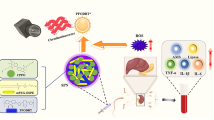
Abstract
Acute pancreatitis (AP) is a common and life-threatening digestive disorder. However, its diagnosis and treatment are still impeded by our limited understanding of its etiology, pathogenesis, and clinical manifestations, as well as by the available detection methods. Fortunately, the progress of microenvironment-targeted nanoplatforms has shown their remarkable potential to change the status quo. The pancreatic inflammatory microenvironment is typically characterized by low pH, abundant reactive oxygen species (ROS) and enzymes, overproduction of inflammatory cells, and hypoxia, which exacerbate the pathological development of AP but also provide potential targeting sites for nanoagents to achieve early diagnosis and treatment. This review elaborates the various potential targets of the inflammatory microenvironment of AP and summarizes in detail the prospects for the development and application of functional nanomaterials for specific targets. Additionally, it presents the challenges and future trends to develop multifunctional targeted nanomaterials for the early diagnosis and effective treatment of AP, providing a valuable reference for future research.
Graphical Abstract

Similar content being viewed by others
Introduction
Acute pancreatitis (AP) is a potentially fatal disease with high morbidity and mortality[1]. The typical clinical symptom is persistent severe pain in the epigastrium with abdominal distension, nausea and vomiting. In recent years, its incidence has increased over time [2, 3]. Gallstones and alcohol are common causes of AP [4], leading to activation of trypsinogen, which further activates other digestive enzymes and causes self-digestion in the pancreas [5]. In terms of the course of the disease, it progresses rapidly and the patient develops moderate AP (MAP) or severe AP (SAP), resulting in infectious necrosis, systemic inflammatory response syndrome (SIRS), and multiple organ dysfunction syndrome (MODS), with a mortality rate of 20–40% [6, 7]. Currently, the diagnosis of AP mainly relies on laboratory tests and imaging examinations, which have lower sensitivity for detecting early AP, leading to a decrease in the diagnostic rate of AP and aggravation of the disease. In terms of treatment, general therapy for AP includes close monitoring of vital signs, fluid balance, pain relief, nutritional support, and infection prevention [7, 8]. However, these methods usually fail to suppress the early response of SIRS and prevent subsequent organ failure, and no effective treatment for AP is currently available [9]. Thus, there is an urgent need for a new strategy for the diagnosis and treatment of AP.
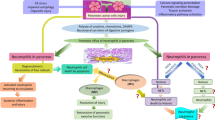
The occurrence of AP is a complex, multifactorial, pathophysiological process. Pathological calcium signaling, mitochondrial dysfunction, impaired unfolded protein response, endoplasmic reticulum (ER) stress, and impaired autophagy are among the multiple factors contributing to the pathophysiological changes in the pancreas [5, 10]. The main pathological changes in the pancreas include the activation of trypsin, aggregation of inflammatory cells, and the excessive release of proinflammatory factors and reactive oxygen species (ROS), along with other factors, producing an abnormal microenvironment. These in turn result in a systemic inflammatory response and extensive pancreatic injury. Therefore, the identification and regulation of relevant indicators of the inflammatory microenvironment may be the key to diagnosing or treating pancreatitis.
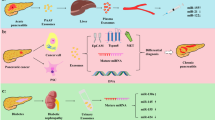
In recent years, researchers have made remarkable progress in both the diagnosis and the treatment of AP by develo** highly sensitive diagnostic tools and drugs targeting microenvironmental changes. Among these approaches, nanotechnology has attracted widespread attention because of its advantages of high sensitivity, specificity, multimeasurement ability, and targeted therapy [11]. Specifically, nanotechnology can target indicators in the microenvironment for the diagnosis and modulation of a disease. For example, Cheng et al. designed an MMP-13/pH-responsive nanoprobe (CMFn@HCQ) for the diagnosis and treatment of inflammation [12]. It was also reported that ferritin nanocages (CMFn) can be used for fluorescence imaging in response to the overexpression of metalloproteinases (MMP-13), a group of protein hydrolases that are related to the degree of inflammation in the microenvironment. It was also shown that the CMFn@HCQ nanocages could release hydroxychloroquine (HCQ) continuously into an acidic microenvironment, which significantly reduced local inflammation. ROS, as free radicals, are closely related to inflammation. The imaging and regulation of ROS can realize the early diagnosis and treatment of AP. Our group also developed a novel nanotheranostic agent (named TMSN@PM) with the ability to target inflammatory sites. Under acidic conditions featuring excessive ROS, TMSN@PM was shown to degrade and release manganese ions for magnetic resonance imaging (MRI) to assess the severity of inflammation. It was found that the T1-weighted signal was enhanced in the pancreatic region, which peaked 3 h after TMSN@PM injection. TMSN@PM also scavenges excess ROS and reduces JNK and hypoxia-inducible factor-1α (HIF-1α) activation, thereby reducing inflammation. Compared with the findings in an untreated group, ROS in the pancreas decreased significantly after TMSN@PM treatment, which attenuated the damage to pancreatic tissue [45]. Nanotechnology can improve the capabilities of MRI by develo** macrophage-targeted-accumulation contrast agents [46]. As reported previously, a novel Gd-containing contrast agent named Gd(III)-dithiolane gold nanoparticles can be phagocytosed by macrophages for targeted accumulation in the pancreas, which showed a very high r1 relaxation rate at both low and high magnetic field strengths for MRI of the pancreas[47]. Decorating nanoparticles with ligands that bind to macrophage surface receptors can improve the targeting of nanoparticles. Since mannose receptors are highly expressed by macrophages, Tian et al. developed novel Gd-DTPA-loaded mannosylated liposomes (named M-Gd-NL) (Fig. 2A). M-Gd-NL can bind to macrophages in a targeted manner in the inflammatory microenvironment and then release Gd-DTPA, resulting in a potent increase in the relaxation rate of Gd-DTPA in macrophages, which substantially enhances the capacity of MRI. This method not only improves the diagnostic capability of MRI, but also enables differentiation between mild and severe AP [48]. Similarly, Long et al. synthesized a P-selectin-targeted, near-infrared fluorescence (NIRF) dye (Cy 5.5)-labeled dual-modal nanoprobe (Gd-DTPA-Cy5.5-PsLmAb) based on the finding that macrophages highly express P-selectin. When PsLmAb of the nanoprobe can bind P-selectin in the microenvironment, the more P-selectin there is, the more Gd-DTPA-Cy5.5-PsLmAb nanoparticles that reside at the site of inflammation, resulting in an enhanced signal in MR/NIRF images (Fig. 2B). This probe can achieve the early diagnosis and treatment of SAP by MR imaging and NIRF imaging, providing a rapid method of visualization for the diagnosis of clinical early-stage SAP [49].
