoxon signed-rank test, P = 0.011) between the period 6 to 12 months and 18 to 24 months (Fig. 5G). However, it is worth noting that the BCVA of two probands (F5-II:1 and F44-II:1) had decreased slightly in 6–12 months, and for the one patient (F44-II:1), there was a slightly worsening from that point to 18–24 months (Fig. 5E–G). Additionally, the BCVA of other two patients (F43-II:1 and F47-II:1) improved within 6–12 months but slightly worsened from that time to 18–24 months (Additional file 1: Table S5 and Fig. 5F, H). The fluctuation of BCVA among these patients may be attributed to the poor compliance as no apparent change on posterior fundus was observed.
Best-corrected visual acuity and refraction progression of unilateral high myopia. Different colored points represent different genes, gray points represent patients without an identified genetic cause, and the red curve represents the best nonlinear fit of all data points. Scatter plots show the steady improvement of best-corrected visual acuity with age in the highly myopic eye (A) and the fellow eye (C). The other scatter plots show the gradual progression of refraction with age in the highly myopic eye (B) and the fellow eye (D). E Paired box plot showing the mean visual acuity of highly myopic eye improving from 0.84 to 0.56 logMAR (P = 4.3E−5) with clinical intervention over 6–12 months (38 patients). F Mean visual acuity of highly myopic eyes improved from 0.72 to 0.39 logMAR (P = 0.003) with clinical intervention in the period from 18 to 24 months (20 patients). G. Mean visual acuity improved from 0.53 to 0.40 with clinical intervention (P = 0.011) over periods from 6 to 12 months and 18 to 24 months (18 patients). H Comparison between the bassline visual acuity and final visual acuity at time points of 6–12 months and 18–24 months after clinical intervention (18 patients). BCVA = best-corrected visual acuity, yrs = years, mo = month
Consistent with the above results, follow-up of 18 uHM probands showed progressive improvement in the mean BCVA at each follow-up time point (baseline vs 6 to 12 months, P = 0.005; baseline vs 18 to 24 months, P = 0.001; 6 to 12 months vs 18 to 24 months, P = 0.011) (Fig. 5H). Among the 18 participants, at the end of 18 to 24 months, the BCVA of 15 (83.3%) uHM patients was 0.52 logMAR or better (Snellen fraction 20/63), and the BCVA of 11 (61.1%) uHM patients improved 3 or more lines.
Discussion
In the current study, 75 probands with referring diagnosis of simplex uHM based on routine examination were recruited from our Pediatric and Genetic Eye Clinic. Extensive clinical and genetic analysis identified peripheral retinal changes with potentially severe consequences in 48% of probands with uHM while causative genetic defects were detected in 26.7% of them. These findings are of clinical significance not only for the complications with the potential to cause blindness in the patients themselves but also for genetic counseling for their siblings or offspring. As far as we know, our data present for the first time a systematic extensive clinical and genetic analysis of a cohort of uHM with important findings that would be valuable in improving the clinical care of patients with uHM.
