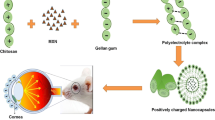
Abstract
Pseudomonas aeruginosa infection is a severe acute suppurative ulcer that engulfs virtually the entire tissue in a short period and leads to devastating destruction. Antibiotic therapy is a common approach for the prophylaxis and treatment of P. aeruginosa infection. However, it is often associated with serious side effects, complications, and multidrug resistance. Therefore, it has been a long-standing challenge to explore safe and effective methods for controlling P. aeruginosa infection. Herein, tannin-coordinated nanozyme composite-based hybrid hydrogels (TCNH) are developed and characterized for the prophylactic treatment of P. aeruginosa and multidrug-resistant P. aeruginosa infections using mouse keratitis as the animal model. The TCNH eye drops are constructed by photoinitiated free radical polymerization of acetylated gelatin solution containing self-synthesized tannin-coordinated Co3O4/Ag nanozyme composite. The as-prepared TCNH displays good dispersibility, peroxidase-like activity and in vitro/in vivo biocompatibility. The nanozyme composite in TCNH seems to penetrate the interior of bacteria and exhibited significant broad-spectrum antibacterial activity owing to its intrinsic and nanozymic catalytic properties. Furthermore, TCNH eye drops can be successfully applied to treat P. aeruginosa and multidrug-resistant P. aeruginosa keratitis. The findings of this study reveal the potential of tannin-coordinated nanozyme composite-based hybrid hydrogel eye drops for treating infectious diseases.
Graphical Abstract

Similar content being viewed by others
Introduction
Bacterial infections, one of the main problems that threaten public health, are spreading all over the world [The peroxidase-like property of TCNH The peroxidase-like activity of TCNH was evaluated based on the catalytic oxidation reaction of the substrate TMB into oxTMB in the presence of H2O2. In brief, H2O2 (20.0 μL, 0.5 M) were added to PBS solution (pH 4.0, 1.0 mL) containing TMB (10.0 μL, 16.0 mM) and TCNH solution (4.0 mg/mL, 5.0 μL) to start the catalytic process. The reaction was monitored by measuring the UV–vis absorbance of oxTMB at 652 nm using a NanoDrop™ One UV–vis spectrophotometer (Thermo Fisher Scientific, USA). In addition, pH-dependent (2.0–12.0) and temperature-dependent (4, 25, 37, and 60 °C) experiments were utilized to study the influence of pH and temperature on the catalytic activity of TCNH. All tests were performed in triplicate. OPD was also adopted to characterize the peroxidase-like activity of TCNH with a similar method as TMB for the UV–vis absorbance of the product 2,3-diaminophenazine (DAP) at 450 nm. Immortalized human corneal epithelial cells (HCECs) and human corneal fibroblasts (HCFs) were adopted to evaluate the cytotoxicity of TCNH using a lactate dehydrogenase (LDH) cytotoxicity assay kit [37]. In brief, cells with densities of 1 × 104 were seeded on 96-well plates, cultured in DMEM/F12 medium supplemented with 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin, and incubated at 37 °C in a 5% CO2 atmosphere for 24 h. Afterwards, TCNH (1.0 μL) was added to the culture (1.0 mL), and 200.0 μL of the mixture was removed and added to the 96-well plates. The cells were incubated for another 24 h. After centrifugation at 1000 rpm for 5 min, the supernatant was discarded, and 150.0 μL of LDH releasing reagent was added for further incubation for 1 h. Then 120.0 μL of supernatant and 60.0 μL of LDH detection solution were added to another well after centrifugation at 1000 rpm for 5 min and incubated at room temperature in the dark for 30 min. The absorbance was measured at 490 nm. In addition, the cytotoxicity of TCNH in the presence of H2O2 was evaluated with the same method as that of TCNH. The effect of TCNH on viability of HCECs and HCFs was examined by a live/ dead cell double staining kit. Briefly, cells with a density of 1 * 105 were grown on glass-bottom cell culture dishes for 24 h, and then cultured with diluted TCNH for another 24 h. The staining solution was prepared by mixing LiveDye (1.0 μL) and NucleiDye (1.0 μL) in DMEM/F12 medium (1.0 mL) supplemented with 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin. Afterwards, the cells were stained at 37 °C for 30 min and washed twice with PBS (pH 7.0) before visualization by CLSM. Live and dead cells were dyed green and red, respectively. In addition, the effect of TCNH in the presence of H2O2 on the viability of cells was surveyed with the abovementioned method. The in vivo biocompatibility of TCNH in the presence of H2O2 was assessed using 8-week-old female C57BL/6J mice. TCNH (4.8 μL) and H2O2 (10 mM, 0.2 μL) were mixed and dripped onto the mouse corneas once every hour for 8 h. The mice were sacrificed at 48 h post saline and TCNH + H2O2 intervention. The corneas were carefully harvested for further histopathological examination by hematoxylin–eosin (H&E) staining and morphological observation by SEM. The antibacterial activity of TCNH was assessed by the plate cultivation method. In brief, bacterial monocolony and LB culture medium (20.0 mL) were mixed and incubated at 37 °C for 16 h. After centrifugation, P. aeruginosa (19,660) was collected and diluted to 2*109 CFU/mL. Then bacterial suspension (1.0 μL), TCNH (191.0 μL), and H2O2 (10 mM, 8.0 μL) were mixed and further incubated at 180 rpm at 37 °C for 30 min. The mixture was diluted by gradient dilution, spread onto the LB agar plates, and then cultured at 37 °C overnight to