Abstract
Pelvic lymph node dissection (PLND) is commonly performed alongside radical prostatectomy. Its primary objective is to determine the lymphatic staging of prostate tumors by removing lymph nodes involved in lymphatic drainage. This aids in guiding subsequent treatment and removing metastatic foci, potentially offering significant therapeutic benefits. Despite varying recommendations from clinical practice guidelines across countries, the actual implementation of PLND is inconsistent, partly due to debates over its therapeutic value. While high-quality evidence supporting the superiority of PLND in oncological outcomes is lacking, its role in increasing surgical time and risk of complications is well-recognized. Despite these concerns, PLND remains the gold standard for lymph node staging in prostate cancer, providing invaluable staging information unattainable by other techniques. This article reviews PLND's scope, guideline perspectives, implementation status, oncologic and non-oncologic outcomes, alternatives, and future research needs.
Similar content being viewed by others
Lymphatic drainage in prostate cancer
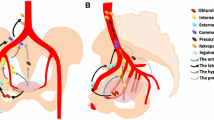
The male pelvic region's lymph nodes can be categorized into five distinct groups based on their placement, specifically the paraaortic nodal group, the common Iliac nodal group, the internal Iliac nodal group, the external Iliac nodal group, and the inguinal lymph nodal group. The various clusters of lymph nodes are linked together to create a lymphatic drainage system in the pelvic area. The male pelvic tissues and organs can be drained lymphatically through three primary routes: the superficial inguinal pathway, the pelvic pathway, and the paraaortic pathway. The pelvic pathway, comprising the common iliac group, the internal iliac group, and the external iliac group, serves as the primary lymphatic drainage pathway for both the bladder and prostate [1]. Lymph nodes situated near the iliac vessels are typically the initial targets of lymph node metastases originating from prostate cancer, with the internal iliac region exhibiting the highest vulnerability, followed by the external iliac region, the obturator region, the common iliac region, and the presacral region [2, 3]. Sentinel lymph nodes are typically seen as the main source of lymphatic drainage from tissues and organs, and they are the most probable spot in the lymphatic drainage chain where tumors are likely to spread first. The Fossa Marcille, situated within the male pelvis, serves as a crucial anatomical framework [4, 5], encompassing lymph nodes from the external iliac, obturator, and internal iliac regions that collectively contribute to its composition. The presence of positive lymph nodes in the fossa Marcille in patients with prostate cancer is often accompanied by negative lymph nodes in other areas [6], indicating a strong association between lymph node invasion in this region and the occurrence of multiple lymph node metastases in other regions [5], implying a high likelihood of sentinel lymph nodes for prostate cancer in the fossa Marcille, which aligns with the lymphatic drainage attributes of prostate cancer. By employing the technique of contrast agent localization, pertinent research indicated that the sentinel lymph nodes of prostate cancer were predominantly clustered in the obturator region (30%-40%), the external iliac region (19%-34%), and the internal iliac region (17%-33%) [7, 8], while subsequent investigations unveiled that the sentinel lymph nodes were predominantly positioned at the intersection of the internal iliac and external iliac vessels, along with the distal extremity of the internal iliac vessels [7], By identifying the sentinel lymph nodes, we can ascertain the severity of the illness, diminish the trauma caused by surgery, and give advice for treatment choices and prognostic evaluation [2].
While prostate cancer exhibits the aforementioned drainage properties, the actual drainage scenario may become more intricate when lymph node metastasis occurs. In light of the potential for lymphatic dissemination in prostate cancer cells, urologists commonly recommend PLND for localized prostate cancer patients exhibiting an elevated risk of lymph node infiltration. In instances where pelvic lymph node metastasis has been conclusively identified, conventional therapeutic interventions such as chemotherapy, radiotherapy, and immunotherapy are generally prioritized. Salvage PLND, proposed as an alternative treatment in select medical centers, remains a subject of ongoing debate regarding its therapeutic efficacy. In summary, the primary objectives of the PLND are to elucidate the classification of the tumor lymph nodes and eliminate potential lymph node metastatic lesions, with the aim of attaining potential therapeutic advantages and enhancing the patient's prognosis [9, 10].
The excision range of PLND
In general, pelvic lymph node dissection (PLND) is a surgical intervention that eliminates lymph nodes from different areas along the iliac vascular distribution, taking into account the manner in which lymphatic drainage occurs in prostate cancer. PLND can be broken down into distinct subcategories depending on the particular regions examined. Nevertheless, there is a lack of consensus among academics regarding the extent of excision within the PLND subgroup. A quadratic division of PLND has been suggested by certain scholars [11]: (1) limited pelvic lymph node dissection (lPLND): external iliac region + obturator region; (2) standard pelvic lymph node dissection (sPLND): lPLND + internal iliac region; (3) extended pelvic lymph node dissection (ePLND): sPLND + common iliac region; (4) super-extended pelvic lymph node dissection ( sePLND): ePLND + presacral region. However, there are also scholars who have proposed a trichotomy of PLND [3]: lPLND (external iliac region + obturator region), ePLND (lPLND + internal iliac region), and sePLND (ePLND + common iliac region + presacral region).
The indications of PLND
The academic community continues to hold differing opinions on the timing of treatment and patient selection for PLND. Proponents of PLND argue that this procedure not only assesses the tumor's lymph node staging but also eliminates metastatic lesions from the lymph nodes for therapeutic purposes [12, 13], Conversely, opponents of PLND argue that it can result in extended surgical duration and increased complications, and that the decision to undergo PLND does not impact the patient's postoperative oncological prognosis. Various nations and territories also offer distinct suggestions for PLND.