Clinical care of uHM, complex or simplex, has been considered to be a challenge in the pediatric eye clinic, in which most attention has been paid to amblyopia and strabismus [4, 29]. Thus, uHM has been described as an accompanying sign associated with other ocular or systemic diseases in which the clinical signs of original diseases are remarkable, including corneal opacity [5], congenital lens abnormalities [2], uveal coloboma [6], morning glory syndrome [7], Straatsma syndrome [8], Stickler syndrome [9], familial exudative vitreoretinopathy [30], and retinopathy of prematurity [10], etc. Unlike the complex uHM cases mentioned above, simplex uHM patients usually lack noticeable suggestive signs of other ocular or systemic diseases except for a posterior myopic fundus on routine ocular examination. Such simplex uHM may mainly be treated as anisometropia alone with attention paid to amblyopia and strabismus in clinical practice [2, 4, 29]. Peripheral retinal changes underlying severe complications may be neglected because of an appearance of simple myopic fundus in uHM, especially when the OCT and ERG results reveal no significant abnormalities or only subtle changes, as they did in most of the probands enrolled in this study. In the current study, we detected obvious peripheral retinal changes including retinal degeneration, lattice degeneration, retinal pigmentary changes or hypopigmentation, peripheral avascular zone, neovascularization, fluorescein leakage, or fibrous proliferation in about half of the probands. In this study, the most common peripheral changes are peripheral avascular zone and neovascularization, which have been identified to be highly associated with variants in genes related to FEVR. Correspondingly, the peripheral retinal degeneration, lattice degeneration, and pigmentary changes have been observed in more than one-third of cases with peripheral retinal changes, highly associated with variants in genes related to Stickler syndrome. These findings indicate that the peripheral retinal changes may underlie vision-threatening diseases and emphasize the importance of systematic evaluation of the entire peripheral retina as soon as possible for children with uHM. Their importance is further shown by the detection in these patients of genetic variants in genes associated with ocular diseases frequently leading to blindness. Therefore, SLO or RetCam screening is recommended as an initial necessary examination for all patients with uHM. Additional specific examinations such as FFA or OCTA would be needed if suggestive signs were observed by SLO or RetCam. In addition, there is no statistically significant difference in the distribution of peripheral retinal changes between the highly myopic eye and the fellow eye, suggesting that the peripheral retinal examination should be performed not only in the highly myopic eye but also in the fellow eye.
There is a high correlation between axial length and spherical equivalent refraction in our patients, indicating that the asymmetric spherical equivalent refraction difference between both eyes in uHM patients can be primarily attributed to the interocular difference in total axial length, which is consistent with previous studies [2, 18, 31]. However, the contribution of other optical components, such as cornea and crystalline lens, cannot be ruled out. In this cohort, FBN1 variants have been identified in two probands, associated with Marfan syndrome. Previous study suggests that the high myopia in Marfan syndrome may be derived from both lenticular as well as axial mechanisms [32]. Consistently, our recently published data also suggests that myopia in patients with Marfan syndrome initially presents as refractive myopia and gradually evolves into axial myopia [33]. This transition is attributed to the normal axial length in the early stages in these patients, which later develops into longer axial length with age and disease progression. Additionally, the minimal axial length difference in this cohort is 1.43 mm (contributed to about 4 diopter difference), observed in the proband F52-II:1. Despite an 8.5 diopter refraction difference in this patient, inconsistent with axial length difference, this might be partly explained by the young age of the proband (examined at 3 years old). Alternatively, it implies a potential contribution of other optical components to refractive asymmetry, as some studies propose that interocular difference in corneal or crystalline lens structure may contribute to the asymmetry in refractive errors. [18, 34,35,36]
There have been many genetic studies on bilateral eoHM in recent years and a number of genetic factors contributing to or associated with eoHM have been identified [12], so that currently genetic variants are seen in about one third of families with monogenic eoHM (about 24% in RetNet genes [13, 14] and 9% in genes for high myopia [15,16,17] based on our previous studies). However, genetic analysis of uHM has been lacking although most uHM is also early-onset, usually occurring in early childhood. In the current study, 21 potential pathogenic variants in 13 genes were identified in 26.7% (20/75) of probands by exome sequencing, with a detection rate comparable to that for eoHM, suggesting a similar genetic contribution to both uHM and eoHM. Similarly, genetic contributions have also been observed in patients with bilateral or unilateral microphthalmia or coloboma [37,38,39]. In previous studies, uHM has been suggested to be primarily influenced by genetic factors rather than environmental factors, especially when it occurs at a young age [2, 18], although exact genetic defects have not been explored. It remains puzzling how genetic defects lead to asymmetric ocular growth between the two eyes. In fact, the asymmetry is not a rare phenomenon in inherited ocular diseases. For the patients with microphthalmia, anophthalmia and coloboma (MAC), the asymmetric development of the two eyes is common [37, 40]. The genetic diagnostic rate of unilateral MAC patients has been found to be similar to that for bilateral MAC patients [37]. In addition, the unilateral retinal changes also have been found in FEVR patients, and causative genetic variants have been identified in more than half of unilateral FEVR patients [41]. Similarly, asymmetry can also be observed in Mendelian inherited systemic disorders such as Goldenhar syndrome [42, 43]. Nevertheless, the exact molecular mechanism for uHM remains unclear. This phenomenon might relate to changes in gene expression due to DNA methylation or other epigenetic control mechanism [44], or perhaps due to modifying genes, a environmental modifier, or developmental stochastic effect [45]. In this study, heterozygous variants in X linked genes, including GPR143, OPN1LW, or CACNA1F, have been identified in three probands. The skewed X-chromosome inactivation in different eyes might account for asymmetric ocular changes in the female patients [2]. Recently, PAX6 and ZFHX1B variants were reported to be involved in developmental anisometropia through regulating the unbalanced elongation of axial length between the eyes [46]. Coincidentally, the variants in both PAX6 and ZFHX1B (ZEB2) have been identified in uHM probands in this study [46]. The other detected genes in this study, such as those related to Stickler syndrome (COL2A1 and COL11A1) or Marfan syndrome (FBN1), which have been proposed to modulate ocular growth through axial mechanism [32, 47]. However, myopia in FEVR has been suggested be through a lenticular mechanism, [32, 48], anisometropia has been observed as a common sign in patients with FEVR [41]. The exact mechanism underlying uHM or asymmetrical development of the eye is unclear [29, 49]. In general, the mechanisms through which genetic defects contribute to the development of uHM and unilateral diseases in general remain a puzzle that requires further studies to unravel in the future.
As genetic defects contribute to a significant portion of uHM, it might be important to provide genetic testing and counseling for patients with uHM during their initial visit. The most common genetic cause of uHM in this study is variants in genes related to Stickler syndrome and FEVR, indicating that uHM had high risk of retinal detachment. In addition, our data suggests that the presence of other underlying inherited ocular diseases might be under-ascertained in simplex uHM. As seen in proband F20-II:2 with uHM and heterozygous CACNA1F p. Ala657Thr variant, subsequent analysis of her son demonstrated the same variant in hemizygous status and congenital stationary night blindness (CSNB) with bilateral high myopia. Thus, genetic screening for uHM patients can provide solid information for genetic counseling not only for the patients themselves, but also for family members, and especially for female carriers with X-linked diseases.
The prevalence and severity of amblyopia has been suggested to be gradually increased with age and with the magnitude of anisometropia during early childhood [50]. Gabai and Zeppieri reported that the optimal treatment window of anisometropia appears to be between 3 and 7 years of age, as the impact of age on treatment effectiveness is minimal during this period, while the efficacy of treatment decreased after this age [51]. Thus, timely recognition and intervention of uHM in pre-school children is important. The basic treatment for uHM is wearing spectacles for optical correction in combination with patch therapy. Several studies have identified the efficiency of such basic treatment on amblyopia associated with uHM in children [4, 52]. In the current study, the BCVA of children with uHM improves through correction of refractive error together with occlusion, consistent with previous studies. Apart from such basic treatment, the posterior scleral reinforcement combined with patching therapy had been suggested to contribute to delaying the myopia progression and improving the visual acuity based on study on 16 preschool uHM children [53]. As nearly half of the uHM probands in the current cohort exhibited peripheral fundus changes underlying risky complication, therefore, surgical interventions for young uHM children should be treated with great caution, emphasizing the necessity of comprehensive preoperative examinations. Further research is essential to validate the benefits and disadvantages of surgical interventions for young uHM children in the future. In addition, low-concentration atropine [54] and orthokeratology treatment [55] might delay the myopia progression on the highly myopic eye in children with myopic anisometropia. However, it's noteworthy that such intervention on myopia is mainly based on low to moderate myopia, other than uHM as seen in the current study. The effectiveness of these interventions in children with uHM requires further studies.
