Abstract
Several studies suggest that topographical patterns influence nerve cell fate. Efforts have been made to improve nerve cell functionality through this approach, focusing on therapeutic strategies that enhance nerve cell function and support structures. However, inadequate nerve cell orientation can impede long-term efficiency, affecting nerve tissue repair. Therefore, enhancing neurites/axons directional growth and cell orientation is crucial for better therapeutic outcomes, reducing nerve coiling, and ensuring accurate nerve fiber connections. Conflicting results exist regarding the effects of micro- or nano-patterns on nerve cell migration, directional growth, immunogenic response, and angiogenesis, complicating their clinical use. Nevertheless, advances in lithography, electrospinning, casting, and molding techniques to intentionally control the fate and neuronal cells orientation are being explored to rapidly and sustainably improve nerve tissue efficiency. It appears that this can be accomplished by combining micro- and nano-patterns with nanomaterials, biological gradients, and electrical stimulation. Despite promising outcomes, the unclear mechanism of action, the presence of growth cones in various directions, and the restriction of outcomes to morphological and functional nerve cell markers have presented challenges in utilizing this method. This review seeks to clarify how micro- or nano-patterns affect nerve cell morphology and function, highlighting the potential benefits of cell orientation, especially in combined approaches.
Graphical Abstract

Similar content being viewed by others
Introduction
Low and non-uniform cell density, disorganization in nerve cell arrangements like nerve coils, and instability of seeded cells can result in sensory-motor problems in healing peripheral nerve tissues [1]. Therefore, rapid repair of neural tissue with grafts or conduits is not the only treatment option. Regenerative methods should prioritize the seeding site, proliferation, differentiation, and cell shape for cell orientation and alignment to ensure that develo** neurons resemble natural structures [2]. Alignment and orientation of neurons during the repair process have been demonstrated to have a positive impact on the function of neural tissue, including increased NCV, reduced neuroma, and improved CMAP [3,4,5].
Nanomedicine-based methods allow tissue engineering to regulate the topography [6], biological or chemical gradients of the microenvironment [35]. This triggers PI3-kinase, phospholipase C, and MAPK signaling pathways in Schwann cells, resulting in neuronal cell growth, migration, and myelination.
Cell-ECM interaction
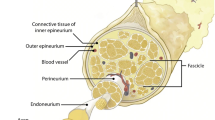
The ECM is essential as the main structural support in the body, performing specialized functions tailored to the needs of each organ. Despite its diverse composition, collagen, elastin, fibronectin, and laminin are the main components of the ECM [36]. Collagen is plentiful in peripheral nerve tissue, which is associated with the layer formed by Schwann cells [36]. The ECM plays a crucial role in regulating neuronal functions, including differentiation, migration, alignment, adhesion, cell–cell communication, and the dispersion of mature neurons. This is achieved through interactions between cell integrins and laminin, fibronectin, collagen, and ECM peptides [37]. Integrins are transmembrane receptors composed of α and β subunits that form noncovalent interactions [38]. These interactions are observed in four ways in neuron-extracellular matrix interactions: RGD (Arg-Gly-Asp) ligands bind to αV, α5β1, α8β1, and αIIbβ3 integrins, LDV (Leu-Asp-Val) ligand binds to integrins α4β1, α4β7, and α9β1, Laminin/collagen binds integrins α1, α2, α10, α11, and laminin binds integrins β1 (α3, α6, and α7) and α6β4 [38, 39]. Integrin-mediated neuron-ECM interactions facilitate two-way signaling (outside-in and inside-out) between cells and ECM (Fig. 1C). These interactions involve talin, paxillin, zyxin, and vinculin, along with actin filaments, and contribute to the formation of different adhesive structures like nascent adhesions, focal complexes, focal adhesions, and fibrillar adhesions [40]. Paxillin-rich, short-lived nascent point-like adhesions are found beneath the lamellipodia at the front of the migrating cell. Focal adhesions containing low levels of paxillin and high levels of vinculin form at the ends of stress fibers containing actin and myosin [41]. This cytoskeleton-derived adhesion is effective in force transmission. The most stable adhesions, fibrillar adhesions containing large amounts of tensin and β1-integrin, form along matrix fibrils beneath the cell center [39]. During cell migration, the cytoplasm expands by filopodia and lamellipodia, adhesions form, creating focal complexes rich in FAK and paxillin, and actin polymerization also takes place. Actomyosin contractile forces release mature focal adhesions on the opposite side, separating the cell from the environment [38, 40, 41].
Supporting structures
Efforts have been made to develop support structures containing neural cells to alleviate the limitations of autograft and allograft in peripheral nerve regeneration, with the most important being biological or polymeric conduits. Despite the significant impact of construction methods and materials on the healing process through biocompatibility, biodegradability, permeability, and mechanical capabilities (tensile strength, pressure resistance, flexibility, etc.) (Table 1), this study specifically examines the role of neuron function (adhesion, proliferation, differentiation, growth) in the physical structure of conduits, including orientation and topology patterns. While it may be challenging to establish criteria based on the physical properties of conduits, a review of existing literature may offer valuable insights to enhance and expedite the repair process of neural tissue.
Aligned fiber structures
Repairing injured peripheral nerves is often challenging, and sometimes even impossible, due to the misdirection of nerve cells and or improper suturing of the two nerve endings. Leveraging the inherent properties of neural tissue, such as its striped structure and directional cell distribution, conduits with aligned fibers have been found to expedite the healing process by facilitating the directional growth of neurons. Numerous studies in this area have indicated that the use of aligned fibers promotes peripheral nerve regeneration and enhances performance (Table 2). Nevertheless, achieving the precise extent and shape of cell alignment remains problematic due to the adverse effects of excessively aligned fibers on cell junctions and the unbalanced growth of axons.