A The preparation procedure of Gd-NL and M-Gd-NL based on lipid film method. Reproduced with permission from reference [48]. Copyright 2017, DOVE Medical Press B Schematic representation of Gd-DTPA-Cy5.5-PsLmAb for NIRF and MR imaging of MAP and SAP. Reproduced with permission from reference [49]. Copyright 2020, American Chemical Society C Schematic illustration of the mechanism of activatable chemiluminescent probes. Reproduced with permission from reference [62]. Copyright 2022, John Wiley and Sons D Fabrication and targeted-therapeutic schematics of ND-MMSNs. Reproduced with permission from reference [63]. Copyright 2018, Springer Nature E Schematic illustration of nanoparticle-encapsulated CQ/TAM combined with MSCs for arresting the increasing severity of AP in mice through iNOS (IDO) signaling. Reproduced with permission from reference [68].
Macrophages also mediate the pathological process of AP through various mechanisms [50, 51]. It has also been reported that macrophages are related to the progression of SAP [52]. During SAP, peritoneal macrophages, alveolar macrophages, and Kupffer cells are activated, which contribute to the damage to various organs. Macrophages can be divided into two subtypes: M1 macrophages and M2 macrophages. M1 macrophages secrete factors related to the proinflammatory stage of AP, while M2 macrophages are mainly involved in pancreatic repair and regeneration [53]. Macrophages can change their phenotype and function spatiotemporally, which is called macrophage polarization. Therefore, regulating the polarization of macrophages is a new direction for the treatment of AP [54, 55]. Kazuaki et al. constructed a nanotechnology-based CO donor (CO-HbV) that can target macrophages and inhibit AP by releasing CO to polarize macrophages toward an M2-like phenotype. CO-HbV was also reported to inhibit neutrophil infiltration in the pancreas and attenuate the subsequent acute lung injury [56]. The degree of severity of AP is related to the number of infiltrating macrophages, which is involved in the development of injuries to the pancreas and multiple other organs. Based on this, researchers have focused particularly on drugs that inhibit macrophage recruitment and deplete macrophages. Tang et al. studied the protective effects of G4.5-COOH and G5-OH on the pancreatic injury of AP mices. It was found that two kinds of dendrimers reduced the inflammatory infiltration of macrophages by inhibiting nuclear translocation of NF-κB in macrophages. Moreover, they also inhibited the expression of proinflammatory cytokines in peritoneal macrophages and significantly decreased the pathological changes of the pancreas [57]. Clodronate liposomes are the most commonly used method to deplete macrophages [58]. Dang et al. loaded liposomes with clodronate and superparamagnetic iron oxide (SPIO), which can be delivered in a targeted manner to macrophages to induce their apoptosis by competing with adenosine triphosphate (ATP), thus inhibiting the release of inflammatory factors and alleviating the renal injury caused by SAP [59]. Different from them, Chen’s team investigated an inflammation-targeted nanoparticle named MU, which was composed of PEG − PLGA and ulinastatin coated by macrophage membrane [60]. In the mouse model of AP, MU can significantly inhibit the secretion of pro-inflammatory cytokines TNF-α and IL-6 by macrophages. In addition, in vitro experiments have proved that MU may play an anti-inflammatory role by reducing the contents of p-IκBα/IκBα and p-p65/p65 through IκBα/NF-κB signaling pathway. Therefore, MU is expected to be an effective targeted drug to inhibit the progress of AP.
Neutrophil infiltration is a hallmark of inflammation. Neutrophils, as a “living” drug delivery carrier, have attracted widespread attention in recent years because of their characteristics of crossing natural barriers, decreasing immune clearance rate and having a long biological half-life [61]. Similar to macrophages, neutrophils can take up nanoparticles [61]. Therefore, researchers have explored various neutrophil tracking probes for disease diagnosis. For example, Huang’s team synthesized three chemiluminescent probes based on benzoazole-phenoxyl-dioxetane for the in vivo imaging of neutrophils in mouse models of peritonitis and psoriasis. These probes activate and prolong chemiluminescence in the presence of neutrophil elastase (NE) (Fig. 2C). In experiments with LPS-induced peritonitis, benzothiazole-phenoxyl-dioxetane (BTPDNE) exhibited more intense brightness and a longer half-life than methyl acrylate-phenoxyl-dioxetane (MPDNE) [62]. Moreover, Wu et al. developed core–shell structured magnetic mesoporous silica nanoparticles (called MMSNs) and constructed a theranostic platform of ND-MMSNs for internalizing MMSNs loaded with doxorubicin (D-MMSNs) by neutrophils [63]. In the inflammatory mouse glioma model, ND-MMSNs are internalized by neutrophils, which can be targeted to accumulate at the inflammatory site of glioma with chemokines. Then, neutrophils release neutrophil extracellular traps (NETs) and D-MMSNs to realize the diagnosis and treatment of residual tumors (Fig. 2D). Similar to the features of the above diseases, there are a large number of neutrophils in the inflammatory microenvironment of AP, and it is expected that nanoparticles used for neutrophil imaging in the future can be used for the diagnosis of AP.
Neutrophils can release ROS to cause tissue damage [64]. Moreover, the production of NETs can speed up the progression of AP [65]. Neutrophils may serve as a target for the treatment of AP because they can mediate local tissue damage in the pancreas and associated damage to other organs when AP occurs [66]. Nanotechnology provides an plausible pathway for neutrophil-related therapeutic intervention. For example, nucleic acid nanoparticles (tFNAs) were recently reported to regulate cell proliferation and migration, and have potent anti-inflammatory and antiapoptotic abilities against AP. Wang et al. found that compared with a saline group, tFNAs significantly decreased neutrophil activity and alleviated pancreatic injury in a treatment group [67]. Additionally, Liu et al. introduced nanoparticle-encapsulated chloroquine/tamoxifen in combination with bone marrow-derived mesenchymal stem cells (BMSCs) that acted synergistically for the treatment of AP. BMSCs prevented the progression of AP by suppressing the recruitment of neutrophils, macrophages, and CD4+ T cells through iNOS signaling (Fig. 2E) [68]. Furthermore, another membrane-encapsulation technology has been applied for targeting inflammation [69, 70]. Membrane-encapsulation technology can confer nanoparticle-derived cell membrane-related functions such as immune evasion [71], crossing barriers [72], and homing to inflammatory sites [73]. Zhou et al. designed neutrophil membrane-coated nanoparticles (NNPs/CLT) that cross the blood-pancreas barrier (BPB), driven to sites of inflammation through chemokine recruitment, which significantly downregulate the level of pancreatic myeloperoxidase and reduce associated lung injuries in AP rats [74].
Macrophages and neutrophils play an important role in the systemic production of inflammatory mediators. Nanodiagnostic and nanotherapeutic agents targeting neutrophils or macrophages can be designed by nanotechnology to assess the severity of AP and suppress overactive inflammatory responses. At present, notable achievements have been made in the research and development of targeted drugs for macrophages and neutrophils. Since macrophages and neutrophils have abundant surface receptors, the development of more nanoparticles targeting these receptors is a promising future strategy.
Oxidative stress and reactive oxygen and nitrogen species
Oxidative stress is an important factor in the progression of AP, and it generates a large number of free radicals including ROS and reactive nitrogen species (RNS), leading to an imbalance between the oxidative and antioxidant systems [75]. ROS, as free radicals, are closely related to inflammation [4C). In mouse models of mild and severe AP, MΦ-NP(L&K) significantly attenuated alveolar necrosis or immune infiltration and effectively reduced the severity of AP[99].