count the bacterial loads. In addition, the mixture was also stained with a live/dead (SYTO 9/PI) bacterial viability kit for fluorescence detection and fixed with 2.5% glutaraldehyde for SEM analysis. Using the abovementioned method, E. coli (25922) and Candida albicans (98001) were adopted to evaluate the broad-spectrum antibacterial performance of TCNH, and the antibacterial activity of TCNH against drug-resistant bacteria was investigated using four strains of MDR P. aeruginosa (21027, 20059, 21173, 21715). The anti-biofilm activity of TCNH was investigated using the crystal violet (CV) staining method. In brief, TCNH (8.0 mg/mL, 500.0 μL), H2O2 (10 mM, 40.0 μL), LB medium (455.0 μL) and bacterial suspension (2*109 CFU/mL, 5.0 μL) were added to 48-well plates, and the mixture was cultured at 37 °C for 48 h. The formed biofilms were washed with PBS (pH 7.0) and stained with 1% CV staining solution for 20 min. The stained biofilms were then washed three times gently to remove unbonded CV and naturally dried. Acetic acid was used to dissolve the attached CV for quantitative analysis of the formed biofilms. The absorbance was measured using a microplate reader at 595 nm. The biofilm was further cultured on a round glass slide with a similar procedure as above. After 48 h, the glass slides were gently removed and washed with PBS (pH 7.0) to remove planktonic bacteria. Then the slides were stained with a live/dead (SYTO 9/PI) bacterial viability kit and observed under a confocal laser scanning microscope (CLSM). Intracellular ROS levels were determined by fluorescence spectroscopy using the 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) method. Briefly, the bacterial suspension (2*109 CFU/mL, 1.0 mL) was incubated with DCFH-DA (10.0 μL) for 30 min and centrifuged at 5000 rpm for 10 min. The sediments were washed with PBS (pH 7.0) 3 times to remove the free DCFH-DA. The collected bacteria were further used for conducting antibacterial experiments using the same procedure as in Biocompatibility Evaluation part. The resulting mixtures were then photographed with a fluorescence microscope (Nikon, Japan). All animal experiments were approved by the Shandong Eye Institute ethics committee and were carried out based on the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6J mice (8 weeks old, female) were purchased from Bei**g Vital River Laboratory Animal Technology Co., Ltd. (Bei**g, China). The P. aeruginosa (19660) keratitis model was selected to evaluate the antibacterial efficacy of TCNH in vivo. Mice (n = 32) were anesthetized through intraperitoneal injection of 0.6% pentobarbital (30.0 mg/kg). Afterwards, three 1-mm incisions were created on mouse corneas using a sterile 25-gauge needle and the mice were randomly divided into four groups (n = 8). The wound surface was inoculated with P. aeruginosa (5.0 μL, 105 CFU), and the mice in the four groups were treated with saline (5.0 μL), H2O2 (5.0 μL), TCNH (5.0 μL), or TCNH + H2O2 (5.0 μL), once every hour for a total of 8 doses. The corneas were observed using a slit lamp at 24 and 48 h to detect the infection progression. After 48 h of the first administration, all mice were sacrificed and the eyeballs were harvested for H&E staining, SEM, flow cytometry, bacterial load counting, and qRT–PCR analysis. In addition, P. aeruginosa (21027) was selected to evaluate the antibacterial efficacy of TCNH on MDR P. aeruginosa keratitis in mice. Mice (n = 32) were randomly divided into four groups (n = 8): saline, tobramycin (0.3 mg/mL), gentamicin (0.3 mg/mL), and TCNH + H2O2. Other experimental processes were performed with the same procedure as that for P. aeruginosa (19,660) keratitis. The mouse eyeballs in the four groups were collected, placed in 96-well plates, and digested overnight at 4 °C with dispase (15.0 mg/mL). The corneal epithelium and stromal layer, separated under a microscope and placed separately, were digested with trypsin (0.25%) and collagenase (2.0 mg/mL) at 37 °C for 1–2 h, respectively. The suspensions were merged, incubated with antibody mixtures (Ly6G-PE, F4/80-PE) for 30 min on ice, and further surveyed using a flow cytometer (BD FACSCalibur, USA). The harvested eyeballs were fixed in 10% neutral buffered formalin for 24 h, embedded in paraffin and cut into slices (5 μm-thickness). The slices were stained with H&E, and further imaged under a light microscope (Nikon, Japan). For immunohistochemistry, tissue slices were stained with primary antibodies (anti-Ly6G + Ly6c, anti F4/80), and subsequently reacted with secondary antibody (GTVisionTM I detection system/Mo&Rb). The hematoxylin was adopted to visualize the nuclei. The staining was analyzed under a light microscope (Nikon, Japan). All tests were performed at least three times. The data were statistically analyzed using Student’s t tests by Prism 8 (GraphPad, San Diego, CA, USA). A value of *P < 0.05 was considered statistically significant between the means. The results are presented as the mean ± standard deviation (SD).Biocompatibility evaluation
Antibacterial assays
Intracellular ROS detection
Pseudomonas aeruginosa keratitis models in mice
Flow cytometry
Histopathological analysis
Statistical analysis
Results and discussion
Fabrication and characterization