Although the current study provides valuable novel findings, there are some limitations. First, as described above, the number of uHM participants enrolled in the follow-up study of the BCVA improvement in highly myopic eye is limited due to variable time of follow-up duration, lack of placebo controls, and compliance of intervention in children. Therefore, the treatment outcome of the current study should be taken with caution. The accurate assessment of the effectiveness of the clinical intervention for uHM patients requires prospective cohort studies in the future. Second, the variants related to Stickler syndrome or Marfan syndrome have been commonly identified in this cohort. Since all subjects are recruited in our Pediatric and Genetic Eye Clinic, the majority of patients in this cohort are children (the age at examination was 6.21 ± 4.70 years) so that some of the valuable examinations (like SLO, FFA) were not performed in all probands. Furthermore, some extra-ocular manifestations related to these syndromes are not obvious in early childhood, which may present or become more obvious in the teenager period [56], so that gene test on these patients in young childhood may provide an early warning marker in the care of these diseases. However, the transition of related clinical manifestations still needs to be further observed in the future.
Conclusions
In summary, to the best of our knowledge, the current study represents the first systematic clinical and genetic analysis of simplex uHM, revealing not only a clear association between monogenic variants and uHM but also potential blinding diseases hidden under classic myopic fundus. These findings emphasize the importance of early extensive examination of peripheral retina in both eyes of uHM patients and of genetic counseling for family members based on genetic testing. Regular follow up visit is crucial not only for care of amblyopia but also for early detection of potential complications, which is the key for effective management of blindness consequence. In addition, this study may enrich our understanding of the molecular basis underlying uHM. The risky factors identified in the current study may also be valuable to be explored in other related eye diseases, such as anisometropia and amblyopia.
Availability of data and materials
All data generated during this study are available in the paper and its supplementary materials. The raw data used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- uHM:
-
Unilateral high myopia
- FFA:
-
Fundus fluorescein angiography
- WES:
-
Whole-exome sequencing
- TES:
-
Targeted exome-sequencing
- SLO:
-
Scanning-laser ophthalmology
- OCT:
-
Optical coherence tomography
- ERG:
-
Electroretinogram
- eoHM:
-
Early-onset high myopia
- HGMD:
-
Human Gene Mutation Database
- HSF:
-
Human splicing finder
- ACMG/AMP:
-
The American College of Medical Genetics and Genomics and the Association for Molecular Pathology
- BCVA:
-
Best corrected visual acuity
- OCTA:
-
Optical coherence tomography angiography
References
Muftuoglu O, Hosal BM, Zilelioglu G. Ciliary body thickness in unilateral high axial myopia. Eye. 2009;23:1176–81.
Weiss AH. Unilateral high myopia: optical components, associated factors, and visual outcomes. Br J Ophthalmol. 2003;87:1025–31.
Kutschke PJ, Scott WE, Keech RV. Anisometropic amblyopia. Ophthalmology. 1991;98:258–63.
Sen DK. Results of treatment in amblyopia associated with unilateral high myopia without strabismus. Br J Ophthalmol. 1984;68:681–5.
Gee SS, Tabbara KF. Increase in ocular axial length in patients with corneal opacification. Ophthalmology. 1988;95:1276–8.
Gregory-Evans CY, Vieira H, Dalton R, Adams GG, Salt A, et al. Ocular coloboma and high myopia with Hirschsprung disease associated with a novel ZFHX1B missense mutation and trisomy 21. Am J Med Genet A. 2004;131:86–90.
Osaguona VB, Momoh RO. Morning glory syndrome in a Nigerian—a case report. J West Afr Coll Surg. 2017;7:128–34.
Jain M, Sharon JM, Anjanamurthy R, Wijesinghe HK. Straatsma syndrome: unilateral myelinated retinal nerve fibre layer, high myopia, strabismus and amblyopia. BMJ Case Rep. 2021;14:e244362.
Liu KY, Sengillo JD, Velez G, Jauregui R, Sakai LY, et al. Missense mutation in SLIT2 associated with congenital myopia, anisometropia, connective tissue abnormalities, and obesity. Orphanet J Rare Dis. 2018;13:138.
Garcia-Valenzuela E, Kaufman LM. High myopia associated with retinopathy of prematurity is primarily lenticular. J AAPOS. 2005;9:121–8.
Adenuga OO. Severe anisometropic myopia in identical twins. Middle East Afr J Ophthalmol. 2014;21:275–8.