As biomarkers, digestive enzymes can be used to diagnose AP. Moreover, as important components of the inflammatory microenvironment, they are important to the development of AP and used to regulate the inflammatory microenvironment. Functional composite nanoparticles have multiple roles in the diagnosis and treatment of AP. First, as drug carriers, they can prolong the half-life of drugs; second, they can react with digestive enzymes in the microenvironment to release drugs; finally, they can bind with enzymes covalently or noncovalently to inhibit enzyme activity. Therefore, the design of functional composite nanoparticles targeting the inflammatory microenvironment using the biocatalytic properties of enzymes is extremely promising.
pH
The decrease of pH is one of the characteristics of the inflammatory microenvironment. When AP occurs, enhanced glycolysis of inflamed tissue leads to increased lactate production and a decrease in pH. Impaired endocrine and/or exocrine function of the pancreas in AP patients inhibits bicarbonate secretion by ductal cells, leading to enhanced acidification of the acinar luminal space. Lowering of pH promotes trypsinogen activation by cathepsin B [100], leading to self-digestion of the pancreas. Furthermore, persistent extracellular acidification can disrupt cell junctions and lead to the leakage of zymogen into the interstitial fluid [101]. Thus, extracellular acidification exacerbates the development of AP.
In recent years, pH-responsive drug carriers have achieved good results in the diagnosis and treatment of various diseases including AP by targeting drugs to sites of inflammation and modulating drug release in response to pH stimuli [102, 103]. In terms of diagnosis, Lu et al. synthesized a pH-responsive MRI contrast agent, SPIO@SiO2@MnO2, which can improve the diagnostic accuracy of MRI in an acidic environment by decomposing manganese dioxide (MnO2) into Mn2+ to increase T1- and T2-weighted signals (Fig. 5A) [104]. Experimental results demonstrated that the contrast sensitivity of diseased tissues is about 12.3 times that of normal tissues. As for treatment, Mei et al. developed porous COS@SiO2 nanocomposites that enable the continuous release COSs and maintain the drug at high concentrations in a pH-controlled manner, which helps to reduce the severity of SAP and its associated lung injury [105]. It was found that the release rate of COS was greater at pH 7.4 than at pH 8.0 (Fig. 5B). Yang’s team used chloroquine diphosphate (CQ) for gene transfection to construct Ca-CQ-pDNA-PLGA-NPs that can deliver targeted genes to the site of pancreatitis and protect the pancreas from deterioration based on pH changes. Compared with the findings at pH 7.4 and pH 6.8, the cumulative pDNA release at pH 4.5 exceeded 30% within 24 h and eventually reached 60% within 4 weeks [106]. Similarly, Hassanzadeh et al. prepared a neutrophil membrane-encapsulated nanoformulation (FA-SF-NPs) using silk fibroin (SF) and ferulic acid (FA). FA-SF-NPs released FA with higher kinetics in a low-pH environment compared with the findings at physiological pH, thereby downregulating serum enzymes and oxidative stress-related indicators to reduce the severity of AP [107]. Moreover, with the development of nanotechnology, pH-responsive theranostic nanoplatforms have been successively developed. Dou et al. constructed metal Fe/Ce-doped mesoporous silica nanoparticles (Fe-Ce-MSN) for the treatment of inflammation and oxidative stress-related diseases [108]. In the mildly acidic environment of inflammatory sites, Fe-/Ce-MSN nanoparticles released Fe ions, which enhanced the T2-weighted signals. Additionally, Fe-Ce-MSN NPs not only scavenged overproduced ROS but also controlled the production of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), with significant antioxidant and anti-inflammatory effects (Fig. 5C).
A SPIO@SiO2@MnO2 shows weak T1 and T2 contrast intensity in normal physiological conditions, as the T2 signal of SPIO is quenched by the MnO2 layer. In the acidic environment of a tumor or inflamed tissue, the MnO2 layer will decompose into magnetically active Mn2+ (T1-weighted), and the T1 and T2 signals are sequentially recovered. Reproduced with permission from reference [104]. Copyright 2022, Springer Nature B The cumulative release of COSs from COS@SiO2 at pH 7.4 and pH 8.0. Reproduced with permission from reference [105]. Copyright 2020, Frontiers Media S.A. C Schematic illustration of biodegradation, ROS scavenging effects, and enhanced theranostic functions by Fe/Ce-MSN-PEG NPs. Reproduced with permission from reference [108].
The inflammatory response of AP fosters a pH gradient between inflamed and healthy tissues, which provides a suitable physiological stimulus for pH-responsive drug delivery. pH-responsive drug delivery systems overcome the shortcomings of conventional drug formulations and show advantages in terms of biocompatibility, stability, size, and structural control. Moreover, they can deliver drugs to specific sites in a controlled manner and at predetermined release rates, reducing drug side effects and improving drug efficacy. Therefore, acid-responsive nanocarriers are of high value for the diagnosis and treatment of AP.
As a brief summary, Table 1 shows the strategies of diagnosis and treatment of AP with various nanomaterials.
Microorganisms
AP can be classified as MAP, moderate-to-severe AP (MSAP), and SAP according to the severity [109]. Patients with AP can develop MSAP and SAP, leading to necrotizing pancreatitis (NP), which has a high mortality rate [110]. In the later stage, patients develop intestinal dysfunction and are at risk of the translocation of intestinal flora and secondary infection of necrotic tissue. Most of the bacteria that cause pancreatic tissue necrosis infections are from the intestinal flora [111], mainly including Gram-negative and Gram-positive bacteria. The gut microbiota exists in the inflammatory microenvironment, which is an important mediator during AP and influences the progression of AP.
New imaging strategies have been developed by those researching infectious diseases [72, 136]. Currently, nanocarriers targeting the inflammatory microenvironment are limited to a single type, resulting in low delivery efficiency. In the future, the advantages of different carriers can be combined to develop carriers with higher delivery efficiency and biosafety. Secondly, the pathogenesis of AP has not been fully clarified, so there are not enough specific targets for AP, resulting in fewer nanodrugs that can specifically enter the pancreatic inflammatory microenvironment. At present, most nanodrugs passively target inflammatory lesions through the ELVIS effect to increase the drug concentration, but there is an off-target effect, which leads to low efficiency of nanodrug delivery [74, 107]. There is an urgent need to develop more active targeted nanodrugs combined with target molecules, instead of passive targeting, and to improve the delivery efficiency. Recently, the emergence of genomics, protein genomics and metabonomics has made it possible to find more specific markers of pancreatitis, thus improving the diagnosis rate and treatment efficiency.