According to the European Association of Urology (EAU) guidelines, PLND is advised for patients with intermediate-risk prostate cancer who have a high likelihood of lymph node invasion (with cut-off values ranging from 5 to 7%), as evaluated by Briganti nomogram, as well as for those with high-risk prostate cancer. It is advisable to use ePLND, which includes the external iliac, obturator, internal iliac, and common iliac regions [14]. According to the American Urological Association (AUA) guidelines, PLND can offer crucial insights into lymph node staging and contribute to the formulation of a post-surgical treatment strategy, yet it does not yield substantial advantages in terms of oncological outcomes. Hence, it is advisable to evaluate the likelihood of lymph node invasion through pertinent nomograms prior to deciding whether to carry out PLND. It is advisable to use ePLND, which includes the external iliac, obturator, and internal iliac regions [15]. In accordance with the National Comprehensive Cancer Network (NCCN) guidelines, PLND assists in elucidating the staging of tumorous lymph nodes and eradicating microscopic lymphatic metastatic lesions. Hence, it is advisable to utilize nomograms for evaluating the likelihood of lymph node infiltration in patients and to conduct ePLND (encompassing the external iliac, obturator, and internal iliac regions) after thoroughly evaluating surgical duration, potential surgical complications, and other variables [16]. According to China's prostate cancer treatment guidelines, there is inadequate evidence to substantiate the positive impact of PLND on on oncological outcomes, nevertheless, PLND can elucidate lymph node staging and offer prognosis guidance. Hence, it is advisable for patients diagnosed with high-risk prostate cancer and those identified as intermediate-risk with an elevated risk of lymph node invasion based on the Memorial Sloan Kettering Cancer Center (MSKCC) nomogram (with a cut-off value of 5%) to undergo ePLND therapy (encompassing external iliac, obturator, and internal iliac regions) (Table 1).
After conducting a thorough analysis of multiple guidelines from various countries and regions, it has been determined that PLND is generally not advisable for patients with low-risk prostate cancer, whereas ePLND is recommended for patients with intermediate-risk prostate cancer who have a higher likelihood of lymph node invasion, as indicated by nomograms such as MSKCC and Briganti. In addition to patients with a high-risk prostate. In relation to the range of lymph node dissection, despite the recommendation of various guidelines for ePLND, the EAU's recommended range of ePLND includes the common iliac region alongside the range of ePLND dissection (external iliac + obturator + internal iliac) recommended by the AUA, NCCN, and China guidelines. It is crucial to delve deeper into the potential variations in lymph node staging and oncological outcomes associated with ePLND of such varying ranges.
The current status of PLND
Numerous guidelines mentioned earlier advocate for the provision of PLND to all patients diagnosed with intermediate- and high-risk prostate cancer, on the condition that the necessary criteria are fulfilled; however, the practical implementation rate of PLND remains relatively inadequate. A study conducted in the UK examined high-risk prostate cancer patients who received surgical treatment in accordance with the EAU guidelines. Among the 3091 high-risk prostate cancer patients, PLND treatment was administered to 66%, while 62.4% either did not undergo PLND or solely underwent obturator lymph node dissection. Furthermore, out of the 66% who received PLND treatment, a mere 56.8% opted for ePLND treatment, with less than one-third of ePLND being performed in its entirety. The findings of this study propose that the diminished rate of PLND performance could be attributed to the abundance of perioperative complications associated with PLND, without any notable improvement in survival outcomes [17]. In accordance with the German S3 guidelines, a multicenter study conducted in Germany and Austria demonstrated an impressive overall implementation rate of 97.2% for PLND [18]. A North American study assessed the adherence rate to guideline-recommended PLND among patients with prostate cancer in North America. The study findings indicated that, as per the NCCN guidelines and the D'Amico risk stratification scheme, the recommended rates for PLND were 63.3% and 64.9% for patients, and 68.8% and 69.1% for those who actually underwent PLND, respectively, indicating a persistently low percentage, despite an increase compared to previous records. The study determined that when making clinical decisions, it is important to take into account not only guideline recommendations but also the clinical characteristics of patients. Patients with a low likelihood of lymphatic metastasis but unfavorable traits of prostate cancer (such as late clinical-stage, high Gleason score, and a significant proportion of positive biopsies) should be considered for PLND, while those with a high risk of lymphatic metastasis but lacking unfavorable characteristics of prostate cancer can be deemed to have PLND disregarded [19].
Taking into account the escalating pattern of lymph node invasion in prostate cancer patients annually [20], the guidelines advocate for the adoption of PLND in screened prostate cancer patients. Nevertheless, the diverse cognitive viewpoints regarding PLND across various centers, surgical methodologies, and other variables have resulted in significant fluctuations in the practical implementation of PLND in clinical settings.
The oncological outcomes of PLND
The variation in PLND implementation rates can be attributed, in part, to the absence of a consistent perspective on the significance of PLND in the clinical context. The question of whether PLND should be conducted, the appropriate level of PLND to be administered, and the actual benefits it provides to patients with prostate cancer has sparked controversy.
Should PLND be implemented?