Kim JI et al. [58] demonstrated that altering the conduit fibers from random to aligned within PLGA and PU (from electrospinning: voltage: 16 kV, flow rate: 1 mL/h) conduits containing NGF enhances the orientation of PC12 cells, aiming to improve nerve cell function. Aligned fibers were found to significantly improve the directional growth of PC12 cells by tripling the length and volume of intracellular actins parallel to the fibers. This enhancement was attributed to the reinforcement of cell adhesion, resulting from increased hydrophilicity that altered the cell contact angle from 128.3 to 120.2°. The alteration of PC12 cell morphology from flat or spherical in random fibers to spindle-shaped and oriented with aligned fibers validates this [58]. In next study, Chen S et al. [59] demonstrated that applying decellularized peripheral nerve matrix gel (pDNM gel) on PLLA fibers (diameter: 650 ± 90 nm) not only enhances axon growth by 30% through increased adhesion and interaction between DRG cells and fibers, but also leads to a 3–sixfold rise in Schwann cells migration in aligned fibers, significantly impacting the orientation of DRG cells. Schwann cell migration on aligned fibers and secretion of biological signals like NGF or neurotrophin-3 on them, have influenced the directional growth of neurons and axons extension. Moreover, it was demonstrated that the growth orientation of DRG cells significantly depends on the substrate pattern. It was observed that augmenting the thickness of the pDNM gel coating (from 0.25 to 1%) and decreasing the resolution of the substrate pattern lead to a more random growth of DRG cells. Surprisingly, the utilization of pDNM gel led to the emergence of fascicle-like axonal bundling in neural tissue, likely due to the increased presence of neuronal ECM molecules [59]. To study the impact of fiber alignment on nerve tissue function, Du J et al. [6C). Despite the potential toxicity of glutaraldehyde, no toxicity was detected in this system. Additionally, the peptide gradient was found to enhance synergistic activity, leading to improved polarization of M1 to M2 macrophages and angiogenesis (Fig. 6C). Furthermore, the synergism of grooves with the peptide gradient was observed to boost the regeneration rate of the transected sciatic nerve (Fig. 6C), resulting in larger fiber diameter, thicker myelin membrane, longer neurites, and increased SFI, NCV, and CMAP [122].
Synergism between support structures and the electrical stimulation
Several studies confirm that electrical stimulation enhances nerve cell proliferation, differentiation, migration, and integration by activating ion channels and influencing growth patterns [123]. The electric field influences the directional growth of neurites/axons, while the electromagnetic field promotes neurogenesis [124]. Hence, the synergism of electrical stimulation with supporting structures is expected to enhance the directional growth of nerve cells and their incorporation into healthy tissue. For instance, it was found that aligned fibers (diameter: 800 nm) composed of PPy-coated PLLA, when subjected to electrical stimulation of 100, 200, and 400 mV/cm, increased neurite extension from 68% along the fiber axis to 76%, 83%, and 71%, respectively [24]. The highest directional growth rate of neurites/axons and cell orientation was observed with electrical stimulation of 200 mV/cm (Fig. 6D). Moreover, the results show increased directional growth of neurites/axons in random fibers with electrical stimulation, indicating a positive response of nerve cells along the electropotential direction (Fig. 6D). Furthermore, the rise in longitudinal fiber growth from 65.44 and 114.73 µm in random and aligned fiber groups to 128.45 µm (100 mV/cm), 149.39 µm (200 mV/cm), and 141.48 µm (400 mV/cm) in aligned fiber groups with electrical stimulation demonstrates the beneficial impact of electrical stimulation on nerve development (Fig. 6D). Electrical stimulation induces cell membrane depolarization on aligned fibers, enhancing the electrical charge in PPy nanoparticles. This leads to the accumulation of adhesive receptors, actin expansion in filopodia, and an increase in growth cones [24]. While actin concentration and self-assembly on one side of the cell dictate neurites/axons directional growth and cell orientation, the main impact of electrical stimulation is the promotion of cell adhesion, which negatively affects their migration. However, Zhang J et al. [53] showed that combining PCL/CNT aligned fibers with electrical stimulation (20 Hz and 100 mV) in the nerve conduit improves the directional growth of neurites/axons and cell orientation more effectively. This also enhances parameters like SFI, amplitude, latency, the number of axons, and myelin sheet thickness in sciatic nerve regeneration, similar to the gold standard autograft. Recently, instead of using electrical stimulation, which faces the challenge of reducing migration due to higher induction of adhesion foci, the use of piezoelectric polymers such as aligned PVDF fibers [125] is of interest. In this regard, after loading 400 nm PLLA nanofibers obtained by electrospinning (Flow rate: 0.5 mL/h, voltage: 12 kV) on PLLA films, Jiang F et al. [126] demonstrated that piezoelectric stimulation (~ 260 mV), not only enhanced cell differentiation (11% more than the non-stimulated group), but also led to a twofold increase in cell length compared to the non-stimulated group (142.7 µm vs. 70.2 µm). It was revealed that piezoelectric stimulation through double activation of Ca2+ channels initiates downstream signaling by increasing intracellular calcium. However, piezoelectric stimulation did not significantly affect cell orientation; instead, cell orientation was primarily determined by aligned fibers.
Challenges and future perspective
Management of PNI treatment remains inadequate despite advances in drugs and surgical techniques. Reports indicate that fewer than 25% of patients undergoing nerve repair achieve optimal sensory and motor function recovery after 5 years. The scientific community is focused on improving nerve cell growth and guidance through new technologies to create conduits to overcome treatment obstacles. Utilizing conduits significantly enhances repair processes for damaged peripheral nerves by promoting nerve cell growth, neurite and axon directional growth, and nerve cell orientation. However, challenges exist in using conduits in medical settings, including:
-
One of the main challenges in using conduits is ensuring biological safety in humans. A thorough comprehension of cell behaviors within conduits, as well as the behavior of nanoparticles loaded and released from conduits, can only be assessed through cell viability, migration, growth, proliferation, adhesion, and differentiation in short-term laboratory settings. The fluctuating nature of conduit degradation in the body, the range of inflammatory responses, the variety of cells affected, the extended human treatment process, the diverse immune system components, patient lifestyle, and medical history all contribute to the challenge of ensuring optimal conduit function. Thus, considering the constraints of the human model, focusing on models such as human organoids that closely resemble original tissue may aid in predicting potential immunogenic strategies.
-
Conduit studies typically aim to promote the directional growth of neurons and align them during development or differentiation. However, the challenge of linearly distributing conductive materials and biological/chemical gradients hinders the targeted transmission of electrical-neural signals and the induction of directional growth of nerve cells, posing a significant issue for peripheral nerve repair. Focusing on accurately regulating the reception of electric-nerve pulses from the proximal end to the distal stump in conduits, based on determining the precise path of conductive material loading, can offer a clearer perspective on repairing damaged peripheral nerves. Additionally, relying solely on the detection of gradients in conduits is concerning. Thus, focusing on the extent of arrangmenet and diffusion of gradients, evaluating the specific quantity and quality of gradients, and considering their potential side effects on other parts or cells can offer valuable insights for their application.