In the study, TCN was constructed by hydrothermal synthesis of cobalt tetroxide nanoparticles, metal-polyphenol coordination for immobilizing tannin into cobalt tetroxide and chemical reduction of silver nitrate for post-decoration of silver nanoparticles on the surface of cobalt tetroxide. Then, the TCNH eye drops were obtained by photoinitiated free radical polymerization of acetylated gelatin suspended with the as-prepared TCN nanoparticles. The morphology, structural properties, and chemical compositions of TCN were characterized by TEM and SEM. The images indicated the nanoscale spherical structure of TCN and the macroporous structure of TCNH with dozens of micrometer-sized pores (Fig. 1A, B, Additional file 1: Figure S1). HAADF-STEM, EDS map** images and EDS analysis (Fig. 1C, D, Additional file 1: Figure S2) presented Ag, C, Co, and O elements in TCN. XPS was adopted to investigate the chemical compositions and electronic states of TCN. The XPS survey spectrum (Fig. 2A) provided typical peaks of Ag, Co, C1s, and O1s. In the high-resolution XPS spectrum of Ag, the peaks at 374.1 and 368.2 eV were ascribed to Ag 3d3/2 and Ag 3d5/2, respectively, which was in accordance with the electron-binding energies of Ag 3d. In the Co spectrum of TCN, the peaks at 797.2, 785.2, 781.0, and 779.1 eV were assigned to Co 2p1/2, Co 2p3/2, Co2+, and Co-metal, respectively. In addition, the high-resolution C1s XPS spectrum exhibited the presence of C = C/C–C (284.5 eV), C = O (288.5 eV), and C-O (286.5 eV) groups. The O1s XPS spectrum could be deconvoluted into three peaks at 533.5 eV (C = O), 532.5 (C-O), and 531.5 eV (metal-oxide). These results confirmed the coordination of tannin with the Co3O4/Ag composite in TCNs. PXRD was then used to determine the crystallographic structure of the hydrogel, TCN and TCNH (Fig. 2B). The PXRD patterns of TCN exhibited diffraction peaks at 36.8, 44.2, 59.4, 64.8, 77.4, and diffraction peak at 38.0, which corresponded to the spinel cobalt tetroxide and cubic metallic Ag, respectively, while the PXRD pattern of the hydrogel exhibited no signals. After incorporation with TCN, TCNH exhibited a PXRD peak at 38.0, which mainly corresponded to TCN in TCNH.
Nitrogen adsorption-desorption experiments were performed to characterize the specific surface area of TCN using the Brunauer-Emmett-Teller model within the relative pressure range of 0.5–1.0, as shown in Fig. 2C. Type IV isotherm characteristics suggested the typical mesoporous structure of TCN, which could be attributed to nanoparticle packing. The specific surface area of TCN was calculated to be 126.7 m2/g. Figure 2D shows the FT-IR spectra of TCN, gelatin hydrogel, and TCNH. The peaks at 3339.4, 1575.1, 1477.0, 1364.9, and 1105.9 cm−1 implied the involvement of phenolic hydroxyls, aromatic rings (C = C stretching), and C-O bonds in TCN, manifesting the immobilization of tannin into cobalt tetroxide [41]. In addition, the characteristic signals of amino groups (1636.6 cm−1) in gelatin hydrogel and CoIII–O vibration (662.7 cm−1) in TCN were present in the spectrum of TCNH, indicating the successful incorporation of TCN into the hydrogel. In addition, ·OH signals were monitored by ESR. As shown in Fig. 2E,·OH signals were detected in the TCN + H2O2 and TCNH + H2O2 groups, but not in the hydrogel + H2O2 group, confirming the generation of ·OH by H2O2 under the catalysis of TCN. These results indicated the successful synthesis of TCN and TCNH.
Noteworthily, several tannic acid modified nanoparticle systems such as Fe2+-tannic acid, Fe3+-tannic acid and Ag-tannic acid have been developed for in vivo disease treatments [42,43,44,45]. Compared with these systems, the TCN nanoparticle in the TCNH system can be homogeneously dispersed in gelatin hydrogels, avoiding the disadvantages of nanoparticle suspensions including excessive sedimentation and aggregation, which could provide a support for the satisfactory peroxidase-like activity, biocompatibility, and antibacterial performance.
Peroxidase-like activity
The peroxidase-like activity of TCNH was spectroscopically evaluated using 3,3',5,5'-tetramethylbenzidine (TMB) and H2O2 as substrates. ·OH was produced by co-incubation of H2O2 with TCNH, which oxidized TMB into ox-TMB and changed the solution color from colorless to blue (Fig. 3A ,B). Blue color with absorption at 652 nm was not produced in the TMB, TMB + H2O2, TMB + H2O2 + hydrogel, TMB + TCN, TMB + TCNH, and TMB + hydrogel groups, while both the TCN + H2O2 + TMB and TCNH + H2O2 + TMB groups exhibited absorption peaks at 652 nm, suggesting that TCNH and TCN possessed peroxidase-like activities (Fig. 3C). The stronger adsorption peak in the TCNH + H2O2 + TMB group than in the TCN + H2O2 + TMB group could be attributed to the good dispersibility of TCN in the hydrogel, which might enhance the catalytic efficacy of TCN. Then the effects of reaction time and TMB concentration on catalytic activity were further investigated (Fig. 3D, E). As the reaction time or TMB concentration increased, the adsorption peaks at 652 nm were intensified, demonstrating the time-dependent and concentration-dependent peroxidase-like catalytic performances. In addition, we further surveyed the effect of temperature on the TMB catalytic activity of TCNH. As shown in Fig. 3F, the intensity of the adsorption peak at 25 °C was stronger than those at 4 °C, 37 °C, and 60 °C. The result was similar with the TMB catalytic activity of Co3O4-reduced graphene oxide nanocomposite in Shi’s previous report [4E). The images of corneal epithelial cells in the TCNH + H2O2 group showed a similar morphology to that in the saline group, indicating no obvious damage to the corneas after TCNH + H2O2 intervention. Both in vitro and in vivo experiments suggested the excellent biocompatibility of TCNH.