The development and application of functional nanomaterials targeting various potential targets in the inflammatory microenvironment is a future trend in the early diagnosis and treatment of AP, but there are still many concerns that have led to the fact that nanomaterials are not yet widely used in the clinic, and one of the most important issue is the biosafety of nanomaterials. On the one hand, we still know little about the risks and their potential threats of nanomaterials, and the fact of lacking regulatory guidance and uniform standards for the toxicological assessment of nanomaterial-based drug delivery systems worsen this situation [137, 138]. On the other hand, most of the experiments conducted to date have been based on animal models, however, it is difficult for animal models to simulate the absorption, distribution, metabolism and excretion of nanomaterials in the human body as well as their effects on organs and tissues due to the complexity of the human immune system in terms of drug metabolism. Moreover, there are many common methods to establish AP models [97, 99, 107], and forms of AP caused by various different factors have their own specific characteristics. Researchers need to know the pathophysiology and limitations of each model in order to choose the appropriate model according to their own experimental needs. The technology for preparing AP models is not fully mature, and it is difficult for animal models to simulate the pathogenesis of human AP due to the diversity and complexity of its causes. Therefore, it is necessary to establish a unified scientific evaluation system, improve animal models, evaluation indexes and testing methods, and vigorously develop nanotoxicology, so as to promote the clinical translation of nanomaterials.
With the joint efforts of researchers in the future, multicenter and large-scale clinical trials can be realized. Additionally, with continuous technological development, artificial intelligence, big data analysis, 3D printing technology, and other fields have emerged, with which nanotechnology can be combined to generate new innovations and improve the ability to diagnose and treat human diseases, thus bringing a new era in the field of medicine.
Availability of data and materials
Not applicable.
Change history
23 January 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12951-023-02232-3
Abbreviations
- AP:
-
Acute pancreatitis
- ROS:
-
Reactive oxygen species
- MAP:
-
Moderate acute pancreatitis
- SAP:
-
Severe acute pancreatitis
- ER:
-
Endoplasmic reticulum
- SIRS:
-
Systemic inflammatory response syndrome
- MODS:
-
Multiple organ dysfunction syndrome
- HCQ:
-
Hydroxychloroquine
- MRI:
-
Magnetic resonance imaging
- HIF-1α:
-
Hypoxia-inducible factor-1α
- NF-κB:
-
Nuclear factor-kappa B
- TLR:
-
Toll-like receptor
- ELVIS effect:
-
The effect of extravasation through leaky vasculature and subsequent inflammatory cell-mediated sequestration
- CUR:
-
Curcumin
- SPION:
-
Superparamagnetic iron oxide nanoparticles
- NIRF:
-
Near-infrared fluorescence
- SPIO:
-
Superparamagnetic iron oxide
- ATP:
-
Adenosine triphosphate
- NE:
-
Neutrophil elastase
- MMSNs:
-
Magnetic mesoporous silica nanoparticles
- NETs:
-
Neutrophil extracellular traps
- BMSCs:
-
Bone marrow-derived mesenchymal stem cells
- RNS:
-
Reactive nitrogen species
- OA:
-
Osteoarthritis
- DEX:
-
Dexamethasone
- TKCP:
-
Thioketal linkers and cartilage-targeting peptide
- AIE:
-
Aggregation-induced emission
- CAPE-loaded NL:
-
CAPE-loaded nanoliposomes
- EMP:
-
Empagliflozin
- NY:
-
Nanoyttria
- PBzyme:
-
Prussian blue nanoenzyme
- NSs:
-
Nanosheets
- CA-NPs:
-
Cinnamic acid nanoparticles
- SST:
-
Somatostatin
- PLA2:
-
Phospholipase A2
- MnO2 :
-
Manganese dioxide
- CQ:
-
Chloroquine diphosphate
- SF:
-
Silk fibroin
- FA:
-
Ferulic acid
- Fe-Ce-MSN:
-
Fe/Ce-doped mesoporous silica nanoparticles
- TNF-α:
-
Tumor necrosis factor-α
- IL-1β:
-
Interleukin-1β
- MSAP:
-
Moderate-to-severe acute pancreatitis
- NP:
-
Necrotizing pancreatitis
- PTT:
-
Photothermal therapy
- GCS:
-
Glycol chitosan
- GNRs:
-
Gold nanorods
- AIBI:
-
2,2-Azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride
- PDA:
-
Polydopamine
- NTR:
-
Nitroreductase
- PDT:
-
Photodynamic therapy
- CPs:
-
Conjugated polymers
- CPT:
-
Camptothecin
- Hb:
-
Hemoglobin
- Ce6:
-
Chlorine e6
- CS:
-
Chondroitin sulfate
- SF:
-
Surface-functionalized
References
Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology Guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400–15.
Sternby H, Bolado F, Canaval-Zuleta HJ, Marra-Lopez C, Hernando-Alonso AI, Del-Val-Antonana A, et al. Determinants of severity in acute pancreatitis: a nation-wide multicenter prospective cohort study. Ann Surg. 2019;270(2):348–55.
Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):122–34.
Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. 2017;17(2):155–65.
Saluja A, Dudeja V, Dawra R, Sah RP. Early intra-acinar events in pathogenesis of pancreatitis. Gastroenterology. 2019;156(7):1979–93.
Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. 2019;68(6):1044–51.
van Dijk SM, Hallensleben NDL, van Santvoort HC, Fockens P, van Goor H, Bruno MJ, et al. Acute pancreatitis: recent advances through randomised trials. Gut. 2017;66(11):2024–32.
Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. 2020;396(10252):726–34.
Kambhampati S, Park W, Habtezion A. Pharmacologic therapy for acute pancreatitis. World J Gastroenterol. 2014;20(45):16868–80.
Biczo G, Vegh ET, Shalbueva N, Mareninova OA, Elperin J, Lotshaw E, et al. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology. 2018;154(3):689–703.
Han S, Chen C, Chen C, Wu L, Wu X, Lu C, et al. Coupling annealed silver nanoparticles with a porous silicon Bragg mirror SERS substrate and machine learning for rapid non-invasive disease diagnosis. Anal Chim Acta. 2023;1254:341116.
Chen H, Qin Z, Zhao J, He Y, Ren E, Zhu Y, et al. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials. 2019;225:119520.
Li X, Liu Y, Qi X, **ao S, Xu Z, Yuan Z, et al. Sensitive activatable nanoprobes for real-time ratiometric magnetic resonance imaging of reactive oxygen species and ameliorating inflammation in vivo. Adv Mater. 2022;34(19):e2109004.
Manohar M, Jones EK, Rubin SJS, Subrahmanyam PB, Swaminathan G, Mikhail D, et al. Novel circulating and tissue monocytes as well as macrophages in pancreatitis and recovery. Gastroenterology. 2021;161(6):2014-29.e14.
**ang H, Guo F, Tao X, Zhou Q, **a S, Deng D, et al. Pancreatic ductal deletion of S100A9 alleviates acute pancreatitis by targeting VNN1-mediated ROS release to inhibit NLRP3 activation. Theranostics. 2021;11(9):4467–82.
Sendler M, Weiss F-U, Golchert J, Homuth G, van den Brandt C, Mahajan UM, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. 2018;154(3):704-18.e10.
**e X, Zhao J, Gao W, Chen J, Hu B, Cai X, et al. Prussian blue nanozyme-mediated nanoscavenger ameliorates acute pancreatitis via inhibiting TLRs/NF-kappaB signaling pathway. Theranostics. 2021;11(7):3213–28.