Many investigations have been carried out to determine if PLND should be put into practice. Badani et al. conducted a retrospective analysis on the survival rates of patients with pT3b prostate cancer who underwent RP, revealing that the risk of postoperative biochemical recurrence was comparable between the two patient groups who did not undergo PLND and those who did; however, in patients who underwent PLND, the augmentation in the number of dissected lymph nodes did not significantly diminish the probability of biochemical recurrence [21]. Preisser et al.’s study discovered that in a cohort of patients diagnosed with intermediate-risk and high-risk prostate cancer who were administered ePLND compared to those who were not, ePLND effectively furnished data regarding the classification of the tumor lymph nodes. Nevertheless, there was no notable disparity between the two cohorts in regards to biochemical recurrence-free survival, metastasis-free survival, and tumour-specific survival [22, 23]. Tomisaki et al's study arrived at a comparable finding: individuals with limited prostate cancer who underwent PLND exhibited no notable disparity in oncological outcomes in comparison to those who did not undergo PLND, and patients who did not undergo PLND circumvented the accompanying surgical hazards and complications [24]. Kodiyan et al. collated information from the US National Cancer Database in accordance with the NCCN guidelines and categorized patients into two categories: those with positive attributes of prostate cancer and those with negative traits of prostate cancer. While the acceptance or rejection of PLND did not significantly impact survival in the group exhibiting favorable traits of prostate cancer, a correlation was observed between the implementation of PLND and improved survival in the group with unfavorable characteristics of prostate cancer [25]. According to the US National Cancer Database, Sood et al. conducting ePLND yields a notable survival benefit for individuals with lymph node metastases (pN1) in contrast to the absence of PLND or ePLND, and for every extra lymph node examined, a patient's tumor-specific survival rate can be enhanced by 7% [26].
Optimal excision range of PLND
Besides the decision of whether or not to receive PLND, the selection of the appropriate level of PLND to be granted is a subject that warrants thorough exploration. Lestingi et al. conducted a phase III randomized controlled clinical trial at a single center, with a prospective design. I examined if there was any disparity in oncological results among 300 intermediate- to high-risk prostate patients who underwent radical prostatectomy with ePLND (including external iliac, obturator, internal iliac, common iliac and presacral regions) and lPLND (only the presacral region) to determine if there was any variation in oncological outcomes. Initial findings indicate that while ePLND offers more conclusive data regarding tumor lymph node staging, there is no statistically significant disparity between the two in relation to 5-year biochemical recurrence-free survival, metastasis-free survival, or tumour-specific survival. Subgroup analyses indicate that ePLND has the potential to be advantageous for patients with high-grade prostate cancer in relation to biochemical recurrence-free survival, although further studies are required to validate these subgroup analyses [27, 28]. Touijer et al. devised a controlled trial with a single center and randomization. A comparison was made between the oncological results of lPLND (external iliac region only) and ePLND (external iliac + obturator + internal iliac) in 1440 patients with prostate cancer. The findings indicated that the disparities in the quantity of dissected lymph nodes and the percentage of positive lymph nodes were negligible between the two groups, and that ePLND did not diminish the likelihood of biochemical recurrence in patients [29]. Wettstein et al. conducted a retrospective study involving two centers, focusing on a cohort. It was proven that ePLND effectively decreased the likelihood of biochemical recurrence and the necessity for supplementary treatment post-surgery by 25%-31% in contrast to non-ePLND [30]. Preisser et aI. performed a retrospective analysis utilizing the SEER database to assess the survival rates of individuals with prostate cancer who had undergone PLND. Patients with no lymph node invasion and postoperative pathology exhibited a decreased tumor-specific mortality rate following a more extensive PLND, in contrast to patients with less extensive PLND. Additionally, each additional lymph node removed by PLND resulted in a reduction of approximately 4.5% in tumour-specific mortality, which was unfavorable for the prostate cancer group [31].
It is evident from the aforementioned studies that there are conflicting findings regarding the potential oncological benefits of PLND. Despite certain studies suggesting the potential oncological benefits of PLND in specific patient groups, such as those with unfavorable characteristics of prostate cancer, these studies have predominantly focused on retrospective research, limited prospective studies, bias in patient selection for PLND, non-standardized templates for PLND, and absence of a comprehensive description of the specific dissection range of PLND, among other factors (Table 2 [27, 29, 30, 32,33,34,35]). In order to bridge the gap between studies, a more effective study design should be implemented in the future to ensure more dependable findings.
The non-oncological outcomes of PLND
Lymph node staging
Regarded as the epitome of lymph node staging, PLND offers accurate data on tumor staging in individuals with prostate cancer, aiding in the evaluation of the patient's state and offering valuable insights for their prognosis. Notwithstanding the utilization of medical imaging technologies like CT, MRI, PET-CT, etc. The utilization of relevant clinical parameters to assess lymph node staging has proven to be effective alternatives; however, the actual pathological information of lymph nodes obtained from these alternatives remains limited when compared to PLND, and there is still potential for further enhancement in determining lymph node staging [36].