In vitro and in vivo biocompatibility of TCNH. Representative CLSM images of HCECs A and HCFs B by live/dead cell double staining after 24 h of cultivation. The quantitation of HCEC C and HCF D viability by LDH cytotoxicity assay kit after saline, H2O2, TCNH, and TCNH + H2O2 interventions. Representative SEM images of mouse corneas after saline and TCNH + H2O2 interventions E
In vitro antibacterial performance
The H2O2 and TCNH concentrations were optimized prior to antibacterial activity evaluation of TCNH against P. aeruginosa (19,660). As shown in Additional file 1: Figure S3A, the log10CFU numbers of P. aeruginosa in both the TCNH + H2O2 and H2O2 groups were over 7.0, and there was no obvious difference between the log10CFU numbers in two groups when the H2O2 and TCNH concentrations were set as 100 μM and 0.4 mg/mL, respectively, indicating the poor antibacterial activities of H2O2 and TCNH + H2O2 against P. aeruginosa under these conditions. With the increase in H2O2 concentration from 100 μM to 400 μM, log10CFU number in the TCNH + H2O2 group significantly decreased, while those in the H2O2 group were slightly reduced, indicating the improved antibacterial activity of TCNH + H2O2 at 400 μM. Then we further investigated the effect of TCN concentration in TCNH on log10CFU numbers with H2O2 concentration at 400 μM (Additional file 1: Figure S3B). With the increase in TCN concentration from 0.4 mg/mL to 2.0 mg/mL, the antibacterial activity of TCNH against P. aeruginosa gradually increased. As the TCN concentration increased to 4.0 mg/mL, log10CFU number dramatically decreased from 3.0 to 0. Based on these results, H2O2 (400 μM) and TCN concentration (4.0 mg/mL) were selected for subsequent antibacterial experiments.
The antibacterial property of TCNH against P. aeruginosa (19,660) was initially evaluated using the plate cultivation method. Figure 5A shows the plate cultivation results of bacteria after different interventions (saline, H2O2, TCNH, and TCNH + H2O2). The TCNH + H2O2 and TCNH groups exhibited high antibacterial activities as nearly no bacterial colonies were observed after interventions. However, bacteria all overgrew the LB agar plate in the saline and H2O2 groups, and there was no significant difference between two groups. In the dead/live (SYTO 9/PI) double staining assays (Fig. 5B), P. aeruginosa exhibited green fluoresce (live) in the saline and H2O2 groups, indicating that the bacteria in both groups were in good condition. After intervention with TCNH + H2O2 and TCNH, the numbers of live bacteria in both groups significantly decreased, which was consistent with the plate cultivation results. The incubated mixtures in the four intervention groups were centrifuged and the residues were further observed by SEM. As shown in Fig. 5C, P. aeruginosa in the saline treated group presented as a long oval rod with a smooth surface. After treatment with a low concentration of H2O2, the bacteria maintained their rod shape, and rare disruptions were observed on the bacterial surface, suggesting the poor antibacterial activity of H2O2 against P. aeruginosa. However, the bacterial cells were always accompanied by deformation and dents after intervention with TCNH + H2O2 and TCNH, indicating the obvious damage to P. aeruginosa caused by TCNH + H2O2 and TCNH. Then ROS levels in the four groups were detected by the DCFH-DA method (Fig. 5E). It was clearly found that there were nearly no ROS in either the saline or H2O2 group, sporadic ROS in the TCNH group and widely distributed ROS in the TCNH + H2O2 group. The omnipresent ROS in the TCNH + H2O2 group could be produced by the reaction of TCNH with exogenous H2O2. In addition, the morphology of TCNH + H2O2 treated bacteria was evaluated by TEM (Fig. 5D). The results revealed that TCN could penetrate the interior of P. aeruginosa due to its small particle size (red arrow), which might explain the process for inhibiting P. aeruginosa using TCNH.