Zhang G, Ma L, Bai L, Li M, Guo T, Tian B, et al. Inflammatory microenvironment-targeted nanotherapies. J Control Release. 2021;334:114–26.
Ke W, Afonin KA. Exosomes as natural delivery carriers for programmable therapeutic nucleic acid nanoparticles (NANPs). Adv Drug Deliv Rev. 2021;176:113835.
Murugesan S, Scheibel T. Copolymer/clay nanocomposites for biomedical applications. Adv Funct Mater. 2020. https://doi.org/10.1002/adfm.201908101.
Ahmad A, Rashid S, Chaudhary AA, Alawam AS, Alghonaim MI, Raza SS, et al. Nanomedicine as potential cancer therapy via targeting dysregulated transcription factors. Semin Cancer Biol. 2023;89:38–60.
Kumar A, Ahmad A, Ansari MM, Gowd V, Rashid S, Chaudhary AA, et al. Functionalized-DNA nanostructures as potential targeted drug delivery systems for cancer therapy. Semin Cancer Biol. 2022;86:54–68.
Ha**ezhad MR, Shahraki S, Nikfarjam Z, Davodabadi F, Mirinejad S, Rahdar A, et al. Development of a new vesicular formulation for delivery of Ifosfamide: evidence from in vitro, in vivo, and in silico experiments. Arab J Chem. 2023;16(9):105086.
Xu XAH, Zhang D, Tao H, Dou Y, Li X, Huang J, Zhang J. A self-illuminating nanoparticle for inflammation imaging and cancer therapy. Sci Adv. 2019;5(1):eaat2953.
Wang Y, Zhai W, Cheng S, Li J, Zhang H. Surface-functionalized design of blood-contacting biomaterials for preventing coagulation and promoting hemostasis. Friction. 2023;11(8):1371–94.
Huang S, Yuan J, **e Y, Qing K, Shi Z, Chen G, et al. Targeting nano-regulator based on metal–organic frameworks for enhanced immunotherapy of bone metastatic prostate cancer. Cancer Nanotechnol. 2023. https://doi.org/10.1186/s12645-023-00200-y.
Gowd V, Ahmad A, Tarique M, Suhail M, Zughaibi TA, Tabrez S, et al. Advancement of cancer immunotherapy using nanoparticles-based nanomedicine. Semin Cancer Biol. 2022;86:624–44.
Zhang Y, Bo S, Feng T, Qin X, Wan Y, Jiang S, et al. A versatile theranostic nanoemulsion for architecture-dependent multimodal imaging and dually augmented photodynamic therapy. Adv Mater. 2019;31(21):e1806444.
Ghosh B, Biswas S. Polymeric micelles in cancer therapy: state of the art. J Control Release. 2021;332:127–47.
Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154–155:102–22.
Li H, Zha S, Li H, Liu H, Wong KL, All AH. Polymeric dendrimers as nanocarrier vectors for neurotheranostics. Small. 2022. https://doi.org/10.1002/smll.202203629.
Yang G, Phua SZF, Bindra AK, Zhao Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv Mater. 2019;31(10):e1805730.
Pan W, Li Z, Qiu S, Dai C, Wu S, Zheng X, et al. Octahedral Pt-MOF with Au deposition for plasmonic effect and Schottky junction enhanced hydrogenothermal therapy of rheumatoid arthritis. Mater Today Bio. 2022;13:100214.
Wang J, Ni Q, Wang Y, Zhang Y, He H, Gao D, et al. Nanoscale drug delivery systems for controllable drug behaviors by multi-stage barrier penetration. J Control Release. 2021;331:282–95.
van der Koog L, Gandek TB, Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthc Mater. 2022;11(5):e2100639.
Wu W, Luo L, Wang Y, Wu Q, Dai HB, Li JS, et al. Endogenous pH-responsive nanoparticles with programmable size changes for targeted tumor therapy and imaging applications. Theranostics. 2018;8(11):3038–58.
Narayanaswamy R, Torchilin VP. Hydrogels and their applications in targeted drug delivery. Molecules. 2019;24(3):603.
Chuang EY, Lin KJ, Huang TY, Chen HL, Miao YB, Lin PY, et al. An intestinal “transformers”-like nanocarrier system for enhancing the oral bioavailability of poorly water-soluble drugs. ACS Nano. 2018;12(7):6389–97.
Su Y, Gao J, Kaur P, Wang Z. Neutrophils and macrophages as targets for development of nanotherapeutics in inflammatory diseases. Pharmaceutics. 2020;12(12):1222.
Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. 2012;30:459–89.
Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129(7):2629–39.
Wang Z, Koenig AL, Lavine KJ, Apte RS. Macrophage plasticity and function in the eye and heart. Trends Immunol. 2019;40(9):825–41.
Cörek ERG, Siegrist S, Einfalt T, Detampel P, Schlepütz CM, Sieber S, Fluder P, Schulz G, Unterweger H, Alexiou C, Müller B, Puchkov M, Huwyler J. Shedding light on metal-based nanoparticles in zebrafish by computed tomography with micrometer resolution. Small. 2020. https://doi.org/10.1002/smll.202000746.
Deng H, Li X, Ju J, Mo X, Ge G, Zhu X. Multifunctional nanoprobes for macrophage imaging. Biomaterials. 2022;290:121824.
Zhang JXDS, Zhang Y, Sha X, Zhang LR, Wei CS, Chen M, Jiang DL. MRI shows clodronate-liposomes attenuating liver injury in rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2010;9(2):192–200.
Yang B, Liu Q, Yao X, Zhang D, Dai Z, Cui P, et al. FePt@MnO-based nanotheranostic platform with acidity-triggered dual-ions release for enhanced MR imaging-guided ferroptosis chemodynamic therapy. ACS Appl Mater Interfaces. 2019;11(42):38395–404.
Holbrook RJ, Rammohan N, Rotz MW, MacRenaris KW, Preslar AT, Meade TJ. Gd(III)-dithiolane gold nanoparticles for T1-weighted magnetic resonance imaging of the pancreas. Nano Lett. 2016;16(5):3202–9.
Tian B, Liu R, Chen S, Chen L, Liu F, Jia G, et al. Mannose-coated gadolinium liposomes for improved magnetic resonance imaging in acute pancreatitis. Int J Nanomed. 2017;12:1127–41.
Long L, Deng L, Wang L, Wen S, Luo L, Liang L, et al. P-selectin-based dual-model nanoprobe used for the specific and rapid visualization of early detection toward severe acute pancreatitis in vivo. ACS Biomater Sci Eng. 2020;6(10):5857–65.
Zhao Q, Wei Y, Pandol SJ, Li L, Habtezion A. STING signaling promotes inflammation in experimental acute pancreatitis. Gastroenterology. 2018;154(6):1822-35.e2.
Iyer S, Bava EP, George J, Tarique M, Sahay P, Edwards DB, et al. Mo1380 pirfenidone treatment ameliorates the severity of acute pancreatitis by reducing macrophage infiltration and modulating its polarization. Gastroenterology. 2020;158(6):S-869.
Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, et al. NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology. 2020;158(1):253-69.e14.
Wu J, Zhang L, Shi J, He R, Yang W, Habtezion A, et al. Macrophage phenotypic switch orchestrates the inflammation and repair/regeneration following acute pancreatitis injury. EBioMedicine. 2020;58:102920.
Sahay P, Bava EP, Iyer S, Dudeja V. Modulation of macrophage polarity for treatment of acute pancreatitis: are we there yet? EBioMedicine. 2020;60:103002.
He Y, Dai J, Niu M, Li B, Husain SZ, Hu G, et al. Su302 inhibition of nampt protects against acute pancreatitis via modulating macrophage polarization and metabolic alteration. Gastroenterology. 2021;160(6):S-666-S−7.
Taguchi K, Nagao S, Maeda H, Yanagisawa H, Sakai H, Yamasaki K, et al. Biomimetic carbon monoxide delivery based on hemoglobin vesicles ameliorates acute pancreatitis in mice via the regulation of macrophage and neutrophil activity. Drug Deliv. 2018;25(1):1266–74.
Tang Y, Han Y, Liu L, Shen W, Zhang H, Wang Y, et al. Protective effects and mechanisms of G5 PAMAM dendrimers against acute pancreatitis induced by caerulein in mice. Biomacromol. 2015;16(1):174–82.
Haider N, Bosca L, Zandbergen HR, Kovacic JC, Narula N, Gonzalez-Ramos S, et al. Transition of macrophages to fibroblast-like cells in healing myocardial infarction. J Am Coll Cardiol. 2019;74(25):3124–35.
Dang SC, Zeng YH, Wang PJ, Chen BD, Chen RF, Kumar Singh A, et al. Clodronate-superparamagnetic iron oxide-containing liposomes attenuate renal injury in rats with severe acute pancreatitis. J Zhejiang Univ Sci B. 2014;15(6):556–65.
Chen Y, Tao H, Chen R, Pan Y, Wang J, Gao R, et al. Biomimetic nanoparticles loaded with ulinastatin for the targeted treatment of acute pancreatitis. Mol Pharm. 2023;20(8):4108–19.
Chu D, Dong X, Shi X, Zhang C, Wang Z. Neutrophil-based drug delivery systems. Adv Mater. 2018;30(22):e1706245.
Huang J, Cheng P, Xu C, Liew SS, He S, Zhang Y, et al. Chemiluminescent probes with long-lasting high brightness for in vivo imaging of neutrophils. Angew Chem Int Ed Engl. 2022;61(30):e202203235.
Wu M, Zhang H, Tie C, Yan C, Deng Z, Wan Q, et al. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat Commun. 2018. https://doi.org/10.1038/s41467-018-07250-6.
Lagnado A, Leslie J, Ruchaud-Sparagano MH, Victorelli S, Hirsova P, Ogrodnik M, et al. Neutrophils induce paracrine telomere dysfunction and senescence in ROS-dependent manner. EMBO J. 2021;40(9):e106048.
Merza M, Hartman H, Rahman M, Hwaiz R, Zhang E, Renstrom E, et al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology. 2015;149(7):1920-31.e8.
Lei Y, Tang L, Liu S, Hu S, Wu L, Liu Y, et al. Parabacteroides produces acetate to alleviate heparanase-exacerbated acute pancreatitis through reducing neutrophil infiltration. Microbiome. 2021;9(1):115.
Wang Y, Li Y, Gao S, Yu X, Chen Y, Lin Y. Tetrahedral framework nucleic acids can alleviate taurocholate-induced severe acute pancreatitis and its subsequent multiorgan injury in mice. Nano Lett. 2022;22(4):1759–68.
Liu H, Liu S, Song X, Jiang A, Zou Y, Deng Y, et al. Nanoparticle encapsulated CQ/TAM combination harmonizes with MSCs in arresting progression of severity in AP mice through iNOS (IDO) signaling. Mater Today Bio. 2022;14:100226.
Zhang Q, Dehaini D, Zhang Y, Zhou J, Chen X, Zhang L, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13(12):1182–90.
Wang H, Liu Y, He R, Xu D, Zang J, Weeranoppanant N, et al. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater Sci. 2020;8(2):552–68.
Chen HY, Deng J, Wang Y, Wu CQ, Li X, Dai HW. Hybrid cell membrane-coated nanoparticles: a multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020;112:1–13.
Cao X, Hu Y, Luo S, Wang Y, Gong T, Sun X, et al. Neutrophil-mimicking therapeutic nanoparticles for targeted chemotherapy of pancreatic carcinoma. Acta Pharm Sin B. 2019;9(3):575–89.
Zhao Y-Z, ZhuGe D-L, Tong M-Q, Lin M-T, Zheng Y-W, Jiang X, et al. Ulcerative colitis-specific delivery of keratinocyte growth factor by neutrophils-simulated liposomes facilitates the morphologic and functional recovery of the damaged colon through alleviating the inflammation. J Control Release. 2019;299:90–106.
Zhou X, Cao X, Tu H, Zhang ZR, Deng L. Inflammation-targeted delivery of celastrol via neutrophil membrane-coated nanoparticles in the management of acute pancreatitis. Mol Pharm. 2019;16(3):1397–405.
Perez S, Pereda J, Sabater L, Sastre J. Redox signaling in acute pancreatitis. Redox Biol. 2015;5:1–14.
Wang S, Huang J, Zhu H, Zhu J, Wang Z, **ng Y, et al. Nanomodulators capable of timely scavenging ROS for inflammation and prognosis control following photothermal tumor therapy. Adv Funct Mater. 2023. https://doi.org/10.1002/adfm.202213151.
Cao Y, Wang J, Tian H, Fu GH. Mitochondrial ROS accumulation inhibiting JAK2/STAT3 pathway is a critical modulator of CYT997-induced autophagy and apoptosis in gastric cancer. J Exp Clin Cancer Res. 2020;39(1):119.
Woodby B, Penta K, Pecorelli A, Lila MA, Valacchi G. Skin health from the inside out. Annu Rev Food Sci Technol. 2020;11:235–54.
Shen C, Gao M, Chen H, Zhan Y, Lan Q, Li Z, et al. Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J Nanobiotechnol. 2021;19(1):395.
Xu H, She P, Ma B, Zhao Z, Li G, Wang Y. ROS responsive nanoparticles loaded with lipid-specific AIEgen for atherosclerosis-targeted diagnosis and bifunctional therapy. Biomaterials. 2022;288:121734.
Shi R, Li H, ** X, Huang X, Ou Z, Zhang X, et al. Promoting Re-epithelialization in an oxidative diabetic wound microenvironment using self-assembly of a ROS-responsive polymer and P311 peptide micelles. Acta Biomater. 2022;152:425–39.
Lei XJHQ, Ge HF, Zhang XYRX, Chen YJ, Hu R, Feng H, Deng J, Huang Y, Li WY. A redox-reactive delivery system via neural stem cell nanoencapsulation enhances white matter regeneration in intracerebral hemorrhage mice. Bioeng Transl Med. 2022. https://doi.org/10.1002/btm2.10451.