Surgical complications
As surgical techniques have progressed, PLND has gone from the early days of open surgery to the current era of laparoscopy and robot-assisted laparoscopy. While there has been a decline in the complication rate of PLND in comparison to previous instances [37], there are still certain complications that necessitate attention. The typical complications associated with PLND encompass lymphocysts, harm to pelvic tissues and organs (such as vessels, obturator nerves, bladder, and rectum), venous thrombosis in the lower extremities, and sexual dysfunction. The disruption of lymphatic drainage pathways in the pelvis caused by PLND makes lymphocysts the prevailing complication. Based on the available literature, the likelihood of lymphocysts following PLND varies between 1.3% and 25% [38,39,40], and their presence is not solely attributed to the enlargement of PLND excision [38, 41], but also to advanced age, elevated BMI (Body Mass Index), and a past occurrence of peripheral vascular/lymphatic lesions [41,42,43,44]. To reduce the number of lymphocysts, preventive measures such as constructing a peritoneal flap [45, 46]、peritoneal opening [47, 48], elastic clam** of lymphatic vessels [39], bipolar electrocoagulation of the trauma, and spraying of haemostatic powder [49] can be taken during operation. Nevertheless, lymphocysts do not consistently manifest symptoms, and interventions are typically limited to symptomatic lymphocysts (such as excessive abdominal-pelvic effusion or secondary infections). Subsequent to PLND, a range of medical interventions including lymphangiography, lymphatic embolisation [50], prolonged pelvic drainage, reoperation for exploration, or puncture and drainage may be undertaken [37]. In contrast to lymphocysts, complications like pelvic tissue and organ damage, lower extremity venous thrombosis, and sexual dysfunction are uncommon and can be prevented through meticulous anatomical separation during PLND and improved perioperative patient care [37, 38].
The alternatives of PLND
Despite PLND being widely regarded as the gold standard for lymph node staging in prostate cancer patients, numerous studies have strived to investigate the utilization of non-PLND techniques in assessing lymph node invasion and preventing superfluous PLND, considering the ongoing controversy surrounding the oncological advantages of PLND and the potential for extended surgical duration and related complications. In the past, a great deal of research has been conducted to evaluate the clinicopathological features of prostate cancer patients, taking into account clinical factors related to the disease. A range of prediction models have been created to forecast the risk of lymph node invasion, with Briganti, MSKCC, Cagiannos, Formulas, Zumsteg, etc. being the most popular ones [51,52,53]. Additionally, there are numerous studies that have been externally validated to determine their predictive power in various populations [52, 54,55,56]. EAU, AUA, NCCN, and other guidelines suggest the use of these prediction models with clinical parameters, which can be adjusted to achieve the best possible results for different patient groups by changing their cut-off values, a widely used method for assessing lymph node invasion.
CT and MRI can be used to evaluate prostate cancer patients before surgery to determine the condition of the primary tumor lesion, however, relying solely on CT or MRI to acquire data on pelvic lymph nodes is quite restricted. Research data indicates that CT scans have a sensitivity of 8.9% and a specificity of 98.3% when identifying positive pelvic lymph nodes, whereas MRI scans have a sensitivity of 14.3% and a specificity of 98.8% [57], indicating that relying solely on CT or MRI to decide whether to proceed with PLND is not dependable. The current recommendation for predicting pelvic lymph node invasion in clinical practice is to use multiparametric MRI in combination with tumour-related clinical parameters. To provide an instance, Brembilla et al. conducted a predictive model, which combined tumour volume (mrV) and T-stage (mrT) from MRI, along with clinical parameters like preoperative PSA and major Gleason scores, to predict pelvic lymph node invasion, and its AUC value is 0.956. By setting the cut-off values of mrT3 and mrV ≥ 1cc for PLND, it was possible to decrease 55.4% of unnecessary PLNDs and only 4.3% of patients with lymph node invasion were overlooked [58]. Gandaglia et al. developed a predictive model to accurately predict pelvic lymph node invasion by utilizing the MRI index lesion's maximum diameter, the pathology grading of the MRI-directed biopsy region, and various clinical parameters such as PSA, clinical stage, and the percentage of biopsy-positive needle counts, resulting in a remarkable 60% reduction in ePLNDs and a mere 1.6% ignorance of lymph node metastases among patients with a cut-off value of 7% [59]. Many clinical prediction models that combine multiparametric MRI and other relevant clinical parameters to predict pelvic lymph node invasion have shown a significant improvement in predictive efficacy when compared to previous models that only incorporate clinical parameters [60,61,62,63]. PET-CT, due to its remarkable ability to visualize tumour foci, has been extensively employed to identify prostate cancer lymph node invasion and can direct the removal of positive lymph nodes during surgery [64]. As an illustration, 68Ga PSMA PET-CT can be employed to guide the excision of positive lymph nodes and prevent the elimination of uninvolved lymph nodes. This method exhibits a 67% sensitivity, 100% specificity, 100% positive predictive value, and 90% negative predictive value in identifying positive lymph nodes at the individual patient level [65].
In the present scenario, where PLND is regarded as the gold standard for assessing lymph node invasion, various techniques for evaluating lymph node invasion have arisen collectively. These techniques have exhibited favorable prognostic outcomes across various cohorts of prostate cancer patients, yet they necessitate additional investigation and investigation in terms of precisely acquiring lymph node pathology data, forecasting tumor survival prognosis, ensuring safety, and assessing cost in comparison to PLND.
Conclusion and outlook
Pelvic lymph node dissection is a surgical procedure that removes pelvic lymph nodes based on the characteristics of pelvic lymphatic drainage in order to clarify the staging of the tumour lymph nodes and obtain potential therapeutic benefits. Numerous national and regional guidelines endorse this procedure as the gold standard for lymph node staging of prostate cancer, yet there remains a certain level of debate regarding its potential oncological advantages. As medical technology advances, there are now a variety of techniques that integrate radiology and clinical parameters to evaluate the danger of lymphatic infiltration in individuals with prostate cancer, and the accuracy of these approaches has been verified in various patient cohorts, however, their actual efficacy in comparison to PLND must be further verified in a broader patient group.