In vitro antibacterial activity of TCNH against P. aeruginosa (19660). Representative photographs of bacterial colonies A, CLSM images of live/dead bacterial staining B and SEM images of bacteria C. TEM image of bacteria treated with TCNH + H2O2 D. Intracellular ROS levels E, CLSM images of biofilms F and CV staining assay G by saline, H2O2, TCNH, TCNH + H2O2 interventions
Biofilms, complexes of DNA, proteases, and polysaccharides, are self-produced and encapsulated by bacteria. It can prevent the penetration of antibiotics and reduce the efficacy of antibiotics in the treatment of bacterial infection [21, 37]. Therefore, it was of significance to investigate the antibiofilm capacity of TCNH before evaluating its performance in inhibiting P. aeruginosa keratitis. As shown in Fig. 5F, the formed biofilms in the four groups were observed by CLSM after SYTO 9/PI staining. Biofilms of P. aeruginosa were well formed after intervention with saline and H2O2 solution. However, there was no obvious biofilm formation in the TCNH + H2O2 and TCNH groups, indicating the significant inhibition efficacy of TCNH + H2O2 and TCNH against P. aeruginosa biofilm formation. The biofilms in the four groups were also quantified by CV staining assay (Fig. 5G). Compared with the saline and H2O2 groups, the TCNH + H2O2 and TCNH groups exhibited significantly reduced peak intensity (590 nm) and were demonstrated to be effective in inhibiting the formation of P. aeruginosa biofilms. The hybrid hydrogel can generate highly toxic ·OH in the presence of H2O2 due to its peroxidase-like activity, which could account for the antibacterial and antibiofilm performances of TCNH.
In addition, plate cultivation assays (Fig. 6A), dead/live (SYTO 9/PI) double staining experiments (Fig. 6B) and SEM (Fig. 6C) were also adopted to assess the efficacy of TCNH in inhibiting E. coli (25922) and C. albicans (98001). The absence of bacterial colonies, the decreased numbers of live bacteria and the deformed bacterial cells clarified the broad-spectrum antibacterial activity of TCNH.
In vivo prophylactic treatment of P. aeruginosa keratitis
The effect of TCNH on prophylactic treatment of P. aeruginosa infection was surveyed in vivo using mouse keratitis as the animal model. The corneas with three 1-mm incisions were inoculated with P. aeruginosa (19660). Saline, H2O2, TCNH and TCNH + H2O2 were dripped on the mouse corneas. At 24 and 48 h post-operation, the mouse corneas were observed using a slit lamp. As shown in Fig. 7A, after intervention with TCNH + H2O2, the mouse corneas were clear, and no obvious infection was found, which was different from the severe corneal infections in the saline and H2O2 groups as well as the mild corneal infection in the TCNH group. The corneal samples in the four groups were further subjected to H&E staining. As presented in Fig. 7B, inflammatory cell aggregation was observed in the saline, H2O2, and TCNH groups. However, no obvious inflammatory cell aggregation was observed in the TCNH + H2O2 group. The corresponding corneal bacterial loads of P. aeruginosa in the four groups were counted. The bacterial number in the TCNH + H2O2 group was significantly lower than those in the saline, H2O2, TCNH groups (Fig. 7C). Neutrophils and macrophages of corneas in the saline, H2O2, TCNH, and TCNH + H2O2 groups were further sorted by a flow sorter (Fig. 7D, E). The TCNH + H2O2 group possessed significantly lower proportions of neutrophils (0.63% ± 0.32%) and macrophages (0.80% ± 0.04%) than the other groups. Similar phenomenon can also be observed by immunostaining analysis of macrophages and neutrophils in the four groups (Additional file 1: Figure S5). Furthermore, the corneas in the saline and TCNH + H2O2 groups were examined by SEM (Fig. 7F). The images showed that the complete and regular morphologies of corneal epithelial cells in the TCNH + H2O2 group, which was similar with those of normal corneal epithelial cells (Fig. 4E). In contrast, corneal epithelial cells in the saline group tended to slough off the cornea, and the shed cells were always accompanied by P. aeruginosa (Fig. 7F, yellow arrow).qRT–PCR was adopted to detect the expression levels of inflammatory factors (IL-1β, TNFα and MCP1) and antioxidant related enzymes (SOD1, NQO1 and catalase) in corneal epithelial cells among the normal, saline, and TCNH groups (Fig. 7G). The results showed that inflammatory factors and antioxidant related enzymes in the saline group were significantly upregulated and downregulated, respectively, compared with those in the normal group. After TCNH + H2O2 intervention, there was dramatic decrease in inflammatory factor expression and an increase in antioxidant–related enzymes, suggesting that TCNH + H2O2 may alleviate inflammation and related oxidative stress injury. These results demonstrated that TCNH + H2O2 possessed positive activity in prophylactic treatment of P. aeruginosa keratitis in mice.