-
Despite the wide range of conduits available (biological, natural, synthetic), they are not yet a dependable choice for axon growth and nerve tissue regeneration over long distances, unlike autologous nerve grafts. To achieve this goal, a thorough comprehension of the physicochemical characteristics of conduits larger than 2.5 cm is essential, particularly in motor-sensory models resembling humans.
-
In vitro studies and clinical trials have been widely conducted on rats, rabbits, dogs, monkeys, pigs, etc. While the generalization of their results to humans based on the complete non-compliance of the aspects of the biology of the repairing nerve, the path of research has become a concern. Focusing on human trials or human organoids that closely resemble original tissue can reduce some of the existing challenges.
-
Conduits are typically designed and created by considering biocompatibility, biodegradability, enhancing cell adhesion, biomarker loading, creating topographical features, and adjusting mechanical properties for nerve tissue. However, the complexity and high cost of production methods pose challenges for commercialization. Focusing on cost-effective designs and simpler production techniques can facilitate their use in both general and personalized clinical applications.
-
Conduit studies mainly rely on autologous cells to prevent transplant rejection, complicating the commercialization and treatment process. The pursuit of a universal neuron or neuron-like cell discovery will enhance the potential for clinical treatments.
-
Conduits are commonly employed to direct axonal growth, inhibit fibrous tissue infiltration and scarring, and discourage axon reinnervation or nerve bundling. Prioritizing the development of the vascular network, promoting the movement of neural stem cells from neighboring areas, and averting uncontrolled swelling of hydrogels within the cavities can enhance treatment outcomes.
Conclusion
Despite the inherent regeneration of peripheral nerves, many nerve injuries encounter challenges like prolonged recovery, inflammation, neuroma, and decreased tissue function. Tissue engineering aims to address these obstacles by incorporating nanotechnologies and cell science to mimic the supportive structures of the ECM. This enhances cell proliferation, migration, and orientation, facilitating the directional growth of neurites/axons. This review suggests that promoting cone growth in a specific direction, achieving even cell distribution through targeted migration, enhancing the longitudinal growth of neurites/axons within conduits, and controlling cell organization to mimic natural tissue structure could be promising strategies. Studies have shown that incorporating micro- or nano-patterns in conduits (such as aligned fibers, grooves, channels, pillars, pits, etc.) along with therapeutic approaches like electrical stimulation and nano-carriers can have a substantial impact on nerve cell behavior, ultimately aiding in the restoration of damaged peripheral nerve functions. Conduits with surface topographies play a significant role in guiding axonal growth and cell orientation, inhibiting fibrous tissue penetration, and preventing axon re-innervation during peripheral nerve regeneration. The utilization of conduits with aligned fibers > multi-channels > grooves has garnered considerable interest due to reduced manufacturing complexity, enhanced ability to direct growth and cell migration, improved accessibility, decreased nerve compression, and control over fiber diameter and myelin thickness. However, the mechanism of action of topography on the development and expansion of peripheral nerves is currently being elucidated. Therefore, thorough research is necessary to understand the mechanism of micro- or nano-patterns and cellular responses based on their physicochemical characteristics, enabling the development of clinical applications with greater confidence. In the following, the focus should be on understanding the mechanism of micro- or nano-patterns in nerve cells to predict cell orientation, neurite/axon growth, and tissue function restoration efficiently. Also, despite various conduit manufacturing techniques, establishing a specific standard or tactic for creating micro or nano patterns is essential to enhance their operational capability in nerve tissue reliably, while addressing issues such as construction complexity, high cost, low reproducibility, and solvent presence. Therefore, besides evaluating conduit surface size and resolution, it is vital to investigate the commercialization process of conduits through biomedical models. This implies that the manufacturing technique should account for the mechanical and chemical changes on conduit surfaces that affect cell migration and adhesion strength, mimicking cell behaviors in the ECM. Recent research indicates that cells develop more focal adhesions on patterned surfaces, which could potentially hinder cell migration and morphological expansion processes, going against their natural tendencies. Finally, this review demonstrates that creating micro- or nano-patterns in the nerve conduit can enhance the peripheral nerve regeneration process by improving motor performance in model mice and tissue structures. Surprisingly, this approach also matches the efficiency of nerve tissue regeneration seen with the gold standard of autograft.
Availability of data and materials
We have included 6 figures. For all of them, copyright permission from the copyright holder was necessary. We have mentioned this in the manuscript with proper citations.
Abbreviations
- 3D:
-
Three dimensional
- CMAP:
-
Compound muscle action potential
- CNT:
-
Carbon nanotube
- DRG:
-
Dorsal root ganglion
- IL:
-
Interleukin
- IONPs:
-
Iron oxide nanoparticles
- MAPK/ERK:
-
Mitogen-activated protein kinase/extracellular signal-regulated kinase
- NCAD:
-
Neural cadherin
- NCAM:
-
Neural cell adhesion molecule
- NCV:
-
Nerve conduction velocity
- NGF:
-
Nerve growth factor
- PCL:
-
Polycaprolactone
- PDMS:
-
Polydimethylsiloxane
- PEG:
-
Polyethylene glycol
- PLCL:
-
Poly(L-lactide-co-ε-caprolactone)
- PLL:
-
Poly-L-lysine
- PLLA:
-
Poly(L-lactide)
- PLGA:
-
Poly lactic-co-glycolic acid
- PNI:
-
Peripheral nerve injury
- PPy:
-
Polypyrrole
- PTFE:
-
Polytetrafluoroethylene
- PVDF:
-
Poly(vinylidene fluoride)
- PU:
-
Polyurethane
- Sema3A:
-
Semaphorin-3A
- SF:
-
Silk fibroin
- SFI:
-
Sciatic functional index
- SPION:
-
Superparamagnetic iron oxide nanoparticle
- TNF:
-
Tumor necrosis factor
References
Sharifi M, Farahani MK, Salehi M, Atashi A, Alizadeh M, Kheradmandi R, Molzemi S. Exploring the physicochemical, electroactive, and biodelivery properties of metal nanoparticles on peripheral nerve regeneration. ACS Biomater Sci Eng. 2022;9:106–38.