Shahin NN, Shamma RN, Ahmed IS. A nano-liposomal formulation of caffeic acid phenethyl ester modulates Nrf2 and NF-kappabeta signaling and alleviates experimentally induced acute pancreatitis in a rat model. Antioxidants. 2022;11(8):1536.
Li Q, Cao Q, Yuan Z, Wang M, Chen P, Wu X. A novel self-nanomicellizing system of empagliflozin for oral treatment of acute pancreatitis: an experimental study. Nanomedicine. 2022;42:102534.
Liu T, **ao B, **ang F, Tan J, Chen Z, Zhang X, et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat Commun. 2020;11(1):2788.
Peng Y, He D, Ge X, Lu Y, Chai Y, Zhang Y, et al. Construction of heparin-based hydrogel incorporated with Cu5.4O ultrasmall nanozymes for wound healing and inflammation inhibition. Bioactive Mater. 2021;6(10):3109–24.
Khurana AAP, Allawadhi P, Kumar V, Sayed N, Packirisamy G, Godugu C. Superoxide dismutase mimetic nanoceria restrains cerulein induced acute pancreatitis. Nanomedicine. 2019;14(14):1805–25.
Khurana A, Anchi P, Allawadhi P, Kumar V, Sayed N, Packirisamy G, et al. Yttrium oxide nanoparticles reduce the severity of acute pancreatitis caused by cerulein hyperstimulation. Nanomedicine. 2019;18:54–65.
**e P, Zhang L, Shen H, Wu H, Zhao J, Wang S, et al. Biodegradable MoSe(2)-polyvinylpyrrolidone nanoparticles with multi-enzyme activity for ameliorating acute pancreatitis. J Nanobiotechnol. 2022;20(1):113.
Zhang L, **e P, Wu H, Zhao J, Wang S. 2D MoSe2@PVP nanosheets with multi-enzyme activity alleviate the acute pancreatitis via scavenging the reactive oxygen and nitrogen species. Chem Eng J. 2022;446:136792.
Abdel-Hakeem EA, Abdel-Hamid HA, Abdel Hafez SMN. The possible protective effect of Nano-Selenium on the endocrine and exocrine pancreatic functions in a rat model of acute pancreatitis. J Trace Elem Med Biol. 2020;60:126480.
Abozaid OAR, Moawed FSM, Ahmed ESA, Ibrahim ZA. Cinnamic acid nanoparticles modulate redox signal and inflammatory response in gamma irradiated rats suffering from acute pancreatitis. Biochim Biophys Acta Mol Basis Dis. 2020;1866(11):165904.
Sendler M, Weiss FU, Golchert J, Homuth G, van den Brandt C, Mahajan UM, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. 2018;154(3):704-18.e10.
Hou S, Feng T, Zhao N, Zhang J, Wang H, Liang N, et al. A carbon nanoparticle-peptide fluorescent sensor custom-made for simple and sensitive detection of trypsin. J Pharm Anal. 2020;10(5):482–9.
Shi J, Deng Q, Wan C, Zheng M, Huang F, Tang B. Fluorometric probing of the lipase level as acute pancreatitis biomarkers based on interfacially controlled aggregation-induced emission (AIE). Chem Sci. 2017;8(9):6188–95.
Zhang HW, Wang LQ, **ang QF, Zhong Q, Chen LM, Xu CX, et al. Specific lipase-responsive polymer-coated gadolinium nanoparticles for MR imaging of early acute pancreatitis. Biomaterials. 2014;35(1):356–67.
Yao Q, Jiang X, Zhai YY, Luo LZ, Xu HL, **ao J, et al. Protective effects and mechanisms of bilirubin nanomedicine against acute pancreatitis. J Control Release. 2020;322:312–25.
Cervin C, Vandoolaeghe P, Nistor C, Tiberg F, Johnsson M. A combined in vitro and in vivo study on the interactions between somatostatin and lipid-based liquid crystalline drug carriers and bilayers. Eur J Pharm Sci. 2009;36(4–5):377–85.
Zhang Q, Zhou J, Zhou J, Fang RH, Gao W, Zhang L. Lure-and-kill macrophage nanoparticles alleviate the severity of experimental acute pancreatitis. Nat Commun. 2021;12(1):4136.
Bhoomagoud M, Jung T, Atladottir J, Kolodecik TR, Shugrue C, Chaudhuri A, et al. Reducing extracellular pH sensitizes the acinar cell to secretagogue-induced pancreatitis responses in rats. Gastroenterology. 2009;137(3):1083–92.
Behrendorff N, Floetenmeyer M, Schwiening C, Thorn P. Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology. 2010;139(5):1711–20, 20.e1-5.
Liu X, Dou G, Li Z, Wang X, ** R, Liu Y, et al. Hybrid biomaterial initiates refractory wound healing via inducing transiently heightened inflammatory responses. Adv Sci. 2022;9(21):e2105650.
Ding H, Tan P, Fu S, Tian X, Zhang H, Ma X, et al. Preparation and application of pH-responsive drug delivery systems. J Control Release. 2022;348:206–38.
Lu H, Chen A, Zhang X, Wei Z, Cao R, Zhu Y, et al. A pH-responsive T(1)-T(2) dual-modal MRI contrast agent for cancer imaging. Nat Commun. 2022;13(1):7948.
Mei Q, Deng G, Huang Z, Yin Y, Li C, Hu J, et al. Porous COS@SiO(2) nanocomposites ameliorate severe acute pancreatitis and associated lung injury by regulating the Nrf2 signaling pathway in mice. Front Chem. 2020;8:720.
Yang C, Hu T, Cao H, Zhang L, Zhou P, He G, et al. Facile construction of chloroquine containing PLGA-based pDNA delivery system for efficient tumor and pancreatitis targeting in vitro and in vivo. Mol Pharm. 2015;12(6):2167–79.
Hassanzadeh P, Arbabi E, Rostami F. Coating of ferulic acid-loaded silk fibroin nanoparticles with neutrophil membranes: a promising strategy against the acute pancreatitis. Life Sci. 2021;270:119128.
Dou Y, Zhang Y, Lin C, Han R, Wang Y, Wu D, et al. pH-responsive theranostic nanoplatform of ferrite and ceria co-engineered nanoparticles for anti-inflammatory. Front Bioeng Biotechnol. 2022;10:983677.
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11.
Garret C, Peron M, Reignier J, Le Thuaut A, Lascarrou JB, Douane F, et al. Risk factors and outcomes of infected pancreatic necrosis: retrospective cohort of 148 patients admitted to the ICU for acute pancreatitis. United Eur Gastroenterol J. 2018;6(6):910–8.
Jiang X, Shi JY, Wang XY, Hu Y, Cui YF. The impacts of infectious complications on outcomes in acute pancreatitis: a retrospective study. Mil Med Res. 2020;7(1):38.
Huang H, Ali A, Liu Y, **e H, Ullah S, Roy S, et al. Advances in image-guided drug delivery for antibacterial therapy. Adv Drug Deliv Rev. 2023;192:114634.