In vivo antibacterial activity of TCNH on P. aeruginosa (19660) keratitis. Representative photographs of treated eyes A, H&E staining images of mouse corneas B, bacterial loads of treated eyes C, and the proportions of neutrophils and macrophages in mouse corneas D, E after saline, H2O2, TCNH, and TCNH + H2O2 interventions. Representative SEM images of mouse corneas in the saline and TCNH + H2O2 groups F
Antibacterial performance of TCNH against MDR P. aeruginosa
Similar with the results of in vitro antibacterial activity against P. aeruginosa (19660), there were nearly no MDR bacterial colonies (21027, 20059, 21173, 21715) in the TCNH and TCNH + H2O2 groups according to the plate cultivation results (Fig. 8A; Additional file 1: Figure S4). Compared with the saline and H2O2 groups, the numbers of live bacteria (green) in the TCNH + H2O2 and TCN groups significantly decreased (Fig. 8B), which was also consistent with the plate cultivation results. The bacterial morphology in the four groups was investigated by SEM (Fig. 8C). Deformed P. aeruginosa was present in the TCNH + H2O2 and TCNH groups, while nearly intact P. aeruginosa was present in the saline and H2O2 treated groups, manifesting the in vitro antibacterial efficacies of TCNH and TCNH + H2O2 against MDR P. aeruginosa. Based on the above results, we further inspected the effect of TCNH on prophylactic treatment of MDR P. aeruginosa (21027) keratitis with gentamicin and tobramycin as the control groups. After administration of gentamicin and tobramycin, clear corneas and no obvious inflammation were observed in the P. aeruginosa (19660) treated group, while similar inflammatory cell infiltration and bacterial loads were observed in the saline (21027) and MDR P. aeruginosa (21027) treated groups (Fig. 8D; Additional file 1: Figure S6), which suggested that gentamicin and tobramycin were effective in controlling common strains such as P. aeruginosa 19660 other than MDR P. aeruginosa strains. Then MDR P. aeruginosa infected corneas were cured with TCNH + H2O2. As a result, there was no inflammatory cell infiltration and an obviously low bacterial load in the corneas (Fig. 8E, F). Furthermore, corneal morphology in the saline and TCNH + H2O2 groups was also investigated by SEM. The images showed that regular corneal epithelial cells were observed in the TCNH + H2O2 group, while the detached epithelial cells and MDR P. aeruginosa coexisted in the saline group (Fig. 8G, yellow arrow). These results demonstrated the satisfactory prophylactic therapeutic efficacy of the nanozyme composite hybrid hydrogel against MDR P. aeruginosa keratitis in mice.
In vitro and in vivo antibacterial activities of TCNH against MDR P. aeruginosa. Representative photographs of bacterial colonies A, CLSM images of live/dead bacteria B, and SEM images of MDR P. aeruginosa C in the saline, H2O2, TCNH, and TCNH + H2O2 groups. Representative photographs D and H&E staining images E, bacterial loads F of treated eyes after saline, H2O2, TCNH, and TCNH + H2O2 interventions. Representative SEM images of mouse corneas in the saline and TCNH + H2O2 groups G
Conclusions
In this study, tannin-coordinated nanozyme-based hybrid hydrogel eye drops were constructed by photoinitiated free radical polymerization of acetylated gelatin for incorporation of a self-prepared tannin-coordinated nanozyme into a gelatin hydrogel. The hybrid hydrogel exhibited good dispersibility, peroxidase-like activity, biocompatibility, and broad-spectrum antibacterial activity. Due to the intrinsic and nanozymic activities, the composite was successfully used to control P. aeruginosa and MDR P. aeruginosa keratitis. This was the first time for prophylactic treatment of P. aeruginosa infection in vivo and ophthalmic infection using nanozyme. Moreover, many efforts should be made to conduct the clinical prophylaxis of P. aeruginosa keratitis using nanozyme hydrogel eye drops. The developed nanozyme hydrogel eye drops provide a promising strategy for the treatment of bacterial and fungal infectious diseases.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Gao F, Shao T, Yu Y, **ong Y, Yang L. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat Commun. 2021;12:745.
Larsson D, Flach C. Antibiotic resistance in the environment. Nat Rev Microbiol. 2021. https://doi.org/10.1038/s41579-021-00649-x.
Makabenta J, Nabawy A, Li C, Schmidt-Malan S, Patel R, Rotello V. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol. 2021;19:23–36.
Pang Z, Raudonis R, Glick B, Lin T, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37:177–92.
Huang R, Li C, Cao-Milán R, He L, Makabenta J, Zhang X, Yu E, Rotello V. Polymer-based bioorthogonal nanocatalysts for the treatment of bacterial biofilms. J Am Chem Soc. 2020;142:10723–9.
Liu Y, Shi L, Su L, van der Mei H, Jutte P, Ren Y, Busscher H. Nanotechnology-based antimicrobials and delivery systems for biofilm-infection control. Chem Soc Rev. 2019;48:428–46.
Shan J, Yang K, **u W, Qiu Q, Dai S, Yuwen L, Weng L, Teng Z, Wang L. Cu2MoS4 nanozyme with NIR-II light enhanced catalytic activity for efficient eradication of multidrug-resistant racteria. Small. 2020;16:2001099.
Wang Z, Liu X, Duan Y, Huang Y. Infection microenvironment-related antibacterial nanotherapeutic strategies. Biomaterials. 2022;280: 121249.
Mujtaba J, Liu J, Dey K, Li T, Chakraborty R, Xu K, Makarov D, Barmin R, Gorin D, Tolstoy V, Huang G, Solovev A, Mei Y. Micro-bio-chemo-mechanical-systems: micromotors, microfluidics, and nanozymes for biomedical application. Adv Mater. 2021;33: e2007465.
Wu J, Wang X, Wang Q, Lou Z, Li S, Zhu Y, Qin L, Wei H. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem Soc Rev. 2019;48:1004–76.
Huang Y, Ren J, Qu X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem Rev. 2019;119:4357–412.
Alizadeh N, Salimi A. Multienzymes activity of metals and metal oxide nanomaterials: applications from biotechnology to medicine and environmental engineering. J Nanobiotechnol. 2021;19:26.
Li Y, Wang L, Liu H, Pan Y, Li C, **e Z, **g X. Ionic covalent-organic framework nanozyme as effective cascade catalyst against bacterial wound infection. Small. 2021;17: e2100756.
Zhang L, Liu Z, Deng Q, Sang Y, Dong K, Ren J, Qu X. Nature-inspired construction of MOF@COF nanozyme with active sites in tailored microenvironment and Pseudopodia-like surface for enhanced bacterial inhibition. Angew Chem Int Edit. 2021;60:3469–74.