Qian Y, Lin H, Yan Z, Shi J, Fan C. Functional nanomaterials in peripheral nerve regeneration: scaffold design, chemical principles and microenvironmental remodeling. Mater Today. 2021;51:165–87.
Liu F, Xu J, Wu L, Zheng T, Han Q, Liang Y, Zhang L, Li G, Yang Y. The influence of the surface topographical cues of biomaterials on nerve cells in peripheral nerve regeneration: a review. Stem Cells Int. 2021. https://doi.org/10.1155/2021/8124444.
Kim SM, Lee MS, Jeon J, Lee DH, Yang K, Cho SW, Han I, Yang HS. Biodegradable nerve guidance conduit with microporous and micropatterned poly (lactic-co-glycolic acid)-accelerated sciatic nerve regeneration. Macromol Biosci. 2018;18:1800290.
Hu Y, Chen Z, Wang H, Guo J, Cai J, Chen X, Wei H, Qi J, Wang Q, Liu H, et al. Conductive nerve guidance conduits based on morpho butterfly wings for peripheral nerve repair. ACS Nano. 2022;16:1868–79.
Zheng T, Wu L, Xu J, Sun S, Guan W, Han Q, Zhang L, Gu X, Yang Y, Li G. YR/DFO@DCNT functionalized anisotropic micro/nano composite topography scaffolds for accelerating long-distance peripheral nerve regeneration. Compos B Eng. 2022;246:110242.
Huang L, Gao J, Wang H, **a B, Yang Y, Xu F, Zheng X, Huang J, Luo Z. Fabrication of 3D scaffolds displaying biochemical gradients along longitudinally oriented microchannels for neural tissue engineering. ACS Appl Mater Interfaces. 2020;12:48380–94.
Huang Z, Sun M, Li Y, Guo Z, Li H. Reduced graphene oxide-coated electrospun fibre: effect of orientation, coverage and electrical stimulation on Schwann cells behavior. J Mater Chem B. 2021;9:2656–65.
Ma Y, Gao H, Wang H, Cao X. Engineering topography: effects on nerve cell behaviors and applications in peripheral nerve repair. J Mater Chem B. 2021;9:6310–25.
Rigby MJ, Gomez TM, Puglielli L. Glial cell-axonal growth cone interactions in neurodevelopment and regeneration. Front Neurosci. 2020;14:203.
Kozulin P, Richards LJ. Axonal guidance: making connections. In: Pfaff DW, Volkow ND, Rubenstein JL, editors. Neuroscience in the 21st century: from basic to clinical. New York: Springer; 2022. p. 383–406.
Dravid A, O’Carroll SJ, Svirskis D. Neurotrophins and their role in axonal outgrowth following spinal cord injury. In: Rajendram R, Preedy VR, Martin CR, editors. Cellular, molecular, physiological, and behavioral aspects of spinal cord injury. Amsterdam: Elsevier; 2022. p. 215–27.
SenGupta S, Parent CA, Bear JE. The principles of directed cell migration. Nat Rev Mol Cell Biol. 2021;22:529–47.
Oh B, Wu YW, Swaminathan V, Lam V, Ding J, George PM. Modulating the electrical and mechanical microenvironment to guide neuronal stem cell differentiation. Adv Sci. 2021;8:2002112.
Chu X-L, Song X-Z, Li Q, Li Y-R, He F, Gu X-S, Ming D. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen Res. 2022;17:2185.
Song S, McConnell KW, Amores D, Levinson A, Vogel H, Quarta M, Rando TA, George PM. Electrical stimulation of human neural stem cells via conductive polymer nerve guides enhances peripheral nerve recovery. Biomaterials. 2021;275:120982.
Bierman-Duquette RD, Safarians G, Huang J, Rajput B, Chen JY, Wang ZZ, Seidlits SK. Engineering tissues of the central nervous system: interfacing conductive biomaterials with neural stem/progenitor cells. Adv Healthcare Mater. 2022;11:2101577.
Thrivikraman G, Boda SK, Basu B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: a tissue engineering perspective. Biomaterials. 2018;150:60–86.
Musselman ED, Cariello JE, Grill WM, Pelot NA. ASCENT (automated simulations to characterize electrical nerve thresholds): a pipeline for sample-specific computational modeling of electrical stimulation of peripheral nerves. PLoS Comput Biol. 2021;17:e1009285.
Eftekhari BS, Eskandari M, Janmey PA, Samadikuchaksaraei A, Gholipourmalekabadi M. Surface topography and electrical signaling: single and synergistic effects on neural differentiation of stem cells. Adv Func Mater. 2020;30:1907792.
Repić T, Madirazza K, Bektur E, Sapunar D. Characterization of dorsal root ganglion neurons cultured on silicon micro-pillar substrates. Sci Rep. 2016;6:39560.
Srinivasan A, Tahilramani M, Bentley JT, Gore RK, Millard DC, Mukhatyar VJ, Joseph A, Haque AS, Stanley GB, English AW, Bellamkonda RV. Microchannel-based regenerative scaffold for chronic peripheral nerve interfacing in amputees. Biomaterials. 2015;41:151–65.
Oh SH, Kang JG, Kim TH, Namgung U, Song KS, Jeon BH, Lee JH. Enhanced peripheral nerve regeneration through asymmetrically porous nerve guide conduit with nerve growth factor gradient. J Biomed Mater Res, Part A. 2018;106:52–64.
Zou Y, Qin J, Huang Z, Yin G, Pu X, He D. Fabrication of aligned conducting PPy-PLLA fiber films and their electrically controlled guidance and orientation for neurites. ACS Appl Mater Interfaces. 2016;8:12576–82.
Lu K, Qian Y, Gong J, Zhu Z, Yin J, Ma L, Yu M, Wang H. Biofabrication of aligned structures that guide cell orientation and applications in tissue engineering. Bio-Des Manuf. 2021;4:258–77.
Antoniadis G. The peripheral nerve: neuroanatomical principles before and after injury. In: Haastert-Talini K, Assmus H, Antoniadis G, editors. Modern concepts of peripheral nerve repair. Cham: Springer; 2017. p. 1–10.
Reina MA, Boezaart AP, Tubbs RS, Zasimovich Y, Fernández-Domínguez M, Fernández P, Sala-Blanch X. Another (internal) epineurium: beyond the anatomical barriers of nerves. Clin Anat. 2020;33:199–206.