Yang Y, Chu B, Cheng J, Tang J, Song B, Wang H, et al. Bacteria eat nanoprobes for aggregation-enhanced imaging and killing diverse microorganisms. Nat Commun. 2022;13(1):1255.
Lu SZ, Guo XY, Zou MS, Zheng ZQ, Li YC, Li XD, et al. Bacteria-instructed in situ aggregation of AuNPs with enhanced photoacoustic signal for bacterial infection bioimaging. Adv Healthc Mater. 2020;9(1):e1901229.
Timmerhuis HC, van den Berg FF, Noorda PC, van Dijk SM, van Grinsven J, Sperna Weiland CJ, et al. Over- and misuse of antibiotics and the clinical consequence in necrotizing pancreatitis: an observational multicenter study. Ann Surg. 2023. https://doi.org/10.1097/SLA.0000000000005790.
Fan X, Yang F, Nie C, Ma L, Cheng C, Haag R. Biocatalytic nanomaterials: a new pathway for bacterial disinfection. Adv Mater. 2021;33(33):e2100637.
Wang Y, Li C, Shen B, Zhu L, Zhang Y, Jiang L. Ultra-small Au/Pt NCs@GOX clusterzyme for enhancing cascade catalytic antibiofilm effect against F. nucleatum-induced periodontitis. Chem Eng J. 2023;466:143292.
Liu M, He D, Yang T, Liu W, Mao L, Zhu Y, et al. An efficient antimicrobial depot for infectious site-targeted chemo-photothermal therapy. J Nanobiotechnol. 2018;16(1):23.
Yu X, He D, Zhang X, Zhang H, Song J, Shi D, et al. Surface-adaptive and initiator-loaded graphene as a light-induced generator with free radicals for drug-resistant bacteria eradication. ACS Appl Mater Interfaces. 2019;11(2):1766–81.
Wang C, Wang Y, Zhang L, Miron RJ, Liang J, Shi M, et al. Pretreated macrophage-membrane-coated gold nanocages for precise drug delivery for treatment of bacterial infections. Adv Mater. 2018;30(46):e1804023.
Lee P, Chandel NS, Simon MC. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268–83.
Lee KE, Spata M, Maduka R, Vonderheide RH, Simon MC. Hif1alpha deletion limits tissue regeneration via aberrant B cell accumulation in experimental pancreatitis. Cell Rep. 2018;23(12):3457–64.
Ji L, Guo X, Lv J, **ao F, Zhang W, Li J, et al. Hypoxia-inducible factor-1alpha knockdown plus glutamine supplementation attenuates the predominance of necrosis over apoptosis by relieving cellular energy stress in acute pancreatitis. Oxid Med Cell Longev. 2019;2019:4363672.
Zhou M-T. Acute lung injury and ARDS in acute pancreatitis: Mechanisms and potential intervention. World J Gastroenterol. 2010;16(17):2094.
Zheng J, Shen Y, Xu Z, Yuan Z, He Y, Wei C, et al. Near-infrared off-on fluorescence probe activated by NTR for in vivo hypoxia imaging. Biosens Bioelectron. 2018;119:141–8.
Zhang X, Wu M, Li J, Lan S, Zeng Y, Liu X, et al. Light-enhanced hypoxia-response of conjugated polymer nanocarrier for successive synergistic photodynamic and chemo-therapy. ACS Appl Mater Interfaces. 2018;10(26):21909–19.
Zhou Y, Maiti M, Sharma A, Won M, Yu L, Miao LX, et al. Azo-based small molecular hypoxia responsive theranostic for tumor-specific imaging and therapy. J Control Release. 2018;288:14–22.
Baik AH, Jain IH. Turning the oxygen dial: balancing the highs and lows. Trends Cell Biol. 2020;30(7):516–36.
Shi X, Yang W, Ma Q, Lu Y, Xu Y, Bian K, et al. Hemoglobin-mediated biomimetic synthesis of paramagnetic O(2)-evolving theranostic nanoprobes for MR imaging-guided enhanced photodynamic therapy of tumor. Theranostics. 2020;10(25):11607–21.
Wang D, Wu H, Lim WQ, Phua SZF, Xu P, Chen Q, et al. A mesoporous nanoenzyme derived from metal-organic frameworks with endogenous oxygen generation to alleviate tumor hypoxia for significantly enhanced photodynamic therapy. Adv Mater. 2019;31(27):e1901893.
Liu P, **e X, Shi X, Peng Y, Ding J, Zhou W. Oxygen-self-supplying and HIF-1alpha-inhibiting core-shell nanosystem for hypoxia-resistant photodynamic therapy. ACS Appl Mater Interfaces. 2019;11(51):48261–70.
Dai L, Li X, Duan X, Li M, Niu P, Xu H, et al. A pH/ROS cascade-responsive charge-reversal nanosystem with self-amplified drug release for synergistic oxidation-chemotherapy. Adv Sci. 2019;6(4):1801807.
Gou S, Huang Y, Wan Y, Ma Y, Zhou X, Tong X, et al. Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials. 2019;212:39–54.
Mandal N, Bhattacharjee M, Chattopadhyay A, Bandyopadhyay D. Point-of-care-testing of alpha-amylase activity in human blood serum. Biosens Bioelectron. 2019;124–125:75–81.
Ramesh A, Kumar S, Brouillard A, Nandi D, Kulkarni A. A nitric oxide (NO) nanoreporter for noninvasive real-time imaging of macrophage immunotherapy. Adv Mater. 2020;32(24):e2000648.
Li J, Zhang J, Fu Y, Sun X, Gong T, Jiang J, et al. Dual pancreas- and lung-targeting therapy for local and systemic complications of acute pancreatitis mediated by a phenolic propanediamine moiety. J Control Release. 2015;212:19–29.
Foulkes R, Man E, Thind J, Yeung S, Joy A, Hoskins C. The regulation of nanomaterials and nanomedicines for clinical application: current and future perspectives. Biomater Sci. 2020;8(17):4653–64.
Halamoda Kenzaoui HB, Van Elk M, Gaitan S, Geertsma R, Gainza E, Lafuente AO, Del Pozo A, Roesslein M, Bremer S. Anticipation of regulatory needs for nanotechnology-enabled health products. Precis Nanomed. 2019. https://doi.org/10.33218/001c.13521.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (82222039, 92168106), the Youth Program of Natural Science Foundation of Sichuan Provincial Department of Science and Technology (23NSFSC6011) and the Affiliated Hospital of North Sichuan Medical College (2022JB001, BS20211116).
Author information
Authors and Affiliations
Contributions
LL and YZ search related documents, draft and modify manuscripts. JD and XL reviewed the paper, proposed amendments and provided financial support.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article has been revised: the table 1 is corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, L., Zhang, Y., Li, X. et al. Microenvironment of pancreatic inflammation: calling for nanotechnology for diagnosis and treatment. J Nanobiotechnol 21, 443 (2023). https://doi.org/10.1186/s12951-023-02200-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-023-02200-x