Cao F, Zhang L, Wang H, You Y, Wang Y, Gao N, Ren J, Qu X. Defect-rich adhesive nanozymes as efficient antibiotics for enhanced bacterial inhibition. Angew Chem Int Edit. 2019;58:16236–42.
** J, Wei G, An L, Xu Z, Xu Z, Fan L, Gao L. Copper/carbon hybrid nanozyme: tuning catalytic activity by the copper state for antibacterial therapy. Nano Lett. 2019;19:7645–54.
Wang X, Shi Q, Zha Z, Zhu D, Zheng L, Shi L, Wei X, Lian L, Wu K, Cheng L. Copper single-atom catalysts with photothermal performance and enhanced nanozyme activity for bacteria-infected wound therapy. Bioact Mater. 2021;6:4389–401.
Wei F, Cui X, Wang Z, Dong C, Li J, Han X. Recoverable peroxidase-like Fe3O4@MoS2-Ag nanozyme with enhanced antibacterial ability. Chem Eng J. 2021;408: 127240.
Mu X, Wang J, Li Y, Xu F, Long W, Ouyang L, Liu H, **g Y, Wang J, Dai H, Liu Q, Sun Y, Liu C, Zhang X. Redox trimetallic nanozyme with neutral environment preference for brain injury. ACS Nano. 2019;13:1870–84.
Liang M, Wang Y, Ma K, Yu S, Chen Y, Deng Z, Liu Y, Wang F. Engineering inorganic nanoflares with elaborate enzymatic specificity and efficiency for versatile biofilm eradication. Small. 2020;16:2002348.
Mu Q, Sun Y, Guo A, Xu X, Qin B, Cai A, Hazard J. A bifunctionalized NiCo2O4-Au composite: Intrinsic peroxidase and oxidase catalytic activities for killing bacteria and disinfecting wound. Mater. 2021;402: 123939.
Sun D, Pang X, Cheng Y, Ming J, **ang S, Zhang C, Lv P, Chu C, Chen X, Liu G, Zheng N. Ultrasound-switchable nanozyme augments sonodynamic therapy against multidrug-resistant bacterial infection. ACS Nano. 2020;14:2063–76.
Wang J, Wang Y, Zhang D, Chen C. Intrinsic oxidase-like nanoenzyme Co4S3/Co(OH)2 hybrid nanotubes with broad-spectrum antibacterial activity. ACS Appl Mater Inter. 2020;12:29614–24.
Zhang L, Zhang L, Deng H, Li H, Tang W, Guan L, Qiu Y, Donovan M, Chen Z, Tan W. In vivo activation of pH-responsive oxidase-like graphitic nanozymes for selective killing of Helicobacter pylori. Nat Commun. 2021;12:2002.
Zhang Y, Li D, Tan J, Chang Z, Liu X, Ma W, Xu Y. Near-infrared regulated nanozymatic/photothermal/photodynamic triple-therapy for combating multidrug-resistant bacterial infections via oxygen-vacancy molybdenum trioxide nanodots. Small. 2020;17:2005739.
Jegel O, Pfitzner F, Gazanis A, Oberländer J, Pütz E, Lange M, von der Au M, Meermann B, Mailänder V, Klasen A, Heermann R, Tremel W. Transparent polycarbonate coated with CeO2 nanozymes repel Pseudomonas aeruginosa PA14 biofilms. Nanoscale. 2022;14:86–98.
Lee J, Zhang L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell. 2015;6:26–41.
Zhang M, Zhang H, Feng J, Zhou Y, Wang B. Synergistic chemotherapy, physiotherapy and photothermal therapy against bacterial and biofilms infections through construction of chiral glutamic acid functionalized gold nanobipyramids. Chem Eng J. 2020;393: 124778.
Wang L, Gao F, Wang A, Chen X, Li H, Zhang X, Zheng H, Ji R, Li B, Yu X, Liu J, Gu Z, Chen F, Chen C. Defect-rich adhesive molybdenum disulfide/rGO vertical heterostructures with enhanced nanozyme activity for smart bacterial killing application. Adv Mater. 2020;32:2005423.
Zhang W, Zhao Y, Wang W, Peng J, Li Y, Shangguan Y, Ouyang G, Xu M, Wang S, Wei J, Wei H, Li W, Yang Z. Colloidal surface engineering: growth of layered double hydroxides with intrinsic oxidase-mimicking activities to fight against bacterial infection in wound healing. Adv Healthc Mater. 2020;9:2000092.
Jia Z, Lv X, Hou Y, Wang K, Ren F, Xu D, Wang Q, Fan K, **e C, Lu X. Mussel-inspired nanozyme catalyzed conductive and self-setting hydrogel for adhesive and antibacterial bioelectronics. Bioact Mater. 2021;6:2676–87.
Bao Q, Hu P, Xu Y, Cheng T, Wei C, Pan L, Shi J. Simultaneous blood–brain barrier crossing and protection for stroke treatment based on edaravone-loaded ceria nanoparticles. ACS Nano. 2018;12:6794–805.
** J, An L, Huang Y, Jiang J, Wang Y, Wei G, Xu Z, Fan L, Gao L. Ultrasmall FeS2 nanoparticles-decorated carbon spheres with laser-mediated ferrous ion release for antibacterial therapy. Small. 2021;17:2005473.