When conducting future research on PLND, it is imperative to prioritize the following crucial aspects: establishing a uniform categorization of PLND subgroups, determining the most suitable excision range for PLND, enhancing the predictive precision of diverse predictive models, elucidating the potential oncological advantages of PLND for prostate cancer patients, and exploring alternative non-PLND treatment options for positive lymph nodes.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- RP:
-
Radical prostatectomy
- RARP:
-
Robotic-assisted radical prostatectomy
- PLND:
-
Pelvic lymph node dissection
- lPLND:
-
Limited PLND
- sPLND:
-
Standard PLND
- ePLND:
-
Extended PLND
- sePLND:
-
Super extended PLND
- EAU:
-
European Association of Urology
- AUA:
-
American Urological Association
- NCCN:
-
National Comprehensive Cancer Network
- MSKCC:
-
Memorial Sloan Kettering Cancer Center
- BMI:
-
Body Mass Index
- PSA:
-
Prostate specific Antigen
References
Paño B, Sebastià C, Buñesch L, et al. Pathways of Lymphatic Spread in Male Urogenital Pelvic Malignancies. Radiographics. 2011;31(1):135–60. https://doi.org/10.1148/rg.311105072.
Joniau S, Van Den Bergh L, Lerut E, et al. Map** of Pelvic Lymph Node Metastases in Prostate Cancer. Eur Urol. 2013;63(3):450–8. https://doi.org/10.1016/j.eururo.2012.06.057.
Roscigno M, Nicolai M, La Croce G, et al. Difference in Frequency and Distribution of Nodal Metastases Between Intermediate and High Risk Prostate Cancer Patients: Results of a Superextended Pelvic Lymph Node Dissection. Front Surg. 2018;5:52. https://doi.org/10.3389/fsurg.2018.00052.
Lee P, Francis KE, Solomon MJ, Ramsey-Stewart G, Austin KKS, Koh C. Triangle of Marcille: the anatomical gateway to lateral pelvic exenteration. ANZ J Surg. 2017;87(7–8):582–6. https://doi.org/10.1111/ans.13872.
Porcaro AB, Cacciamani GE, Sebben M, et al. Lymph Nodes Invasion of Marcille’s Fossa Associates with High Metastatic Load in Prostate Cancer Patients Undergoing Extended Pelvic Lymph Node Dissection: The Role of “Marcillectomy.” Urol Int. 2019;103(1):25–32. https://doi.org/10.1159/000500330.
Sebben M, Tafuri A, Porcaro AB, Artibani W, Cacciamani G. Response to: Bando et al. Diagnostic and therapeutic value of pelvic lymph node dissection in the fossa of Marcille in patients with clinically localized high-risk prostate cancer: Histological and molecular analyses. Prostate. 2020;80(10):795–6. https://doi.org/10.1002/pros.23987.
Miki J, Yanagisawa T, Tsuzuki S, et al. Anatomical localization and clinical impact of sentinel lymph nodes based on patterns of pelvic lymphatic drainage in clinically localized prostate cancer. Prostate. 2018;78(6):419–25. https://doi.org/10.1002/pros.23486.
Staník M, Macík D, Čapák I, Marečková N, Lžíčařová E, Doležel J. Sentinel lymph node dissection in prostate cancer using superparamagnetic particles of iron oxide: early clinical experience. Int Urol Nephrol. 2018;50(8):1427–33. https://doi.org/10.1007/s11255-018-1903-0.
Haiquel L, Cathelineau X, Sanchez-Salas R, Macek P, Secin F. Pelvic lymph node dissection in high-risk prostate cancer. Int Braz J Urol Off J Braz Soc Urol. 2022;48(1):54–66. https://doi.org/10.1590/S1677-5538.IBJU.2020.1063.
Fossati N, Willemse PPM, Van den Broeck T, et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur Urol. 2017;72(1):84–109. https://doi.org/10.1016/j.eururo.2016.12.003.
Ploussard G, Briganti A, De La Taille A, et al. Pelvic Lymph Node Dissection During Robot-assisted Radical Prostatectomy: Efficacy, Limitations, and Complications—A Systematic Review of the Literature. Eur Urol. 2014;65(1):7–16. https://doi.org/10.1016/j.eururo.2013.03.057.
Touijer KA, Mazzola CR, Sjoberg DD, Scardino PT, Eastham JA. Long-term Outcomes of Patients with Lymph Node Metastasis Treated with Radical Prostatectomy Without Adjuvant Androgen-deprivation Therapy. Eur Urol. 2014;65(1):20–5. https://doi.org/10.1016/j.eururo.2013.03.053.
Banapour P, Schumacher A, Lin JC, Finley DS. Radical Prostatectomy and Pelvic Lymph Node Dissection in Kaiser Permanente Southern California: 15-Year Experience. Perm J. 2019;23(1):17–233. https://doi.org/10.7812/TPP/17-233.
Mottet N, Van Den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2021;79(2):243–62. https://doi.org/10.1016/j.eururo.2020.09.042.