Zhang Q. Genetics of pathologic myopia. In: Spaide RF, Ohno-Matsui K, Yannuzzi LA, editors. Pathologic myopia. Cham: Springer International Publishing; 2021. p. 43–58.
Zhou L, **ao X, Li S, Jia X, Zhang Q. Frequent mutations of RetNet genes in eoHM: Further confirmation in 325 probands and comparison with late-onset high myopia based on exome sequencing. Exp Eye Res. 2018;171:76–91.
Sun W, Huang L, Xu Y, **ao X, Li S, et al. Exome sequencing on 298 probands with early-onset high myopia: approximately one-fourth show potential pathogenic mutations in RetNet genes. Invest Ophthalmol Vis Sci. 2015;56:8365–72.
Wang Y, **ao X, Li X, Yi Z, Jiang Y, et al. Genetic and clinical landscape of ARR3-associated MYP26: the most common cause of Mendelian early-onset high myopia with a unique inheritance. Br J Ophthalmol. 2022. https://doi.org/10.1136/bjo-2022-321511.
Wang Y, Sun W, **ao X, Jiang Y, Ouyang J, et al. Unique haplotypes in OPN1LW as a common cause of high myopia with or without protanopia: a potential window into myopic mechanism. Invest Ophthalmol Vis Sci. 2023;64:29.
Jiang Y, Zhou L, Wang Y, Ouyang J, Li S, et al. The genetic confirmation and clinical characterization of LOXL3-associated MYP28: a common type of recessive extreme high myopia. Invest Ophthalmol Vis Sci. 2023;64:14.
Vincent SJ, Collins MJ, Read SA, Carney LG. Myopic anisometropia: ocular characteristics and aetiological considerations. Clin Exp Optom. 2014;97:291–307.
Feng L, Candy TR, Yang Y. Severe myopic anisometropia in a Chinese family. Optom Vis Sci. 2012;89:507–11.
Park SJ, Kim JY, Baek SH, Kim ES, Kim US. One sister and brother with mirror image myopic anisometropia. Korean J Ophthalmol. 2010;24:62–4.
Chen Y, Zhang Q, Shen T, **ao X, Li S, et al. Comprehensive mutation analysis by whole-exome sequencing in 41 Chinese families with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2013;54:4351–7.
Sharma P, Shareef I, Kalaw FGP, Kako RN, Lin A, et al. Prevalence of peripheral retinal findings in retinal patients using ultra-widefield pseudocolor fundus imaging. Sci Rep. 2023;13:20515.
Yamao S, Tsujioka T, Takada R, Matsumoto F, Kusaka S. Utility of oral fluorescein angiography with ultra-widefield imaging system for evaluation of various retinal disorders. Retina. 2021;41:1338–45.
De Silva SR, Arno G, Robson AG, Fakin A, Pontikos N, et al. The X-linked retinopathies: physiological insights, pathogenic mechanisms, phenotypic features and novel therapies. Prog Retin Eye Res. 2021;82:100898.
Cheung R, Ly A, Katalinic P, Coroneo MT, Chang A, et al. Visualisation of peripheral retinal degenerations and anomalies with ocular imaging. Semin Ophthalmol. 2022;37:554–82.
Ohno-Matsui K, Kawasaki R, Jonas JB, Cheung CM, Saw SM, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol. 2015;159:877-883.e877.
Thomas MG, Kumar A, Mohammad S, Proudlock FA, Engle EC, et al. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity? Ophthalmology. 2011;118:1653–60.
Pendergast SD, Trese MT. Familial exudative vitreoretinopathy: results of surgical management. Ophthalmology. 1998;105:1015–23.
Barrett BT, Bradley A, Candy TR. The relationship between anisometropia and amblyopia. Prog Retin Eye Res. 2013;36:120–58.
Elhusseiny AM, Jabroun M, Rajabi F, Gonzalez E, Alkharashi M. A novel variant in the TSPAN12 gene-presenting as unilateral myopia, pediatric cataract, and heterochromia in a patient with familial exudative vitreoretinopathy. Eur J Ophthalmol. 2022;32:Np6-np9.