Pestronk A, Schmidt RE, Bucelli R, Sim J. Schwann cells and myelin in human peripheral nerve: major protein components vary with age, axon size and pathology. Neuropathol Appl Neurobiol. 2023;49:e12898.
Wilson ER, Della-Flora Nunes G, Weaver MR, Frick LR, Feltri ML. Schwann cell interactions during the development of the peripheral nervous system. Dev Neurobiol. 2021;81:464–89.
Papagiannis G, Triantafyllou A, Stasi S, Yiannopoulou KG, Papathanasiou G, Mitsiokapa E, Papadopoulos EC, Papagelopoulos PJ, Koulouvaris P. Biomechanical behavior and viscoelastic properties of peripheral nerves subjected to tensile stress: common injuries and current repair techniques. Crit Rev Phys Rehab Med. 2020. https://doi.org/10.1615/CritRevPhysRehabilMed.2020034798.
Chooi WH, Chew SY. Modulation of cell-cell interactions for neural tissue engineering: potential therapeutic applications of cell adhesion molecules in nerve regeneration. Biomaterials. 2019;197:327–44.
Gärtner A, Fornasiero EF, Dotti CG. Cadherins as regulators of neuronal polarity. Cell Adh Migr. 2015;9:175–82.
Guan X, Guan X, Dong C, Jiao Z. Rho GTPases and related signaling complexes in cell migration and invasion. Exp Cell Res. 2020;388:111824.
Sowparani S, Mahalakshmi P, Sweety JP, Francis AP, Dhanalekshmi U, Selvasudha N. Ubiquitous neural cell adhesion molecule (NCAM): potential mechanism and valorisation in cancer pathophysiology, drug targeting and molecular transductions. Mol Neurobiol. 2022;59:5902–24.
Kataria H, Alizadeh A, Karimi-Abdolrezaee S. Neuregulin-1/ErbB network: an emerging modulator of nervous system injury and repair. Prog Neurobiol. 2019;180:101643.
Han G-H, Peng J, Liu P, Ding X, Wei S, Lu S, Wang Y. Therapeutic strategies for peripheral nerve injury: decellularized nerve conduits and Schwann cell transplantation. Neural Regen Res. 2019;14:1343.
Liu F, Xu J, Wu L, Zheng T, Han Q, Liang Y, Zhang L, Li G, Yang Y. The influence of the surface topographical cues of biomaterials on nerve cells in peripheral nerve regeneration: a review. Stem Cells Int. 2021;2021:1–13.
Romano NH, Madl CM, Heilshorn SC. Matrix RGD ligand density and L1CAM-mediated Schwann cell interactions synergistically enhance neurite outgrowth. Acta Biomater. 2015;11:48–57.
Stukel JM, Willits RK. Mechanotransduction of neural cells through cell–substrate interactions. Tissue Eng Part B Rev. 2016;22:173–82.
Goldmann WH. Role of vinculin in cellular mechanotransduction. Cell Biol Int. 2016;40:241–56.
Wang H, Wang X, Qu J, Yue Q, Yn Hu, Zhang H. VEGF enhances the migration of MSCs in neural differentiation by regulating focal adhesion turnover. J Cell Physiol. 2015;230:2728–42.
Zhang H, Guo J, Wang Y, Shang L, Chai R, Zhao Y. Natural polymer-derived bioscaffolds for peripheral nerve regeneration. Adv Func Mater. 2022;32:2203829.
Gregory H, Phillips JB. Materials for peripheral nerve repair constructs: natural proteins or synthetic polymers? Neurochem Int. 2021;143:104953.
Riccio M, Marchesini A, Pugliese P, De Francesco F. Nerve repair and regeneration: biological tubulization limits and future perspectives. J Cell Physiol. 2019;234:3362–75.
Heinzel JC, Quyen Nguyen M, Kefalianakis L, Prahm C, Daigeler A, Hercher D, Kolbenschlag J. A systematic review and meta-analysis of studies comparing muscle-in-vein conduits with autologous nerve grafts for nerve reconstruction. Sci Rep. 2021;11:11691.
Sun P, Guan Y, Yang C, Hou H, Liu S, Yang B, Li X, Chen S, Wang L, Wang H. A bioresorbable and conductive scaffold integrating silicon membranes for peripheral nerve regeneration. Adv Healthcare Mater. 2023. https://doi.org/10.1002/adhm.202301859.
Redondo-Gomez C, Leandro-Mora R, Blanch-Bermudez D, Espinoza-Araya C, Hidalgo-Barrantes D, Vega-Baudrit J. Recent advances in carbon nanotubes for nervous tissue regeneration. Adv Polym Technol. 2020;2020:1–16.
Sharifi M, Kheradmandi R, Salehi M, Alizadeh M, ten Hagen TLM, Falahati M. Criteria, challenges, and opportunities for acellularized allogeneic/xenogeneic bone grafts in bone repairing. ACS Biomater Sci Eng. 2022;8:3199–219.
Jeong H-J, Nam H, Jang J, Lee S-J. 3D bioprinting strategies for the regeneration of functional tubular tissues and organs. Bioengineering. 2020;7:32.
Askari M, Naniz MA, Kouhi M, Saberi A, Zolfagharian A, Bodaghi M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques. Biomater Sci. 2021;9:535–73.
Yan Y, Yao R, Zhao J, Chen K, Duan L, Wang T, Zhang S, Guan J, Zheng Z, Wang X. Implantable nerve guidance conduits: material combinations, multi-functional strategies and advanced engineering innovations. Bioactive Mater. 2022;11:57–76.
Sarker M, Naghieh S, McInnes AD, Schreyer DJ, Chen X. Strategic design and fabrication of nerve guidance conduits for peripheral nerve regeneration. Biotechnol J. 2018;13:1700635.
Zhang J, Zhang X, Wang C, Li F, Qiao Z, Zeng L, Wang Z, Liu H, Ding J, Yang H. Conductive composite fiber with optimized alignment guides neural regeneration under electrical stimulation. Adv Healthcare Mater. 2021;10:2000604.
Du J, Liu J, Yao S, Mao H, Peng J, Sun X, Cao Z, Yang Y, **ao B, Wang Y, et al. Prompt peripheral nerve regeneration induced by a hierarchically aligned fibrin nanofiber hydrogel. Acta Biomater. 2017;55:296–309.