Eastham JA, Auffenberg GB, Barocas DA, et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J Urol. 2022;208(1):19–25. https://doi.org/10.1097/JU.0000000000002758.
Schaeffer EM, Srinivas S, Adra N, et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN. 2023;21(10):1067–96. https://doi.org/10.6004/jnccn.2023.0050.
Aning JJ, Reilly GS, Fowler S, Challacombe B, McGrath JS, Sooriakumaran P. Perioperative and oncological outcomes of radical prostatectomy for high‐risk prostate cancer in the UK: an analysis of surgeon‐reported data. 2019.
On behalf of the GeSRU Academics Prostate Cancer Group, Borkowetz A, Bruendl J, et al. Multicenter evaluation of guideline adherence for pelvic lymph node dissection in patients undergoing open retropubic vs. laparoscopic or robot assisted radical prostatectomy according to the recent German S3 guideline on prostate cancer. World J Urol. 2018;36(6):855–61. https://doi.org/10.1007/s00345-018-2195-9.
Leyh-Bannurah SR, Budäus L, Zaffuto E, et al. Adherence to pelvic lymph node dissection recommendations according to the National Comprehensive Cancer Network pelvic lymph node dissection guideline and the D’Amico lymph node invasion risk stratification. Urol Oncol Semin Orig Investig. 2018;36(2):81.e17–24. https://doi.org/10.1016/j.urolonc.2017.10.022.
Preisser F, Nazzani S, Bandini M, et al. Increasing rate of lymph node invasion in patients with prostate cancer treated with radical prostatectomy and lymph node dissection. Urol Oncol Semin Orig Investig. 2018;36(8):365.e1–7. https://doi.org/10.1016/j.urolonc.2018.05.019.
Badani KK, Reddy BN, Moskowitz EJ, et al. Lymph node yield during radical prostatectomy does not impact rate of biochemical recurrence in patients with seminal vesicle invasion and node-negative disease. Urol Oncol Semin Orig Investig. 2018;36(6):310.e1–6. https://doi.org/10.1016/j.urolonc.2018.03.004.
Preisser F, van den Bergh RCN, Gandaglia G, et al. Effect of Extended Pelvic Lymph Node Dissection on Oncologic Outcomes in Patients with D’Amico Intermediate and High Risk Prostate Cancer Treated with Radical Prostatectomy: A Multi-Institutional Study. J Urol. 2020;203(2):338–43. https://doi.org/10.1097/JU.0000000000000504.
Skolarikos A. Re: Effects of Extended Pelvic Lymph Node Dissection on Oncologic Outcomes in Patients with D’Amico Intermediate and High Risk Prostate Cancer Treated with Radical Prostatectomy: A Multi-institutional Study. 2019.
Tomisaki I, Ikuta H, Higashijima K, Onishi R, Minato A, Fujimoto N. Oncological Outcome After Radical Prostatectomy without Pelvic Lymph Node Dissection for Localized Prostate Cancer: Follow-up Results in a Single Institution. Cancer Invest. 2019;37(10):524–30. https://doi.org/10.1080/07357907.2019.1675076.
Kodiyan J, Guirguis A, Ashamalla H. Radical Prostatectomy Without Pelvic Lymph Node Dissection Is Widely Practiced in High-Risk Patients Despite Poorer Survival. Clin Genitourin Cancer. 2020;18(5):395–401.e8. https://doi.org/10.1016/j.clgc.2020.03.008.
Sood A, Keeley J, Palma-Zamora I, et al. Extended pelvic lymph-node dissection is independently associated with improved overall survival in patients with prostate cancer at high-risk of lymph-node invasion. BJU Int. 2020;125(6):756–8. https://doi.org/10.1111/bju.15034.
Lestingi JFP, Guglielmetti GB, Trinh QD, et al. Extended Versus Limited Pelvic Lymph Node Dissection During Radical Prostatectomy for Intermediate- and High-risk Prostate Cancer: Early Oncological Outcomes from a Randomized Phase 3 Trial. Eur Urol. 2021;79(5):595–604. https://doi.org/10.1016/j.eururo.2020.11.040.
Lestingi JFP. Reply to Alberto Briganti, Giorgio Gandaglia, Markus Graefen, Steven Joniau, R. Jeffrey Karnes, and Francesco Montorsi’s Letter to the Editor re: Jean F.P. Lestingi, Giuliano B. Guglielmetti, Quoc-Dien Trinh, et al. Extended Versus Limited Pelvic Lymph Node Dissection During Radical Prostatectomy for Intermediate- and High-risk Prostate Cancer: Early Oncological Outcomes from a Randomized Phase 3 Trial. Eur Urol. 2021;79:595–604 Time for a Change? Clinically Meaningful Reasons Why We Will Continue Performing Extended Pelvic Lymph Node Dissection in Selected Patients with Prostate Cancer. Eur Urol. Published online 2021.
Touijer KA, Sjoberg DD, Benfante N, et al. Limited versus Extended Pelvic Lymph Node Dissection for Prostate Cancer: A Randomized Clinical Trial. Eur Urol Oncol. 2021;4(4):532–9. https://doi.org/10.1016/j.euo.2021.03.006.
Wettstein MS, David LA, Pazhepurackel C, et al. Benefit of a more extended pelvic lymph node dissection among patients undergoing radical prostatectomy for localized prostate cancer: A causal mediation analysis. Prostate. 2021;81(5):286–94. https://doi.org/10.1002/pros.24105.