Clasky A, Watchorn J, Chen P, Gu F. From prevention to diagnosis and treatment: biomedical applications of metal nanoparticle-hydrogel composites. Acta Biomater. 2021;122:1–25.
Koide H, Okishima A, Hoshino Y, Kamon Y, Yoshimatsu K, Saito K, Yamauchi I, Ariizumi S, Zhou Y, **ao T, Goda K, Oku N, Asai T, Shea K. Synthetic hydrogel nanoparticles for sepsis therapy. Nat Commun. 2021;12:5552.
Li X, He L, Li Y, Chao M, Li M, Wan P, Zhang L. Healable, degradable, and conductive MXene nanocomposite hydrogel for multifunctional epidermal sensors. ACS Nano. 2021;15:7765–73.
Wang F, Zhao L, Song F, Wu J, Zhou Q, **e L. Hybrid natural hydrogels integrated with voriconazole-loaded microspheres for ocular antifungal applications. J Mater Chem B. 2021;9:3377–88.
Fleiszig S, Kroken A, Nieto V, Grosser M, Wan S, Metruccio M, Evans D. Contact lens-related corneal infection: intrinsic resistance and its compromise. Prog Retin Eye Res. 2020;76: 100804.
Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124:1678–89.
Gao N, Me R, Dai C, Yu F. ISG15 acts as a mediator of innate immune response to Pseudomonas aeruginosa infection in C57BL/6J mouse corneas. Invest Ophthalmol Vis Sci. 2020;61:26.
Lin S, Cheng Y, Zhang H, Wang X, Zhang Y, Zhang Y, Miao L, Zhao X, Wei H. Copper tannic acid coordination nanosheet: a potent nanozyme for scavenging ROS from cigarette smoke. Small. 2020. https://doi.org/10.1002/smll.201902123.
Mu D, Wang W, Li J, Lv P, Liu R, Tan Y, Zhong C, Qi Y, Sun X, Liu Y, Shen S, Li Y, Xu B, Zhang B. Ultrasmall Fe(III)-tannic acid nanoparticles to prevent progression of atherosclerotic plaques. ACS Appl Mater Interfaces. 2021;13:33915–25.
He Z, Hu Y, Gui Z, Zhou Y, Nie T, Zhu J, Liu Z, Chen K, Liu L, Leong K, Cao P, Chen Y, Mao H. Sustained release of exendin-4 from tannic acid/Fe (III) nanoparticles prolongs blood glycemic control in a mouse model of type II diabetes. J Control Release. 2019;301:119–28.
Qin J, Liang G, Cheng D, Liu Y, Cheng X, Yang P, Wu N, Zhao Y, Wei J. Controllable synthesis of iron-polyphenol colloidal nanoparticles with composition-dependent photothermal performance. J Colloid Interface Sci. 2021;593:172–81.
He X, Gopinath K, Sathishkumar G, Guo L, Zhang K, Lu Z, Li C, Kang E, Xu L. UV-assisted deposition of antibacterial Ag-tannic acid nanocomposite coating. ACS Appl Mater Interfaces. 2021;13:20708–17.
**e J, Cao H, Jiang H, Chen Y, Shi W, Zheng H, Huang Y. Co3O4-reduced graphene oxide nanocomposite as an effective peroxidase mimetic and its application in visual biosensing of glucose. Anal Chim Acta. 2013;796:92–100.
Funding
This work was supported by Shandong Provincial Key Research and Development Program (Major Scientific and Technological Innovation Project, 2022CXGC010505), Innovation Project of Shandong Academy of Medical Sciences (2019PT002), National Natural Science Foundation of China (82000852) and the Academic Promotion Programme of Shandong First Medical University (2019ZL001).
Author information
Authors and Affiliations
Contributions
HW and FS wrote the manuscript and conducted most of the experiments and data analysis. JF designed the scheme and assisted in preparing figures in the manuscript. XQ carried out the hematoxylin–eosin staining and qPCR experiments. LM performed the flow cytometry and analysed the data. LX, WS and QZ conceived and revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Shandong Eye Institute ethics committee and were carried out based on the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Consent for publication
All authors agree to be published.
Competing interests
The authors declared no confict of interests in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
SEM of TCNH. Figure S2. EDS of TCN. Figure S3. Optimization of the H2O2 and TCNH concentrations. Figure S4. In vitro antibacterial activity of TCNH against MDR P. aeruginosa (20059, 21173, 21715). Representative photographs of bacterial colonies after intervened by saline, H2O2, TCNH, and TCNH+H2O2. Figure S5. Immunostaining analysis of macrophages and neutrophils in mouse corneas after saline, H2O2, TCNH, and TCNH+H2O2 interventions. Figure S6. In vivo antibacterial activities of gentamicin and tobramycin on P. aeruginosa (19660) keratitis. Representative photographs (A) and hematoxylin–eosin staining images (B) of treated eyes and bacterial loads of treated mouse eyes (C) after gentamicin and tobramycin interventions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, H., Song, F., Feng, J. et al. Tannin coordinated nanozyme composite-based hybrid hydrogel eye drops for prophylactic treatment of multidrug-resistant Pseudomonas aeruginosa keratitis. J Nanobiotechnol 20, 445 (2022). https://doi.org/10.1186/s12951-022-01653-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-022-01653-w