Singh A, Asikainen S, Teotia AK, Shiekh PA, Huotilainen E, Qayoom I, Partanen J, Seppälä J, Kumar A. Biomimetic photocurable three-dimensional printed nerve guidance channels with aligned cryomatrix lumen for peripheral nerve regeneration. ACS Appl Mater Interfaces. 2018;10:43327–42.
Zheng C, Yang Z, Chen S, Zhang F, Rao Z, Zhao C, Quan D, Bai Y, Shen J. Nanofibrous nerve guidance conduits decorated with decellularized matrix hydrogel facilitate peripheral nerve injury repair. Theranostics. 2021;11:2917.
Lu Q, Zhang F, Cheng W, Gao X, Ding Z, Zhang X, Lu Q, Kaplan DL. Nerve guidance conduits with hierarchical anisotropic architecture for peripheral nerve regeneration. Adv Healthcare Mater. 2021;10:2100427.
Kim JI, Hwang TI, Aguilar LE, Park CH, Kim CS. A controlled design of aligned and random nanofibers for 3D bi-functionalized nerve conduits fabricated via a novel electrospinning set-up. Sci Rep. 2016;6:23761.
Chen S, Du Z, Zou J, Qiu S, Rao Z, Liu S, Sun X, Xu Y, Zhu Q, Liu X. Promoting neurite growth and schwann cell migration by the harnessing decellularized nerve matrix onto nanofibrous guidance. ACS Appl Mater Interfaces. 2019;11:17167–76.
Quan Q, Meng H-Y, Chang B, Liu G-B, Cheng X-Q, Tang H, Wang Y, Peng J, Zhao Q, Lu S-B. Aligned fibers enhance nerve guide conduits when bridging peripheral nerve defects focused on early repair stage. Neural Regen Res. 2019;14:903.
Hu N, Wu H, Xue C, Gong Y, Wu J, **ao Z, Yang Y, Ding F, Gu X. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials. 2013;34:100–11.
Dong X, Yang Y, Bao Z, Midgley AC, Li F, Dai S, Yang Z, Wang J, Liu L, Li W, et al. Micro-nanofiber composite biomimetic conduits promote long-gap peripheral nerve regeneration in canine models. Bioactive Mater. 2023;30:98–115.
Wang L, Wu Y, Hu T, Ma PX, Guo B. Aligned conductive core-shell biomimetic scaffolds based on nanofiber yarns/hydrogel for enhanced 3D neurite outgrowth alignment and elongation. Acta Biomater. 2019;96:175–87.
Zhang Z, Jørgensen ML, Wang Z, Amagat J, Wang Y, Li Q, Dong M, Chen M. 3D anisotropic photocatalytic architectures as bioactive nerve guidance conduits for peripheral neural regeneration. Biomaterials. 2020;253:120108.
Dong X, Liu S, Yang Y, Gao S, Li W, Cao J, Wan Y, Huang Z, Fan G, Chen Q, et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials. 2021;272:120767.
Muangsanit P, Roberton V, Costa E, Phillips JB. Engineered aligned endothelial cell structures in tethered collagen hydrogels promote peripheral nerve regeneration. Acta Biomater. 2021;126:224–37.
Fan J, Zhang Y, Liu Y, Wang Y, Cao F, Yang Q, Tian F. Explanation of the cell orientation in a nanofiber membrane by the geometric potential theory. Results Phys. 2019;15:102537.
Simitzi C, Ranella A, Stratakis E. Controlling the morphology and outgrowth of nerve and neuroglial cells: the effect of surface topography. Acta Biomater. 2017;51:21–52.
Yang K, Jung K, Ko E, Kim J, Park KI, Kim J, Cho S-W. Nanotopographical manipulation of focal adhesion formation for enhanced differentiation of human neural stem cells. ACS Appl Mater Interfaces. 2013;5:10529–40.
Baek J, Cho S-Y, Kang H, Ahn H, Jung W-B, Cho Y, Lee E, Cho S-W, Jung H-T, Im SG. Distinct mechanosensing of human neural stem cells on extremely limited anisotropic cellular contact. ACS Appl Mater Interfaces. 2018;10:33891–900.
Cai L, Zhang L, Dong J, Wang S. Photocured biodegradable polymer substrates of varying stiffness and microgroove dimensions for promoting nerve cell guidance and differentiation. Langmuir. 2012;28:12557–68.
Liguori GR, Zhou Q, Liguori TTA, Barros GG, Kühn PT, Moreira LFP, Van Rijn P, Harmsen MC. Directional topography influences adipose mesenchymal stromal cell plasticity: prospects for tissue engineering and fibrosis. Stem Cells Int. 2019. https://doi.org/10.1155/2019/5387850.
Tonazzini I, Jacchetti E, Meucci S, Beltram F, Cecchini M. Schwann cell contact guidance versus boundary interaction in functional wound healing along nano and microstructured membranes. Adv Healthcare Mater. 2015;4:1849–60.
Huang C, Ouyang Y, Niu H, He N, Ke Q, ** X, Li D, Fang J, Liu W, Fan C, Lin T. Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Appl Mater Interfaces. 2015;7:7189–96.
Polo Y, Luzuriaga J, Iturri J, Irastorza I, Toca-Herrera JL, Ibarretxe G, Unda F, Sarasua J-R, Pineda JR, Larrañaga A. Nanostructured scaffolds based on bioresorbable polymers and graphene oxide induce the aligned migration and accelerate the neuronal differentiation of neural stem cells. Nanomed: Nanotechnol, Biol Med. 2021;31:102314.
Zhang D, Yao Y, Duan Y, Yu X, Shi H, Nakkala JR, Zuo X, Hong L, Mao Z, Gao C. Surface-anchored graphene oxide nanosheets on cell-scale micropatterned poly(d, l-lactide-co-caprolactone) conduits promote peripheral nerve regeneration. ACS Appl Mater Interfaces. 2020;12:7915–30.
Li G, Zheng T, Wu L, Han Q, Lei Y, Xue L, Zhang L, Gu X, Yang Y. Bionic microenvironment-inspired synergistic effect of anisotropic micro-nanocomposite topology and biology cues on peripheral nerve regeneration. Sci Adv. 2021;7:5812.
Lu S, Chen W, Wang J, Guo Z, **ao L, Wei L, Yu J, Yuan Y, Chen W, Bian M, et al. Polydopamine-decorated plcl conduit to induce synergetic effect of electrical stimulation and topological morphology for peripheral nerve regeneration. Small Methods. 2023;7:2200883.