Preisser F, Bandini M, Marchioni M, et al. Extent of lymph node dissection improves survival in prostate cancer patients treated with radical prostatectomy without lymph node invasion. Prostate. 2018;78(6):469–75. https://doi.org/10.1002/pros.23491.
Fergany A, Kupelian PA, Levin HS, Zippe CD, Reddy C, Klein EA. No difference in biochemical failure rates with or without pelvic lymph node dissection during radical prostatectomy in low-risk patients. Urology. 2000;56(1):92–5. https://doi.org/10.1016/S0090-4295(00)00550-1.
Furubayashi N, Negishi T, Uozumi T, et al. Eliminating microscopic lymph node metastasis by performing pelvic lymph node dissection during radical prostatectomy for prostate cancer. Mol Clin Oncol. 2020;12(2):104–10. https://doi.org/10.3892/mco.2019.1965.
Iwamura H, Hatakeyama S, Narita T, et al. Significance of pelvic lymph node dissection during radical prostatectomy in high-risk prostate cancer patients receiving neoadjuvant chemohormonal therapy. Sci Rep. 2022;12(1):9675. https://doi.org/10.1038/s41598-022-13651-x.
Namiki S, Kawase M, Ebara S, et al. Pelvic Lymphadenectomy May Not Improve Biochemical Recurrence-Free Survival in Patients with Prostate Cancer Treated with Robot-Assisted Radical Prostatectomy in Japan (The MSUG94 Group). Cancers. 2022;14(23). https://doi.org/10.3390/cancers14235803.
Briganti A, Blute ML, Eastham JH, et al. Pelvic lymph node dissection in prostate cancer. Eur Urol. 2009;55(6):1251–65. https://doi.org/10.1016/j.eururo.2009.03.012.
Keegan, K.A., Cookson, M.S. Complications of Pelvic Lymph Node Dissection for Prostate Cancer. Curr Urol Rep. 2011;12:203–8. https://doi.org/10.1007/s11934-011-0179-z.
Cacciamani GE, Maas M, Nassiri N, et al. Impact of Pelvic Lymph Node Dissection and Its Extent on Perioperative Morbidity in Patients Undergoing Radical Prostatectomy for Prostate Cancer: A Comprehensive Systematic Review and Meta-analysis. Eur Urol Oncol. 2021;4(2):134–49. https://doi.org/10.1016/j.euo.2021.02.001.
Deutsch S, Hadaschik B, Lebentrau S, Ubrig B, Burger M, May M. Clinical Importance of a Peritoneal Interposition Flap to Prevent Symptomatic Lymphoceles after Robot-Assisted Radical Prostatectomy and Pelvic Lymph Node Dissection: A Systematic Review and Meta-Analysis. Urol Int. 2022;106(1):28–34. https://doi.org/10.1159/000512960.
Fossati N, Willemse PPM, Van den Broeck T, et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur Urol. 2017;72(1):84–109. https://doi.org/10.1016/j.eururo.2016.12.003.
Goßler C, May M, Breyer J, Stojanoski G, Weikert S, Lenart S, et al. High BMI, Aggressive Tumours and Long Console Time Are Independent Predictive Factors for Symptomatic Lymphocele Formation after Robot-Assisted Radical Prostatectomy and Pelvic Lymph Node Dissection. Urol Int. 2021;105(5-6):453–9. https://doi.org/10.1159/000514439.
Sforza S, Tellini R, Grosso AA, et al. Can we predict the development of symptomatic lymphocele following robot-assisted radical prostatectomy and lymph node dissection? Results from a tertiary referral Centre. Scand J Urol. 2020;54(4):328–33. https://doi.org/10.1080/21681805.2020.1784270.
Porcaro AB, Sebben M, Tafuri A, et al. Body mass index is an independent predictor of Clavien–Dindo grade 3 complications in patients undergoing robot assisted radical prostatectomy with extensive pelvic lymph node dissection. J Robot Surg. 2019;13(1):83–9. https://doi.org/10.1007/s11701-018-0824-3.
Thomas C, Ziewers S, Thomas A, et al. Development of symptomatic lymphoceles after radical prostatectomy and pelvic lymph node dissection is independent of surgical approach: a single-center analysis. Int Urol Nephrol. 2019;51:633–40. https://doi.org/10.1007/s11255-019-02103-7.
Deutsch S, Hadaschik B, Lebentrau S, Ubrig B, Burger M, May M. Clinical Importance of a Peritoneal Interposition Flap to Prevent Symptomatic Lymphoceles after Robot-Assisted Radical Prostatectomy and Pelvic Lymph Node Dissection: A Systematic Review and Meta-Analysis. Urol Int. 2022;106(1):28–34. https://doi.org/10.1159/000512960.
Neuberger M. Peritoneal flap for lymphocele prophylaxis following robotic-assisted laparoscopic radical prostatectomy with pelvic lymph node dissection: study protocol and trial update for the randomized controlled PELYCAN study. 2021.
Pose RM, Knipper S, Wu C, Maurer T, Graefen M, Steuber T. Significant reduction of lymphoceles after radical prostatectomy and pelvic lymph node dissection. 2021.
Stolzenburg JU. Four-point Peritoneal Flap Fixation in Preventing Lymphocele Formation Following Radical Prostatectomy. 2018.