Tong L, Saw SM, Chia KS, Tan D. Anisometropia in Singapore school children. Am J Ophthalmol. 2004;137:474–9.
Ghoraba HH, Ludwig CA, Moshfeghi DM. Biometric variations in high myopia associated with different underlying ocular and genetic conditions. Ophthalmol Sci. 2023;3:100236.
Guo D, Li S, **ao X, Jiang Y, Wang Y, et al. Clinical and genetic landscape of ectopia lentis based on a cohort of patients from 156 families. Invest Ophthalmol Vis Sci 2024;65(1):20
Patel VS, Simon JW, Schultze RL. Anisometropic amblyopia: axial length versus corneal curvature in children with severe refractive imbalance. J AAPOS. 2010;14:396–8.
Zaka-Ur-Rab S. Evaluation of relationship of ocular parameters and depth of anisometropic amblyopia with the degree of anisometropia. Indian J Ophthalmol. 2006;54:99–103.
Cass K, Tromans C. A biometric investigation of ocular components in amblyopia. Ophthalmic Physiol Opt. 2008;28:429–40.
Harding P, Gore S, Malka S, Rajkumar J, Oluonye N, et al. Real-world clinical and molecular management of 50 prospective patients with microphthalmia, anophthalmia and/or ocular coloboma. Br J Ophthalmol. 2022. https://doi.org/10.1136/bjo-2022-321991.
Schneider A, Bardakjian T, Reis LM, Tyler RC, Semina EV. Novel SOX2 mutations and genotype-phenotype correlation in anophthalmia and microphthalmia. Am J Med Genet A. 2009;149a:2706–15.
Reis LM, Costakos D, Wheeler PG, Bardakjian T, Schneider A, et al. Dominant variants in PRR12 result in unilateral or bilateral complex microphthalmia. Clin Genet. 2021;99:437–42.
Hornby SJ, Adolph S, Gilbert CE, Dandona L, Foster A. Visual acuity in children with coloboma: clinical features and a new phenotypic classification system. Ophthalmology. 2000;107:511–20.
Tian T, Chen C, Zhang X, Zhang Q, Zhao P. Clinical and genetic features of familial exudative vitreoretinopathy with only-unilateral abnormalities in a Chinese cohort. JAMA Ophthalmol. 2019;137:1054–8.
Gonzalez-Rodriguez B, Gonzalez-Rodriguez M. Goldenhar syndrome. Am J Ophthalmol. 2021;232: e3.
Bogusiak K, Puch A, Arkuszewski P. Goldenhar syndrome: current perspectives. World J Pediatr. 2017;13:405–15.
Wang L, Liu Z, Lin H, Ma D, Tao Q, et al. Epigenetic regulation of left-right asymmetry by DNA methylation. EMBO J. 2017;36:2987–97.
Vogt G. Stochastic developmental variation, an epigenetic source of phenotypic diversity with far-reaching biological consequences. J Biosci. 2015;40:159–204.
Wang YY, Zhang XJ, Kam KW, Chen ZJ, Zhang Y, et al. Association of polymorphisms in ZFHX1B and PAX6 with anisometropia in Chinese Children: the Hong Kong Children Eye Genetics Study. Invest Ophthalmol Vis Sci. 2023;64:6.
Chen ZX, Chen TH, Zhang M, Chen JH, Lan LN, et al. Correlation between FBN1 mutations and ocular features with ectopia lentis in the setting of Marfan syndrome and related fibrillinopathies. Hum Mutat. 2021;42:1637–47.
Qi D, Liu S, Yu T. Characterization of unique lens morphology in a cohort of children with familial exudative vitreoretinopathy. Curr Eye Res. 2020;45:1222–7.
Gilmour DF. Familial exudative vitreoretinopathy and related retinopathies. Eye. 2015;29:1–14.
Leon A, Donahue SP, Morrison DG, Estes RL, Li C. The age-dependent effect of anisometropia magnitude on anisometropic amblyopia severity. J AAPOS. 2008;12:150–6.