Zheng T, Wu L, Sun S, Xu J, Han Q, Liu Y, Wu R, Li G. Co-culture of Schwann cells and endothelial cells for synergistically regulating dorsal root ganglion behavior on chitosan-based anisotropic topology for peripheral nerve regeneration. Burns Trauma. 2022;10:030.
Gu X, Chen X, Tang X, Zhou Z, Huang T, Yang Y, Ling J. Pure-silk fibroin hydrogel with stable aligned micropattern toward peripheral nerve regeneration. Nanotechnol Rev. 2021;10:10–9.
Mobasseri A, Faroni A, Minogue BM, Downes S, Terenghi G, Reid AJ. Polymer scaffolds with preferential parallel grooves enhance nerve regeneration. Tissue Eng Part A. 2015;21:1152–62.
Scaccini L, Mezzena R, De Masi A, Gagliardi M, Gambarotta G, Cecchini M, Tonazzini I. Chitosan micro-grooved membranes with increased asymmetry for the improvement of the schwann cell response in nerve regeneration. Int J Mol Sci. 2021;22:7901.
Zhang D, Xu S, Wu S, Gao C. Micropatterned poly(d, l-lactide-co-caprolactone) films entrapped with gelatin for promoting the alignment and directional migration of Schwann cells. J Mater Chem B. 2018;6:1226–37.
Zhang D, Wu S, Feng J, Duan Y, **ng D, Gao C. Micropatterned biodegradable polyesters clicked with CQAASIKVAV promote cell alignment, directional migration, and neurite outgrowth. Acta Biomater. 2018;74:143–55.
Wang J, **ong H, Zhu T, Liu Y, Pan H, Fan C, Zhao X, Lu WW. Bioinspired multichannel nerve guidance conduit based on shape memory nanofibers for potential application in peripheral nerve repair. ACS Nano. 2020;14:12579–95.
You R, Zhang Q, Li X, Yan S, Luo Z, Qu J, Li M. Multichannel bioactive silk nanofiber conduits direct and enhance axonal regeneration after spinal cord injury. ACS Biomater Sci Eng. 2020;6:4677–86.
Jiang Z, Zhang Y, Wang Y, Wang S, Chang J, Liu W, Han B. Multichannel nerve conduit based on chitosan derivates for peripheral nerve regeneration and schwann cell survival. Carbohyd Polym. 2023;301:120327.
Ye W, Li H, Yu K, **e C, Wang P, Zheng Y, Zhang P, **u J, Yang Y, Zhang F, et al. 3D printing of gelatin methacrylate-based nerve guidance conduits with multiple channels. Mater Des. 2020;192:108757.
Ankam S, Suryana M, Chan LY, Moe AAK, Teo BK, Law JB, Sheetz MP, Low HY, Yim EK. Substrate topography and size determine the fate of human embryonic stem cells to neuronal or glial lineage. Acta Biomater. 2013;9:4535–45.
Lunghi A, Mariano A, Bianchi M, Dinger NB, Murgia M, Rondanina E, Toma A, Greco P, Di Lauro M, Santoro F, et al. Flexible neural interfaces based on 3D PEDOT:PSS micropillar arrays. Adv Mater Interfaces. 2022;9:2200709.
Vinzons LU, Lin S-P. Hierarchical micro-/nanotopographies patterned by tandem nanosphere lens lithography and uv–led photolithography for modulating pc12 neuronal differentiation. ACS Applied Nano Mater. 2022;5:6935–53.
Bucaro MA, Vasquez Y, Hatton BD, Aizenberg J. Fine-tuning the degree of stem cell polarization and alignment on ordered arrays of high-aspect-ratio nanopillars. ACS Nano. 2012;6:6222–30.
Tse K-H, Sun M, Mantovani C, Terenghi G, Downes S, Kingham PJ. In vitro evaluation of polyester-based scaffolds seeded with adipose derived stem cells for peripheral nerve regeneration. J Biomed Mater Res, Part A. 2010;95A:701–8.
Zhu M, Li W, Dong X, Yuan X, Midgley AC, Chang H, Wang Y, Wang H, Wang K, Ma PX. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat Commun. 2019;10:4620.
Rao Z, Lin T, Qiu S, Zhou J, Liu S, Chen S, Wang T, Liu X, Zhu Q, Bai Y, Quan D. Decellularized nerve matrix hydrogel scaffolds with longitudinally oriented and size-tunable microchannels for peripheral nerve regeneration. Mater Sci Eng, C. 2021;120:111791.
Pawelec KM, Yoon C, Giger RJ, Sakamoto J. Engineering a platform for nerve regeneration with direct application to nerve repair technology. Biomaterials. 2019;216:119263.
Park D, Kim D, Park SJ, Choi JH, Seo Y, Kim D-H, Lee S-H, Hyun JK, Yoo J, Jung Y. Micropattern-based nerve guidance conduit with hundreds of microchannels and stem cell recruitment for nerve regeneration. NPJ Regen Med. 2022;7:62.
Zhang K, **ao X, Wang X, Fan Y, Li X. Topographical patterning: characteristics of current processing techniques, controllable effects on material properties and co-cultured cell fate, updated applications in tissue engineering, and improvement strategies. J Mater Chem B. 2019;7:7090–109.
Kundu A, Micholt L, Friedrich S, Rand DR, Bartic C, Braeken D, Levchenko A. Superimposed topographic and chemical cues synergistically guide neurite outgrowth. Lab Chip. 2013;13:3070–81.
Micholt L, Gärtner A, Prodanov D, Braeken D, Dotti CG, Bartic C. Substrate topography determines neuronal polarization and growth in vitro. PLoS ONE. 2013;8:e66170.
Fan S, Qi L, Li J, Pan D, Zhang Y, Li R, Zhang C, Wu D, Lau P, Hu Y. Guiding the patterned growth of neuronal axons and dendrites using anisotropic micropillar scaffolds. Adv Healthcare Mater. 2021;10:2100094.
Vedaraman S, Perez-Tirado A, Haraszti T, Gerardo-Nava J, Nishiguchi A, De Laporte L. Anisometric microstructures to determine minimal critical physical cues required for neurite alignment. Adv Healthcare Mater. 2021;10:2100874.
Baranes K, Chejanovsky N, Alon N, Sharoni A, Shefi O. Topographic cues of nano-scale height direct neuronal growth pattern. Biotechnol Bioeng. 2012;109:1791–7.