Motterle G, Morlacco A, Zanovello N, et al. Surgical Strategies for Lymphocele Prevention in Minimally Invasive Radical Prostatectomy and Lymph Node Dissection: A Systematic Review. J Endourol. 2020;34(2):113–20. https://doi.org/10.1089/end.2019.0716.
Chu HH, Shin JH, Kim JW, Noh SY, Yang WJ, Park S. Lymphangiography and Lymphatic Embolization for the Management of Pelvic Lymphocele After Radical Prostatectomy in Prostatic Cancer. Cardiovasc Intervent Radiol. 2019;42(6):873–9. https://doi.org/10.1007/s00270-019-02209-6.
Branger N, Pignot G, Lannes F, et al. Comparison between Zumsteg classification and Briganti nomogram for the risk of lymph-node invasion before radical prostatectomy. World J Urol. 2020;38(7):1719–27. https://doi.org/10.1007/s00345-019-02965-7.
Hueting TA. External Validation of Models Predicting the Probability of Lymph Node Involvement in Prostate Cancer Patients. 2018.
Bandini M. A Head-to-head Comparison of Four Prognostic Models for Prediction of Lymph Node Invasion in African American and Caucasian Individuals. 2017.
Peilleron N, Seigneurin A, Herault C, et al. External evaluation of the Briganti nomogram to predict lymph node metastases in intermediate-risk prostate cancer patients. World J Urol. 2021;39(5):1489–97. https://doi.org/10.1007/s00345-020-03322-9.
Oderda M, Diamand R, Albisinni S, et al. Indications for and complications of pelvic lymph node dissection in prostate cancer: accuracy of available nomograms for the prediction of lymph node invasion. BJU Int. 2021;127(3):318–25. https://doi.org/10.1111/bju.15220.
Sahin A, Urkmez A, Yildirim C, Ali Kutluhan M, Topaktaş R, Verit A. Sensitivity and specificity of Briganti nomogram in Turkish patients undergoing radical prostatectomy and pelvic lymph node dissection. Aging Male. 2020;23(5):836–40. https://doi.org/10.1080/13685538.2019.1601176.
For the Michigan Urological Surgery Improvement Collaborative, Peabody H, Lane BR, et al. Limitations of abdominopelvic CT and multiparametric MR imaging for detection of lymph node metastases prior to radical prostatectomy. World J Urol. 2021;39(3):779–85. https://doi.org/10.1007/s00345-020-03227-7.
Brembilla G, Dell’Oglio P, Stabile A, et al. Preoperative multiparametric MRI of the prostate for the prediction of lymph node metastases in prostate cancer patients treated with extended pelvic lymph node dissection. Eur Radiol. 2018;28(5):1969–76. https://doi.org/10.1007/s00330-017-5229-6.
Gandaglia G. A Novel Nomogram to Identify Candidates for Extended Pelvic Lymph Node Dissection Among Patients with Clinically Localized Prostate Cancer Diagnosed with Magnetic Resonance Imaging-targeted and Systematic Biopsies. 2018.
Hatano K, Tanaka J, Nakai Y, et al. Utility of index lesion volume assessed by multiparametric MRI combined with Gleason grade for assessment of lymph node involvement in patients with high-risk prostate cancer. Jpn J Clin Oncol. 2019.
Hu B, Deng Y, Chen J, et al. Evaluation of MR elastography for prediction of lymph node metastasis in prostate cancer. Abdom Radiol. 2021;46(7):3387–400. https://doi.org/10.1007/s00261-021-02982-4.
Huang C, Song G, Wang H, et al. Preoperative PI-RADS Version 2 scores helps improve accuracy of clinical nomograms for predicting pelvic lymph node metastasis at radical prostatectomy. Prostate Cancer Prostatic Dis. 2020;23:116–26. https://doi.org/10.1038/s41391-019-0164-z.
Trapani ED, Cordima G, Matei DV, Cobelli OD. A novel nomogram predicting lymph node invasion among patients with prostate cancer: The importance of extracapsular extension at multiparametric magnetic resonance imaging. Urol Oncol: Published online; 2020.
Molecular and functional imaging for detection of lymph node metastases in prostate cancer - PubMed. https://pubmed.ncbi.nlm.nih.gov/23823804/. Accessed 8 Nov 2023.
Gandaglia G, Mazzone E, Stabile A, et al. Prostate-specific membrane antigen Radioguided Surgery to Detect Nodal Metastases in Primary Prostate Cancer Patients Undergoing Robot-assisted Radical Prostatectomy and Extended Pelvic Lymph Node Dissection: Results of a Planned Interim Analysis of a Prospective Phase 2 Study. Eur Urol. 2022;82(4):411–8. https://doi.org/10.1016/j.eururo.2022.06.002.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (No.82060464) and the Yunnan Science and Technology Department and Kunming Medical University Special Fund (Grant No. 202001AY070001-148).
Author information
Authors and Affiliations
Contributions
Baonan Dong (M.M.): Author, Data Collect, Writing-Original Draft. Hui Zhan (M.D.): Writing-Review & Editing. Ting Luan (M.D.): Writing-Review & Editing, Funding acquisition. Jiansong Wang (M.D.): Supervision, Funding acquisition. All authors approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dong, B., Zhan, H., Luan, T. et al. The role and controversy of pelvic lymph node dissection in prostate cancer treatment: a focused review. World J Surg Onc 22, 68 (2024). https://doi.org/10.1186/s12957-024-03344-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-024-03344-2