Gabai A, Zeppieri M. Anisometropia. Treasure Island: StatPearls Publishing LLC; 2023.
Pang Y, Allison C, Frantz KA, Block S, Goodfellow GW. A prospective pilot study of treatment outcomes for amblyopia associated with myopic anisometropia. Arch Ophthalmol. 2012;130:579–84.
Shen ZM, Zhang ZY, Zhang LY, Li ZG, Chu RY. Posterior scleral reinforcement combined with patching therapy for pre-school children with unilateral high myopia. Graefes Arch Clin Exp Ophthalmol. 2015;253:1391–5.
Lin L, Lan W, Liao Y, Zhao F, Chen C, et al. Treatment outcomes of myopic anisometropia with 1% atropine: a pilot study. Optom Vis Sci. 2013;90:1486–92.
Lu W, ** W. Clinical observations of the effect of orthokeratology in children with myopic anisometropia. Cont Lens Anterior Eye. 2020;43:222–5.
Liberfarb RM, Levy HP, Rose PS, Wilkin DJ, Davis J, et al. The Stickler syndrome: genotype/phenotype correlation in 10 families with Stickler syndrome resulting from seven mutations in the type II collagen gene locus COL2A1. Genet Med. 2003;5:21–7.
Acknowledgements
We sincerely thank all patients and their family members for their participation in this study.
Funding
This work is supported by Grants from the National Natural Science Foundation of China (81770965), the Science and Technology Planning Projects of Guangzhou (202102010271), and the Fundamental Research Funds of the State Key Laboratory of Ophthalmology.
Author information
Authors and Affiliations
Contributions
YJ, XX, and QZ conceptualized and designed the study. YJ, XX, WS, PW, SL, XJ, YW, and QZ conducted the data acquisition and analysis. YJ and QZ performed the statistical analysis. YJ and QZ drafted the manuscript. QZ and FH revised the manuscript for important intellectual content. All authors have readed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of Zhongshan Ophthalmic Center and in accordance with the principles of the Declaration of Helsinki. Before their enrollment, written informed consent documents have been obtained from all participants or their legal guardians.
Consent for publication
Consents for publication were obtained from all participants or their legal guardians.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. The gene list of the ophthalmic targeted exome sequencing panel included 736 genes. Table S2. Clinical phenotype and molecular diagnosis of 75 patients with unilateral high myopia. Table S3-1. The distribution of peripheral fundus findings in unilateral high myopia children. Table S3-2. The distribution of peripheral fundus findings in 20 unilateral high myopia children with genetic defects. Table S3-3. The distribution of peripheral fundus findings in unilateral high myopia children with or without genetic defects. Table S4. Comparison of baseline characteristics between groups with and without genetic defects. Table S5. The follow-up study of refraction in patients with unilateral high myopia in this study.
Additional file 2
: Figure S1. The optical coherence tomography (OCT) scans and the electroretinogram (ERG) recordings of eight unilateral high myopia probands in this cohort. A–D The OCT scans demonstrated normal structure in three eyes of two patients (F4-II:1 and F5-II:2). The grade 1 or grade 2 foveal hypoplasia was observed in five eyes of three patients (F1-II:1, F5-II:2, and F12-II:2). E-H The ERG findings of the highly myopic eye of four patients showed normal cone and rod response in two patients (F8-II:1 and F14-II:1), moderate to severe reduction in cone response, and severe reduction in rod response in the other two patients (F9-II:1 and F13-II:2). Figure S2. Family Pedigrees and Sanger sequencing chromatograms results for 20 unilateral high myopia families. The DNA sequencing results of affected patients and normal controls were presented in the right column. While the corresponding family pedigrees were displayed in the left column.
Additional file 3
: Figure S3. Representative image of each peripheral retinal change identified in the study. Table S6. List of peripheral retinal changes in this cohort.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Y., **ao, X., Sun, W. et al. Clinical and genetic risk factors underlying severe consequence identified in 75 families with unilateral high myopia. J Transl Med 22, 75 (2024). https://doi.org/10.1186/s12967-024-04886-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-04886-5