Milos F, Belu A, Mayer D, Maybeck V, Offenhäusser A. Polymer nanopillars induce increased paxillin adhesion assembly and promote axon growth in primary cortical neurons. Adv Biol. 2021;5:2000248.
Milos F, Tullii G, Gobbo F, Lodola F, Galeotti F, Verpelli C, Mayer D, Maybeck V, Offenhäusser A, Antognazza MR. High aspect ratio and light-sensitive micropillars based on a semiconducting polymer optically regulate neuronal growth. ACS Appl Mater Interfaces. 2021;13:23438–51.
Xu X, Wang W, Kratz K, Fang L, Li Z, Kurtz A, Ma N, Lendlein A. Controlling major cellular processes of human mesenchymal stem cells using microwell structures. Adv Healthcare Mater. 2014;3:1991–2003.
Mobasseri S, Terenghi G, Downes S. Micro-structural geometry of thin films intended for the inner lumen of nerve conduits affects nerve repair. J Mater Sci: Mater Med. 2013;24:1639–47.
Yang A, Huang Z, Yin G, Pu X. Fabrication of aligned, porous and conductive fibers and their effects on cell adhesion and guidance. Colloids Surf, B. 2015;134:469–74.
Zhang Z, Wang Y, Chen Z, Xu D, Zhang D, Wang F, Zhao Y. Tailoring conductive inverse opal films with anisotropic elliptical porous patterns for nerve cell orientation. J Nanobiotechnol. 2022;20:1–11.
Piscioneri A, Morelli S, Ritacco T, Giocondo M, Peñaloza R, Drioli E, De Bartolo L. Topographical cues of PLGA membranes modulate the behavior of hMSCs, myoblasts and neuronal cells. Colloids Surf, B. 2023;222:113070.
Kim JA, Lee N, Kim BH, Rhee WJ, Yoon S, Hyeon T, Park TH. Enhancement of neurite outgrowth in PC12 cells by iron oxide nanoparticles. Biomaterials. 2011;32:2871–7.
Seo J, Kim J, Joo S, Choi JY, Kang K, Cho WK, Choi IS. Nanotopography-promoted formation of axon collateral branches of hippocampal neurons. Small. 2018;14:1801763.
Cho Y, Choi Y, Seong H. Nanoscale surface coatings and topographies for neural interfaces. Acta Biomater. 2024;175:55–75.
Antman-Passig M, Shefi O. Remote magnetic orientation of 3d collagen hydrogels for directed neuronal regeneration. Nano Lett. 2016;16:2567–73.
Antman-Passig M, Giron J, Karni M, Motiei M, Schori H, Shefi O. Magnetic assembly of a multifunctional guidance conduit for peripheral nerve repair. Adv Func Mater. 2021;31:2010837.
**a L, Zhang C, Su K, Fan J, Niu Y, Yu Y, Chai R. Oriented growth of neural stem cell-derived neurons regulated by magnetic nanochains. Front Bioeng Biotechnol. 2022;10:895107.
Rose JC, Cámara-Torres M, Rahimi K, Köhler J, Möller M, De Laporte L. Nerve cells decide to orient inside an injectable hydrogel with minimal structural guidance. Nano Lett. 2017;17:3782–91.
Omidinia-Anarkoli A, Boesveld S, Tuvshindorj U, Rose JC, Haraszti T, De Laporte L. An injectable hybrid hydrogel with oriented short fibers induces unidirectional growth of functional nerve cells. Small. 2017;13:1702207.
Alsmadi NZ, Patil LS, Hor EM, Lofti P, Razal JM, Chuong C-J, Wallace GG, Romero-Ortega MI. Coiled polymeric growth factor gradients for multi-luminal neural chemotaxis. Brain Res. 2015;1619:72–83.
Hsu RS, Chen PY, Fang JH, Chen YY, Chang CW, Lu YJ, Hu SH. Adaptable microporous hydrogels of propagating NGF-gradient by injectable building blocks for accelerated axonal outgrowth. Advanced Science. 2019;6:1900520.
Zhu L, Jia S, Liu T, Yan L, Huang D, Wang Z, Chen S, Zhang Z, Zeng W, Zhang Y. Aligned PCL fiber conduits immobilized with nerve growth factor gradients enhance and direct sciatic nerve regeneration. Adv Func Mater. 2020;30:2002610.
Zhang D, Li Z, Shi H, Yao Y, Du W, Lu P, Liang K, Hong L, Gao C. Micropatterns and peptide gradient on the inner surface of a guidance conduit synergistically promotes nerve regeneration in vivo. Bioactive Mater. 2022;9:134–46.
Bertucci C, Koppes R, Dumont C, Koppes A. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Res Bull. 2019;152:265–84.
Zhu R, Sun Z, Li C, Ramakrishna S, Chiu K, He L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp Neurol. 2019;319:112963.
Liu Z, Cai M, Zhang X, Yu X, Wang S, Wan X, Wang ZL, Li L. Cell-traction-triggered on-demand electrical stimulation for neuron-like differentiation. Adv Mater. 2021;33:2106317.
Jiang F, Shan Y, Tian J, Xu L, Li C, Yu F, Cui X, Wang C, Li Z, Ren K. Poly(l-Lactic Acid) nanofiber-based multilayer film for the electrical stimulation of nerve cells. Adv Mater Interfaces. 2023;10:2202474.
Acknowledgements
Not applicable.
Funding
The present study was supported by Shahroud University of Medical Sciences, Shahroud, Iran as a Ph.D. thesis (Grant number: 200159). This research was carried out with the ethical code of IR.SHMU.AEC.1402.008.
Author information
Authors and Affiliations
Contributions
MS: Conceptualization, methodology, visualization, resources, writing-original draft. MK-F: Conceptualization, formal analysis, methodology, writing-original draft. MS: Conceptualization, formal analysis, methodology, writing-original draft. SE-B: Conceptualization, investigation, project administration, supervision, writing-original draft. MA: Conceptualization, resources, writing-original draft. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sharifi, M., Kamalabadi-Farahani, M., Salehi, M. et al. Recent advances in enhances peripheral nerve orientation: the synergy of micro or nano patterns with therapeutic tactics. J Nanobiotechnol 22, 194 (2024). https://doi.org/10.1186/s12951-024-02475-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-024-02475-8